Background
Foot infections can be difficult problems for physicians to treat because of the biomechanical complexities of the extremity and the underlying circumstances that cause the infections. With the notable exception of patients without diabetes, peripheral vascular disease (PVD), or other metabolic conditions, these infections typically follow a traumatic event or tissue loss with contamination by foreign materials, colonization by bacteria, or both. However, the more common presentation is that of a patient whose health is compromised with a metabolic or peripheral vascular defect that complicates optimal successful treatment. [1, 2, 3]
Treatment strategies for foot infections have been changing and evolving as a result of pharmacologic and technical breakthroughs. Plastic and reconstructive techniques for limb salvage have altered the course of treatment for foot infections, with the goals of functional restoration and a major decrease in amputation rates.
Physicians need to be aware of the many different types of foot infections that exist. Some foot infections are very simple, but others are quite complex, and no two are exactly alike. They are categorized into the following three groups:
-
Soft-tissue foot infections
-
Bone infections in the foot
-
Diabetic foot infections
Soft-tissue infections in the foot consist of any infectious process affecting the skin, subcutaneous tissue, adipose tissue, superficial or deep fascia, ligaments, tendons, tendon sheaths, joints, or joint capsules. Considering that there are more than 20 joints, 44 tendons, approximately 100 ligaments, four major compartments, and numerous fascial planes in the normal foot, one can readily appreciate the potential for complex problems.
Many events can be responsible for these soft-tissue infections. A description of soft-tissue infection includes simple, moderate, and severe infection, which includes but is not limited to infections in the immunocompromised patient, infections associated with PVD, emergency soft-tissue infections, and infections associated with trauma.
Given the limited soft-tissue envelope, it is common for bone to be involved when an infectious process is present in the foot.
Diabetes mellitus (DM) has been diagnosed in more than 25 million people in the United States. (See Diabetic Foot Infections, below.) It can produce a complex clinical picture because of its involvement in numerous different organ systems. The coexisting diabetic peripheral neuropathy and compromised distal vascularity act synergistically, putting these patients at high risk for pedal complications. Diabetics tend to develop ulcerations in the feet, which often lead to infection of the soft tissue and bones. Foot infections in diabetic patients can be unpredictable and are typically polymicrobial.
Many factors can complicate the treatment of foot infections. Patients who are immunocompromised and those with peripheral vascular, metabolic, or biomechanical defects can have complicated courses that lead to delayed healing time. When conservative and noninvasive treatment measures fail to resolve foot infections, surgical intervention is required. Infections often stem from chronic ulcerations in the feet.
To achieve the final goals of a functional foot that is free from infections and will not succumb to further breakdown, it is frequently necessary to consult various surgical specialists. Foot ulcerations are commonly associated with decreased perfusion to the lower extremity. Peripheral vascular surgeons or interventional radiologists should be consulted for aggressive revascularization or other interventions to increase perfusion and optimize healing. It is also important to consult plastic surgeons when skin grafts, local flaps, or free tissue flaps are needed for final coverage of defects.
Pharmacologic advances and newer reconstructive techniques have changed the way in which physicians treat infected lower extremities. Treatment goals today are directed toward salvaging limbs and restoring function while decreasing major amputation rates.
Guidelines for the management of complicated skin and soft-tissue infections have been published by the Surgical Infection Society (SIS). [4] Guidelines for the management of diabetic foot infections have been published by the International Working Group on the Diabetic Foot (IWGDF) [5] and by a multispecialty group assembled by the Jiangsu Medical Association and the Diabetes Society of the Chinese Medical Association. [6]
Soft-Tissue Infections in Foot
Cellulitis
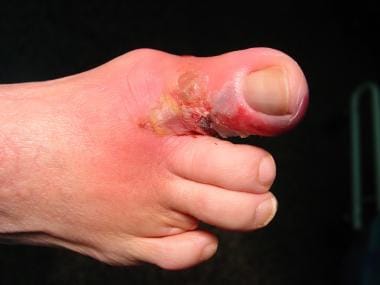
Cellulitis is often the first sign of a soft-tissue infection of the foot. In most cases, this marker represents an isolated localized skin infection, but it may represent a more severe process (see the images below). Cellulitis usually originates from minor irritation of the skin, such as cuts or abrasions, but also develops from more severe puncture wounds or trauma. Group A streptococci are the most common bacterial contaminants responsible for soft-tissue infections, with Staphylococcus aureus being the second most common. Each is present in natural skin flora. [1]
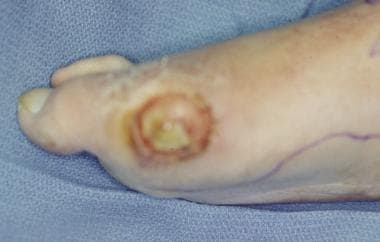
 Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
 Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
The clinical presentation for cellulitis typically includes blanching erythema in the skin that is generally bright red and angry. On examination, a break in the skin is usually present; however, patients with venous stasis or lymphatic disease can develop cellulitis with no apparent tissue loss. Increased temperature relative to the contralateral foot and leg is often noted.
