Practice Essentials
Antithrombin III (ATIII) is a non-vitamin K–dependent protease that inhibits coagulation by neutralizing the enzymatic activity of thrombin; enzymatic targets include factors IIa, IXa, and Xa. Antithrombin III activity is markedly potentiated by heparin, the principal mechanism by which both heparin and low–molecular-weight heparin (LMWH) result in anticoagulation. [1, 2, 3]
Congenital antithrombin III deficiency is an autosomal dominant disorder in which an individual inherits one copy of the SERPINC1 (also called AT3) gene on chromosome 1q25.1, which encodes antithrombin III. This condition leads to increased risk of venous and arterial thrombosis, with an onset of clinical manifestations typically appearing in young adulthood. This form is most commonly diagnosed during childhood by screening after an affected family member has been identified or after a child has had a thrombotic event.
Severe congenital antithrombin III deficiency, in which the individual inherits two defective genes, is a rare autosomal recessive condition associated with increased thrombogenesis, typically noted in the neonatal period or early infancy. This condition is rarely compatible with life. Most affected neonates, however, have heterozygous antithrombin III deficiency rather than the homozygous state.
Acquired antithrombin III deficiency is a deficiency of antithrombin primarily due to consumption or decreased production. It is observed in situations in which activation of the coagulation system is abnormal. Common conditions that result in acquired antithrombin III deficiency include disseminated intravascular coagulation (DIC), microangiopathic hemolytic anemias due to endothelial damage (ie, hemolytic-uremic syndrome), veno-occlusive disease (VOD) (in patients undergoing bone marrow transplantation), sepsis, liver disease, and nephrotic syndrome. Oral contraceptive use and even heparin administration have also been associated with antithrombin III deficiency.
Diagnosis of anthrombin III deficiency
Laboratory studies that can be performed in the workup for antithrombin III deficiency include the following:
-
Antithrombin assays
-
Prothrombin time (PT) and activated partial thromboplastin time (aPTT)
-
Protein C (antigen and activity tests) and protein S (total and free tests)
-
Factor V Leiden testing
-
Homocysteine level
-
Anticardiolipin antibodies (immunoglobulin G [IgG] and IgM class)
Imaging studies that can be used in the evaluation of antithrombin III deficiency include the following:
-
Echocardiography: This should be performed in all patients with antithrombin III deficiency, especially if they have evidence of arterial thrombus
-
Doppler ultrasonography: Doppler ultrasonography of the affected extremity with compression should be performed at diagnosis and then used in follow-up to determine resolution of an acute thrombus
-
Ventilation-perfusion scanning: Pulmonary thrombosis can be imaged with this modality
Management of anthrombin III deficiency
In neonates who have homozygous AT3 mutations, arterial and venous thrombosis are seen, particularly if vascularly invasive procedures (eg, extracorporeal membrane oxygenation [ECMO], umbilical vessel catheterization) are performed. In these patients, replacement of antithrombin III using antithrombin III concentrates or fresh frozen plasma is recommended.
Enoxaparin (Lovenox), a low–molecular-weight heparin (LMWH), is frequently used to prevent thrombi, as well as to prevent the propagation of thrombi that have already occurred. In antithrombin III deficiency, however, the activity of LMWH is not as reliable as in an otherwise healthy person.
Once a patient with congenital antithrombin III deficiency has developed thrombosis, anticoagulation is more strongly indicated. Warfarin (Coumadin) is the principal anticoagulant used. This vitamin K antagonist is administered at a dose to maintain an international normal ratio (INR) on PT of 1.5-2.5.
Pathophysiology
A study by Yilmaz and Gunaydin indicated that in the absence of major acquired risk factors, patients under age 45 years with venous thromboembolism (VTE) commonly have inherited risk factors for the condition. The study involved 58 VTE patients under age 45 years, 45 of whom had at least one inherited risk factor, including 14 with antithrombin III deficiency. (Factor V Leiden mutation was the most common risk factor, occurring in 30 patients.) [4]
See the image below.
Antithrombin III deficiency is usually inherited in an autosomal dominant fashion.
Several different genetic abnormalities have been identified in separate kindreds. The defects most frequently affect the proteins translation or post-translational processing, which results in decreased functional antithrombin III.
A pediatric study by Kumar et al indicated that null-sense mutations in AT3 can lead to a more severe thrombotic phenotype than do missense mutations in the gene. The investigators reported that patients in the study with null-sense AT3 mutations had a 66.7% probability of 5-year VTE-free survival, compared with a 92.0% probability in those with missense mutations. [5]
Two types of antithrombin III deficiency have been described. Type I (classic) is the result of decreased synthesis of biologically normal antithrombin III, resulting in quantitative deficiency of the enzyme; thus, both antigen and activity levels are similarly low. Type II (functional) is the result of a discrete molecular defect within the antithrombin III itself, resulting in a normal antigen level associated with a reduction in heparin cofactor activity levels of about 50%.