Dependent rubor, observed in patients with PVD, and gout are the two differential diagnoses most commonly mistaken for cellulitis in the foot. However, in neuropathic patients with loss of protective sensation (eg, diabetics), Charcot arthropathy should be included in the differential diagnosis early; it is often difficult to differentiate the rubor of Charcot from the rubor of cellulitis, and the two conditions can coexist. [7] Complications include lymphadenitis and contiguous inoculation to adjacent osseous structures, joints, deep structures, or tissue planes.
Initial treatment for cellulitis as a result of an abrasion in a host who is not immunocompromised includes oral antibiotic therapy using first-generation cephalosporins, aminopenicillins, or quinolones. Knowledge of local community and hospital antibiograms helps guide empiric therapy. [8, 9]
Group A streptococci, the most common pathogens, are usually susceptible to penicillin V and cephalexin. In more severe cases, oxacillin (2 g IV q4hr) or cefazolin (1 g IV q8hr) can be used. [10] Cellulitis usually responds well to antibiotics, rest, and elevation of the extremity. In more severe cases where septic embolization occurs, invasive treatment with debridement of necrotic tissue becomes necessary. In complex cases, early consultation with infectious disease specialists is highly recommended.
Paronychia
Paronychia is a common soft-tissue infection, with inflammation of the periungual area adjacent to the nail grooves and borders (see the images below). It can be initiated by a traumatic event such as dropping objects on the toes, having them stepped on, or improper fitting of shoes. More frequently, paronychia results from an ingrown toenail (onychocryptosis). Recurrent ingrown toenail or ulceration has been reported to occur in association with Marjolin squamous cell carcinoma (SCC). [11]
 Onychocryptosis with paronychia of medial and lateral nail folds. Treatment consists of oral antibiotic therapy and removal of involved nail borders. Nail borders puncture skin and act as foreign bodies. These must be removed in order for infection to completely resolve.
Onychocryptosis with paronychia of medial and lateral nail folds. Treatment consists of oral antibiotic therapy and removal of involved nail borders. Nail borders puncture skin and act as foreign bodies. These must be removed in order for infection to completely resolve.
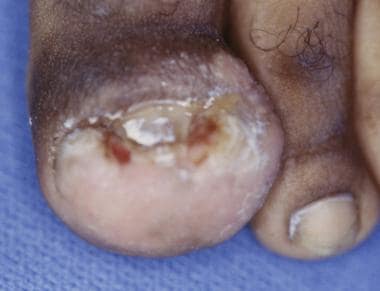
 Onychocryptosis in 13-year-old boy that has gone untreated for several months. Removal of ingrown nail has not resolved infection in this case, because underlying osteomyelitis has developed. Note remaining erythema and edema of hallux.
Onychocryptosis in 13-year-old boy that has gone untreated for several months. Removal of ingrown nail has not resolved infection in this case, because underlying osteomyelitis has developed. Note remaining erythema and edema of hallux.
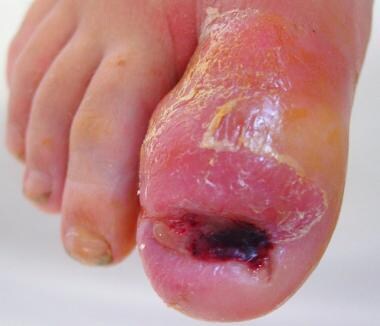
 Osteomyelitis of the distal phalanx secondary to untreated onychocryptosis. This ia rare situation demonstrates importance of prompt treatment. Patient underwent partial hallux amputation.
Osteomyelitis of the distal phalanx secondary to untreated onychocryptosis. This ia rare situation demonstrates importance of prompt treatment. Patient underwent partial hallux amputation.
Underlying onychomycosis also can be a predisposing factor, which results in paronychia. [12]
Initial treatment should include antibiotic therapy and warm soaks to the affected digit. Antibiotic therapy should be directed toward the offending pathogens, which commonly derive from the skin flora. [13] When onychocryptosis is the underlying etiology, that portion of the nail should be removed to address the soft-tissue reaction. A partial nail avulsion involves removing the border of the ingrown nail. Chronic recurrent paronychias can be treated with a chemical matrixectomy or via local surgical excision.
Puncture wounds
Puncture wounds occur more than 50% of the time on the plantar surface of the foot, with more than 90% of these involving penetration by a nail. [14] Other objects that can cause these wounds include wood, metal, plastic, glass, and animal or human teeth. Puncture wounds can lead to serious complications by inoculating deep spaces of the foot, including bones, joints, tendons, and deep fascia. Therefore, the depth of penetration is one of the most important factors in determining whether a wound will resolve without complex intervention. Degree of infection can depend on location, type of penetrating object, retained foreign bodies, and penetration through shoes and socks.
The most common organisms inoculating penetrating wounds are S aureus, beta-hemolytic streptococci, and various other anaerobic bacteria. Pasteurella multocida is typical in dog and cat bites or claw puncture wounds. Viridans streptococci are responsible for most problems related to human bites. Pseudomonas aeruginosa is usually responsible for infection when the injury is the result of object penetration through shoes and socks. [15]
Signs of erythema, edema, and pain at the site of injury usually suggest that an infectious process has begun. The most common presenting complaint in the emergency department (ED) is persistent pain. [16] A complete history can aid in determining the age of the wound in addition to the mechanism, the type of contamination, and the possibility of a retained foreign body. Pain out of proportion to the injury strongly indicates that an infection has developed. Inoculation into a joint can lead to septic arthritis with rapid destruction of the articular structures.
P aeruginosa is the most common pathogen associated with osteochondritis following puncture wounds in the foot. Foreign-body penetration into bone can lead to direct-extension osteomyelitis. [17] If the wound can be probed directly to bone or joint capsule, deep inoculation is more likely, and the wound should be explored and debrided in surgery.
Diagnostic imaging is indicated for all puncture wounds. [18, 19] When foreign bodies become lodged within the foot, they must be removed. If the object is visible or readily accessible through the wound, an attempt can be made in the ED to retrieve it. If the object is deeply embedded, it should be addressed in surgery and retrieved under fluoroscopic guidance. Deep penetrating injuries should be debrided to remove all contaminants and nonviable tissue. Superficial wounds can be irrigated with saline either in the ED or the physician's office. Drains are often helpful for deep penetrating wounds in addition to open or closed systems.
Empiric antibiotic therapy should be directed toward the suspected pathogen, with S aureus being the most common bacterial contaminant. A complete history aids in antibiotic choice against other pathogens (eg, P multocida, P aeruginosa, viridans streptococci). Puncture wounds that occur in contaminated environments (eg, farms and industrial areas) can result in polymicrobial infections, which include gram-negative and anaerobic bacteremia.
Deep wound cultures and sensitivities help guide the antibiotic choice. Superficial infections usually can be treated adequately with oral antibiotics. Deep wounds or those with suspected bone and joint involvement should include IV therapy and appropriate surgical debridement.
Immunocompromise
Pedal infections in patients who are immunocompromised can be difficult to diagnose and treat because of comorbidities, which often alter the presentation and require treatment. This group of patients includes persons with HIV infection, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and high-dose corticosteroid use, DM, and asplenia. These patients can have impaired host defenses and are at higher risk for acquiring infections.
Human immunodeficiency virus
Patients with HIV are more susceptible to fungal and viral pedal infections. [20] Tinea pedis and onychomycosis are often observed in this population. Human papillomavirus (HPV), manifesting itself as verruca plantaris, occurs at a higher rate than in the non-HIV population.
Fungal infections usually can be managed with a variety of antifungal creams. Verrucae can be more challenging to treat. The condition often requires several modalities for complete resolution. Common therapies include salicylic acid application, cryotherapy, blistering agents, and surgical excision.
Systemic lupus erythematosus
Individuals with SLE are predisposed to infection by virtue of their impaired humoral immunity and lowered T cell–mediated immunity, which results from immunosuppressive therapy. [21] Lupus flares can be mistaken for an infection, and the differential diagnosis must be evaluated carefully. SLE skin and soft-tissue infections most commonly are caused by S aureus and less commonly by group A streptococci. The absence of a true leukocytosis may create a confusing environment for the diagnostician.
Rheumatoid arthritis
Patients with systemic arthropathies such as RA are often on long-term corticosteroid therapy, which suppresses cell-mediated immunity and puts them at higher risk for infection. [22] Classic presentations include synovitis, pannus formation, and periarticular bone and cartilage destruction. These local manifestations of systemic disease can be confused with soft-tissue infection.
Approximately 20% of patients with RA have rheumatoid nodules. They frequently occur over pressure areas in the foot and can rupture, causing skin breakdown, erythema, and infection. Therefore, patients with RA presenting with possible pedal infections require a thorough workup to exclude the conditions discussed above.
Diabetes mellitus
Persons with DM are truly compromised as a consequence of their impaired host defenses, decreased perfusion to the lower extremities, and diminished protective sensation in the feet secondary to peripheral neuropathy. This group of patients is very challenging to treat (see Diabetic Foot Infections, below).