Two functional assays for antithrombin III deficiency identified two separate subclasses of the type 2 defect. The assays are the antithrombin-heparin cofactor activity and the progressive antithrombin activity. The first measures the ability of heparin to neutralize the enzymatic activity of thrombin and factor Xa and the second measures the ability of antithrombin III to neutralize thrombin in the absence of heparin. Heterozygotes of type 2 antithrombin III deficiency that exhibit diminished levels of both thrombin-heparin cofactor and progressive antithrombin activity level usually carry a mutation near the thrombin binding site (active site), while those with diminished thrombin-heparin cofactor and normal progressive antithrombin activity are carriers of a defect in the heparin binding site. The distinction between the two is clinically important, since the second group of patients rarely develop any symptoms unless they are homozygous for the defect; then they will have significant thrombotic events early in life and their thrombin-heparin cofactor level will be less than 10%. Both parents will be affected.
Numerous discrete point mutations of the antithrombin gene have been identified. [6, 7] The type I deficiency is the most common phenotype however.
Two defects, wibble and wobble, have been characterized as resulting in substitutions of a single amino acid at the beginning of the beta sheet of the peptide. Substitutions that result in polar amino acids in this location result in decreased activity and survival of the enzyme (Wibble), whereas others cause amino acid substitutions and result in less severe decreases. Clinically, the Wibble gene is associated with a greater risk of thrombosis early in life (second decade).
Other regions of the gene (eg, the "shutter" region) are also associated with clinically significant thrombosis. The shutter region of the antithrombin III protein is centrally located near the "A" B sheet and facilitates opening and closing of the enzyme's active site. These examples are all part of the conformational diseases that may occur in all SERPIN class enzymes (see Alpha-1 Antitrypsin Deficiency).
Acquired deficiencies are commonly due to increased coagulation activity secondary to endothelial injury or the presence of antiphospholipid (AP) antibodies (eg, lupus anticoagulant). In both of these situations, antithrombin III is consumed at increased rates because of excessive activation of the coagulation pathway. Other reported mechanisms of acquired antithrombin III deficiency include chronic liver disease, with resultant synthetic failure, and protein loss due to ascites or nephrotic syndrome, as indicated earlier.
A study by Di Minno et al indicated that in patients with unprovoked VTE, even mild antithrombin deficiency significantly increases the risk of VTE recurrence. In the report, which involved 823 patients who had suffered a first VTE, the investigators found that after adjusting for major VTE risk factors and anticoagulation duration, patients with antithrombin levels as high as 80% had a significantly higher risk of VTE recurrence than did those with antithrombin levels above 80%. [8]
A study by Sokol et al further supported evidence that mild antithrombin deficiency can be linked to recurrent VTE. The report found that when patients with antithrombin activity of less than 70% were compared with those with antithrombin activity of over 80%, VTE recurrence in the lower group had a hazard ratio of 3.7. [9]
Epidemiology
Frequency
United States
The prevalence of antithrombin III deficiency has been estimated as approximately 1 per 2,000 individuals. [10] However, in patients who develop thrombosis, the prevalence is increased to between 1 in 20 individuals to 1 in 200 individuals. [11]
International
In some regions, for example Scotland, the frequency of type I antithrombin III deficiency in blood donors has been reported to be about 0.2 per 1,000 population; type II has a frequency of about 2-3 per 1,000 population. [12]
Mortality/Morbidity
The incidence of thrombotic events is increased in patients with antithrombin III deficiency. In the hereditary form, the lifetime risk has been estimated to be 50-85%, whereas in other groups, the risk may be as low as 20%. [13, 11]
A study by Lenz et al in women with pregnancy-related complications and VTE found antithrombin III deficiency in just 3% of the group, although other thrombophilic risk factors were present. [14]
A literature review by Rhéaume et al indicated that women with asymptomatic antithrombin deficiency have a greater risk of pregnancy-associated VTE. The estimated odds ratio for VTE in these women, using pooled results from case-control studies, was 6.09. [15]
Race
Congenital antithrombin III deficiency is recognized in all racial and ethnic groups.
Sex
No sex-related difference is noted in terms of the prevalence of congenital antithrombin III deficiency. Women of childbearing age present special risk factors. Antithrombin III deficiency, like other congenital procoagulant defects, may contribute to an increased risk of spontaneous abortions. Particularly in cases of fetal or umbilical thrombosis as the cause of the miscarriage, it is important to consider antithrombin III deficiency, along with protein C or protein S deficiency and AP antibody syndrome.
Oral contraceptives (OCs) contain estrogen, which is a stimulator of coagulation. Women who are antithrombin III–deficient heterozygotes are at an increased risk of thrombosis when taking OCs, which have also been implicated in causing some decrease in antithrombin III level.
Parents of newborns who have a thrombotic event are at increased risk of having a procoagulant disorder themselves. These individuals should be referred for further assessment of their own risk factors.
Age
Patients who are homozygous for gene defects often present in the neonatal period; individuals who are heterozygous for gene defects may remain asymptomatic well into middle age. A thrombotic challenge, such as placement of a central venous catheter or other vascular catheter, frequently unmask disease due to heterozygotic gene mutations. Individuals who have multiple catheter-related thrombotic events, or life/organ threatening events with no other risk factor, should be evaluated for an underlying procoagulant condition.
-
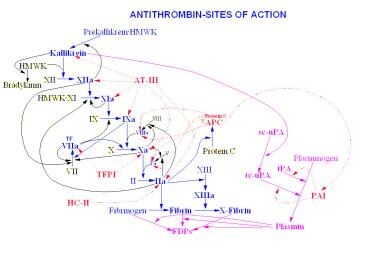
Antithrombin (AT) sites of action.