Asplenia
The spleen is the primary site for immunoglobulin M (IgM) synthesis. Patients with asplenia thus are at higher risk for all infections, particularly for infection with encapsulated bacterial organisms. [21] It is important to determine if these patients have joint implants or internal fixation in the feet because of the organisms' affinity for seeding these areas. For fractures requiring open reduction and external fixation (ORIF), prophylaxis against these pathogens is appropriate. [23] Typical pedal infections in these patients are clinically indistinguishable from those in healthy hosts, except that they are often more severe. Judicious and early consultation of infectious disease specialists is highly recommended.
Antibiotic coverage should be directed toward the encapsulated pathogens and should consist of third- or fourth-generation cephalosporins. Imipenem should be used in the patient who is allergic to penicillin. If hardware is present and has become colonized, it should be removed for complete resolution of the infection.
Peripheral vascular disease
The poorly perfused or ischemic foot is prone to more frequent and severe infections as a consequence of low oxygen tension. [1] The inflammatory response to stress can be impaired. Decreased local perfusion, edema formation, and neutrophil infiltration are present. Small abrasions in these patients can become quite problematic because they do not have sufficient blood supply to heal properly. Prolonged healing time leaves the patient more susceptible to infection and complications. Ischemic feet are also prone to ischemic-type ulcerations that have an extended healing time and an increased chance of infection.
Evaluation of a patient with PVD who presents with an apparent foot infection can be quite challenging, and one must distinguish dependent rubor from cellulitis (see the image below). Dependent rubor is very common in this group and consists of a foot that is erythematous while dependent, closely mimicking cellulitis. The two conditions differ clinically in that dependent rubor does not have increased temperature, can be bilateral, and, most important, completely resolves with elevation of the extremity. Therefore, when a patient presents with absent pedal pulses or known PVD and has apparent cellulitis in the foot, the leg should be elevated to observe if the erythema subsides.
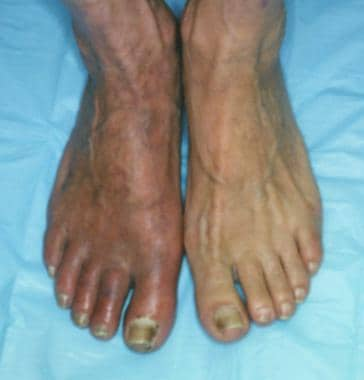 Dependent rubor in lower extremity of patient with peripheral vascular disease. This condition can mimic cellulitis, which must be excluded during physical examination. Erythema completely resolves when extremity is elevated.
Dependent rubor in lower extremity of patient with peripheral vascular disease. This condition can mimic cellulitis, which must be excluded during physical examination. Erythema completely resolves when extremity is elevated.
Injured tissue in the critically ischemic foot can proceed to gangrene because the metabolic requirements to heal the wound are much greater than those needed to maintain normal tissue viability. [24] This event is typically not an emergency and is described as dry gangrene (see the image below). Clinically, the lesion is dry and stable with no local or systemic signs of infection. A small amount of erythema may be present around the demarcation line between healthy and nonviable tissue, and again, dependent rubor must be ruled out. If this wound remains stable and does not become grossly infected, allowing the digit to autoamputate is often the management of choice.
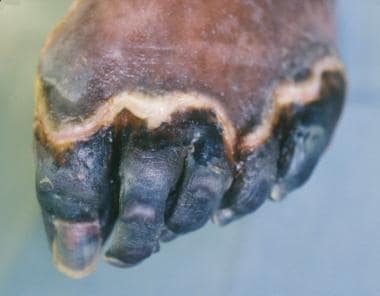 Dry gangrene of digits 1-5. Digits are stable and have no signs of infection. Note line of demarcation between healthy and nonviable tissue. Treatment options include autoamputation and surgical amputation. This does not represent urgent surgical situation unless it becomes infected.
Dry gangrene of digits 1-5. Digits are stable and have no signs of infection. Note line of demarcation between healthy and nonviable tissue. Treatment options include autoamputation and surgical amputation. This does not represent urgent surgical situation unless it becomes infected.
Conversely, gas gangrene is an emergency, and its pathophysiology differs from that of dry gangrene. [25] It has a wet appearance, and the patient usually has local and systemic signs of infections requiring surgical debridement (see the images below). (See Surgical Emergencies in Soft-Tissue Infection below.)
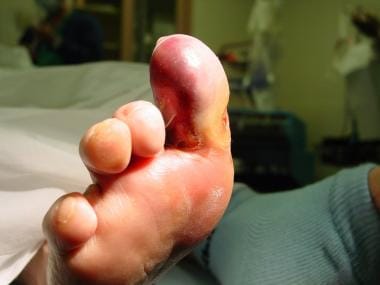 Early wet gangrene of hallux. This began secondary to embolic event with showering to hallux. Patient has history of peripheral vascular disease and previously lost her fourth toe because of it.
Early wet gangrene of hallux. This began secondary to embolic event with showering to hallux. Patient has history of peripheral vascular disease and previously lost her fourth toe because of it.
Antibiotic therapy should be directed toward the offending organisms. Ischemic tissues may not respond to antibiotics and therefore may require débridement (often aggressive). In managing deep-space infections, debridement in surgery is the only option for infection control. Superficial wound infections not probing to deep structures may respond to topical dressings and antibiotic therapy.
Trauma
Foot wounds associated with various traumatic events are inherently contaminated. Potential infecting organisms vary, depending on how, when, and where the injury occurred. Ideally, a thorough history clarifies these questions. If rapid signs of infection develop, group A streptococcal and clostridial species must be excluded because they occur within hours of injury. Tetanus prophylaxis should be reviewed with all patients, and vaccine is administered when appropriate. [26]
Burn wounds
Burn wounds to the feet, like burns to other parts of the body, are susceptible to becoming infected by various microorganisms. This represents a major complication for the newly exposed tissue. Common pathogens include P aeruginosa, S aureus, Escherichia coli, Klebsiella, and Enterococcus species. [27]
Treatment includes aggressive debridement of all nonviable tissue, administration of systemic antibiotics, and application of topical antimicrobial agents. Silver sulfadiazine cream 1% remains one of the more popular topical agent because of its effectiveness in controlling P aeruginosa, as well as many other wound pathogens. [28] Once wounds are stable, coverage of the area should be sought using synthetic skin equivalents, porcine xenografts, allografts, or autografts.
Degloving injuries
Degloving injuries result from a shearing force on the skin, which causes it to be undermined and elevated through the subcutaneous plane. A flap of tissue is created with a margin remaining intact or becoming completely avulsed. This represents a potentially serious situation that can lead to significant infection.
Initial treatment consists of high-pressure irrigation and debridement of all necrotic tissue. The soft-tissue defect is similar to a burn wound; thus, common pathogens can be expected. Because these wounds on the feet typically occur in contaminated environments such as motor vehicle accidents or farm-type injuries, anaerobic pathogens also should be expected.
As with burn injuries, topical and systemic antibiotics should be administered. If a tissue flap remains intact, it is replaced over the wound without tension and allowed to demarcate. Once the margins are defined, the nonviable tissue is debrided, and plans for final reconstruction using split-thickness skin graft or flap coverage are initiated.
Crush injuries
Crush injuries are common with foot trauma, especially in industrial settings. The widespread use of safety boots has decreased the incidence and severity of these injuries, but they still occur on a regular basis. The degree of injury can include mild contusions, skin lacerations, fractures, disruption of vascular supply, or combinations thereof. Crush injuries to the digits commonly include a nail-bed laceration with fracture of the underlying phalanx. These are treated as open fractures with irrigation, local wound care, tetanus prophylaxis, and antibiotics. Industrial settings generally are contaminated; therefore, surgical debridement and delayed closure should be considered.
The vascular supply to the digits is easily compromised and often damaged in crush injuries. Decreased tissue perfusion and oxygen tension occur, causing an ischemic digit. Therefore, in addition to typical pathogens, anaerobic organisms should be suspected and prophylaxis against these organisms employed. Crush injuries to the midfoot and hindfoot have a lower incidence of soft-tissue infection relative to similar injuries to the digits. Open fractures in these areas still have a heightened risk for osteomyelitis (this is discussed later in this article). [29]
Gunshot wounds
Gunshot wounds generally are described as being of either low or high velocity. The speed at which the bullet is traveling as it exits the barrel or enters the body determines the group under which it is classified; it also serves as an indicator for the amount of damage induced by the projectile, with low velocity yielding relatively more benign injuries. [30] Handguns, which typically use low-velocity ammunition, cause most civilian gunshot wounds.
All gunshot wounds are contaminated with bacteria. The old myth that a bullet becomes sterilized by the high temperatures reached in the barrel is false. [31] The newly inoculated bacteria multiply in proportion to the amount of devitalized tissue. The tissue surrounding the bullet path becomes devitalized with compromised blood flow. It takes a few days for new capillaries to grow into the disrupted area. The interim period of decreased blood results in poor delivery of antibiotics to necrotic regions.
Boucree et al demonstrated Staphylococcus epidermidis to be the most common pathogen cultured in a large study of gunshot wounds to the foot. A wide range of gram-negative and gram-positive organisms as well as aerobes and anaerobes also have been observed. [32]
Early debridement and IV antibiotics are the recommended treatments of choice. All nonviable tissue should be debrided, and the original wound should be left open for 4-7 days. The wound is debrided as often as needed and finally is closed by delayed primary closure. All periwound erythema and edema must be resolved prior to reconstruction.
Lawn mower injuries
Upward of 60,000 lawn mower injuries occur annually in the United States, most of them involving the lower extremities. Injuries can range from simple lacerations to more severe fractures and amputations. Because the setting for these injuries is inherently dirty, a wide variety of pathogens will be present. The combination of pathogens and severity of injury leads to a prolonged and expensive treatment course, with an estimated annual cost of $475 million.
A good history is important for determining the mechanism of injury, the setting in which the injury occurred, and tetanus status. The injuries often occur after patients fall off or are struck by a riding mower; thus, lesions away from the lower extremity also must be excluded on examination. Lacerations should be inspected in depth to ensure that all tendon, bone, and neurovascular structures are intact. These can easily be compromised because of their superficial nature.
After a complete history is obtained and a thorough physical examination is performed, treatment should begin with broad-spectrum antibiotics covering both gram-positive and gram-negative organisms. These accidents are considered farm-style injuries; the most common organisms cultured are Enterobacter cloacae, P aeruginosa, Enterococcus species, coagulase-negative Staphylococcus, diphtheroids, and S aureus. [33]
In addition to antibiotic therapy, the patient is taken to the operating room for irrigation and debridement. The grass, water, dirt, and debris that frequently contaminate these wounds are removed, and all nonviable tissue is excised. As with most contaminated wounds, these are left open and packed until the wound converts to healthy tissue. Several staged debridements are commonly needed before the damaged limb is definitively reconstructed.
Surgical Emergencies in Soft-Tissue Foot Infection
Fortunately, only a small group of potentially life-threatening situations require immediate and aggressive treatment to preserve a limb or a life. Necrotizing fasciitis and gas gangrene are the two most common pedal surgical emergencies. Local tissue damage, progressive gangrene, and potential systemic toxemia and/or death can result from these infections. [2]
Necrotizing fasciitis
Necrotizing fasciitis is characterized by widespread necrosis of fascia and deeper subcutaneous tissues, with initial sparing of skin and muscle. Eventually, skin involvement is noted, with cellulitis evolving into cutaneous gangrene. The most common underlying risk factor is being a patient with DM. A study by Elliott reported that foot ulcerations and infections associated with diabetes were the second most common cause of necrotizing fasciitis; 15.2% of cases of necrotizing fasciitis were due to foot ulcerations and infections associated with diabetes. [34]
Surgical wounds and infections resulting from IV drug abuse or "skin popping" can also lead to necrotizing infections. Aerobic streptococci are typical pathogens, in addition to Bacteroides species, staphylococci, and enterococci, which all play a role in the infectious process. E coli and Proteus species are facultative anaerobic gram-negative rods that often are cultured from these wounds.
Patients usually present with nonspecific inflammatory signs of pain, swelling, and erythema. Crepitus in the soft tissue is almost diagnostic for this condition but is not always present at the initial presentation. Blistering of the skin and soft-tissue gas on radiographic examination are also highly indicative. The average time from onset of symptoms until the patient is observed in the ED is 4 days.
The keys to successful management of necrotizing fasciitis and associated low mortality are as follows [35] :
-
Early diagnosis - A classification model has been proposed that may facilitate prompt discrimination of necrotizing fasciitis and osteomyelitis [36]
-
Appropriate antibiotic therapy - Antibiotics must be broad-spectrum to cover polymicrobial flora; piperacillin-tazobactam and ticarcillin-clavulanate are used frequently and are very effective; combination therapy with an antipseudomonal, a third-generation cephalosporin, and clindamycin is also indicated
-
Appropriate medical management
-
Prompt surgical intervention, which often involves multiple debridements - Aggressive debridement is mandatory to control local factors such as tissue necrosis and anaerobic bacterial overgrowth
Gas gangrene
Gas gangrene (clostridial myonecrosis) is considered a surgical emergency (see the images below). It includes a rapid fulminating course, severe toxin-related systemic toxicity, a vast level of tissue destruction, and a high mortality. Rapid diagnosis and aggressive management are crucial for limb preservation.
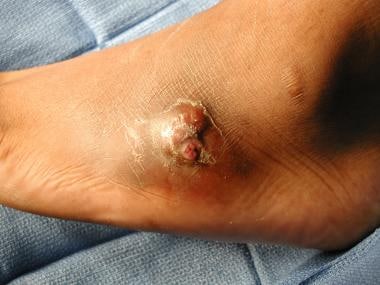
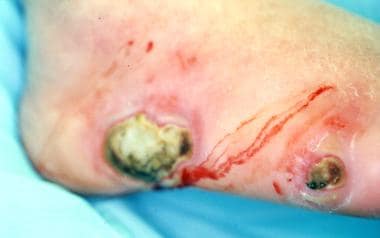 Gangrene with underlying deep-space infection. "Milking" wound produced purulent exudate from the abscess.
Gangrene with underlying deep-space infection. "Milking" wound produced purulent exudate from the abscess.
 Early wet gangrene of hallux. This began secondary to embolic event with showering to hallux. Patient has history of peripheral vascular disease and previously lost her fourth toe because of it.
Early wet gangrene of hallux. This began secondary to embolic event with showering to hallux. Patient has history of peripheral vascular disease and previously lost her fourth toe because of it.
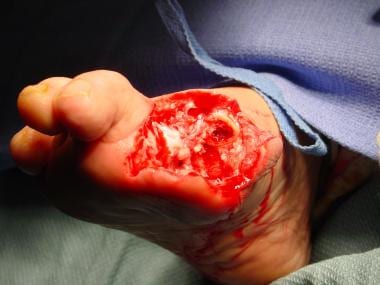 Wet gangrene is surgical emergency. Intraoperative photo was taken after irrigation and debridement with partial first-ray resection. Note healthy tissue margins and high level of perfusion once necrotic tissue has been removed.
Wet gangrene is surgical emergency. Intraoperative photo was taken after irrigation and debridement with partial first-ray resection. Note healthy tissue margins and high level of perfusion once necrotic tissue has been removed.
Six different species of Clostridium can be responsible for the soft-tissue destruction; however, Clostridium perfringens is the most common. C perfringens produces 12 active tissue toxins responsible for the syndromes of gas gangrene. Clostridia are saprophytes and are ubiquitous. Infections leading to gas gangrene require an opportunistic environment. Prerequisites include a wound, contamination with clostridia, and a depressed oxygen state at the site of inoculation. This accounts for the increased incidence of gangrene noted in diabetics and patients with PVD. A decreased oxygen state also can be observed postoperatively from local edema and tight dressings.
The time to onset is typically in the range of 12-24 hours but can be as short as 6 hours; thus, a high initial index of suspicion is necessary. Excruciating progressive muscular pain is the first and most important symptom. Systemic findings are more frequent and severe in individuals with gas gangrene than in those with necrotizing fasciitis. Diaphoresis, low-grade fevers, tachycardia, and mental status changes are often observed with this population.
The skin initially has a pale-white, shiny, and tense tone. Eventually, an orange discoloration develops with hemorrhagic bullae, leading to a brown serosanguineous discharge that is foul-smelling; the smell is distinctly different from the typical foul smell of an anaerobic infection. Microscopic examination of the discharge exhibits a dense smear of club-shaped gram-positive rods, numerous red blood cells (RBCs), and rare polymorphonuclear leukocytes (PMNs). Crepitus is felt in the tissue with progression of the disease but is not always palpable early on. Radiographs demonstrate gas distributed in the myofascial bundles. [37]
Successful management includes high-dose IV antibiotic therapy with aggressive surgical debridement. The antibiotic of choice is penicillin G 10-12 million U/day. Chloramphenicol 4 g/day can be used in patients who have a penicillin allergy. Surgical debridement is performed on an emergency basis and confirms the diagnosis suspected clinically when necrotic muscle is found beyond the area of trauma. Multiple debridements are performed every 24-48 hours, often requiring fasciotomies and/or amputations.
Hyperbaric oxygen (HBO) therapy is an accepted adjunct to—but not a replacement for—antibiotic and surgical treatments. High oxygen concentrations reduce the germination rate of activated C perfringens spores and inhibit the production of alpha-toxins. [38, 39] However, most studies have demonstrated similar survival rates in patients treated with and without HBO. When used, HBO therapy is administered with 100% oxygen at 3 atm for 1-2 hours every 8-12 hours for a total of six to eight treatments. [40, 41]
Bone Infections in Foot
The normal adult anatomy of the foot includes 26 bones and two sesamoids. The foot can be divided into tarsal bones, metatarsals (MTs), and phalanges. The greater tarsus, or hindfoot, comprises the calcaneus and talus. The lesser tarsus, or midfoot, includes the cuboid, navicular, medial cuneiform, intermediate cuneiform, and lateral cuneiform. The forefoot comprises the five MTs and the 14 phalanges.
Osteomyelitis is relatively common, especially in conjunction with other infectious processes in the foot. The typically thin soft-tissue layer and close proximity to the outside environment place an individual at risk for direct inoculation via puncture wounds and spread from overlying soft-tissue infection. The classic route of hematogenous spread is not common in the foot. Direct-extension osteomyelitis is typical in the diabetic population. Treatment for pedal osteomyelitis can involve 6-8 weeks of IV antibiotic therapy. However, surgical debridement with combined antibiotic therapy is the treatment of choice and often reduces the duration of antibiotic therapy to 2-4 weeks.
Acute osteomyelitis
Hematogenous spread of infection is one of the three classic routes of bone contamination and is the most common means of acquiring osteomyelitis in the skeletally immature patient. The other two forms are contiguous spread and direct implantation. Hematogenous osteomyelitis is uncommon in the foot. Patterns of infection differ for children and adults.
Hematogenous spread
The vascular anatomy of the undeveloped osseous structures determines the extent and involvement of bone infection. Neonates have diaphyseal vessels that can penetrate growth plates, creating a vascular connection with the epiphysis, which allows the spread of infection. Epiphyseal infections are common in neonates for this reason. Metaphyseal capillaries in children older than 1 year do not cross the physis but often create sinusoidal lakes in the metaphysis. This predisposes to infection in the metaphyseal bone. There is also a 25% incidence of hematogenous spread to nontubular bones, often involving the calcaneus.
The closure of the growth plates in adults results in a vascular connection between the metaphysis and epiphysis that is once again established. Therefore, adults are at higher risk for joint contamination; however, tubular bone involvement is not observed very often. Hematogenous spread in adults occurs in the spine, pelvis, and, to a lesser extent, the small irregular bones of the foot.
Acute osteomyelitis results causes inflammation within the bone, leading to edema, cellular infiltration, and eventual abscess formation. The increased pressure allows organisms to spread into cortical bone and eventually into the subperiosteal space. Elevation of the periosteum and subsequent lying down of new bone creates an involucrum, which is often the first sign of osteomyelitis on radiographic examination. It is generally more pronounced in neonates and children because of the looser attachment of the periosteum. Conversely, sinus tracts are more common in adults, with abscess formation in adjacent soft tissues.
The progression of cortical osteolysis in the tubular bones of the foot can lead to stress risers and pathologic fractures. Radiographs in this group, especially in children, are not specific and can mimic a variety of malignant bone tumors, which need to be evaluated further.
Contiguous spread
Contiguous spread of infection involves contamination from the soft tissue inward toward bone. The method of inoculation differs from hematogenous spread. Microbes invade the periosteum, causing disruption of the cortex and dissemination into bone marrow. Direct-extension osteomyelitis is the most common form of bone infection in the foot, and the diabetic population accounts for most cases.
Periosteal reaction and involucrum indicate infection radiographically. Periostitis without osteomyelitis can be observed with adjacent soft-tissue infection, and periostitis with new bone formation can be observed in traumatic induced soft-tissue infections. Early radiographic findings can be misleading and often confusing, especially when complicated by preexisting trauma.
Direct implantation
Direct implantation of pathogens from deep puncture wounds is responsible for many cases of osteomyelitis in the feet of persons who are not diabetic. Puncture wound trauma from stepping on nails accounts for most of these infections, with P aeruginosa being present in as many as 90% of cases. [10] Surgical reconstruction can lead to infection and subsequent osteomyelitis; however, in most cases, it is observed in conjunction with repair of open fractures. [42]
Chronic osteomyelitis
Chronic osteomyelitis is defined as the presence of bone infection for more than 6 weeks. In the foot, it is most commonly observed in diabetic individuals because their compromised immunity and vascular insufficiency predispose them to deep bone infections. Osteomyelitis also can be associated with open fractures, PVD, immunocompromised hosts, neurologic deformity of the ankle and foot, [43] and improper treatment of the acute condition.
Chronic osteomyelitis can be either active or inactive. Both states can demonstrate bone destruction, cortical disruption, and sclerosis; thus, they can be difficult to distinguish from a bone tumor. [44, 45] Inactive infection does not harbor viable pathogens within the bone. Thickening of the cortex and resolving osteolysis can be observed and are signs of healing.
Several different signs can indicate an active chronic osteomyelitis. Brodie abscess more often is associated with hematogenous spread and is observed on radiographs as a lucent area surrounded by sclerosis and periostitis. The tibia is the most common site of this lesion, but it also can infect the tarsal bones of the foot. Staphylococci are the most common pathogens observed and usually exhibit diminished virulence.
On plain films, a Brodie abscess can mimic osteoid osteoma, especially when it is intracortical. Another sign of active infection on radiographs is the presence of sequestra, focal areas of sclerosis that can harbor viable pathogens. Periostitis and osteolysis are still observed, as well as increased edema in the overlying soft tissue.
Osteomyelitis in trauma
Patients who are acutely traumatized are at considerable risk for bone infections. Traumatic injuries usually are subject to contamination with skin flora, with the most common one being S aureus. Ubiquitous organisms can include anaerobes associated with farm or soil injuries and other more common gram-positives or gram-negative organisms observed in relatively cleaner situations. [46] A thorough history of the accident helps determine which pathogens to suspect.
Infections are the primary cause of instability and nonunion in open fractures. It has been estimated that 70% of open fractures are contaminated at the time of injury. [47] Open fractures in the foot are often observed in persons with crush injuries, individuals involved in motor vehicle and pedestrian accidents, and persons with lawn mower–type injuries.
Gustilo et al developed the following classification system for open fractures and demonstrated that the incidence of infection was directly correlated with the extent of soft-tissue damage [48] :
-
Type I fractures are low-energy injuries with minimal soft-tissue damage and a surface wound smaller than 1 cm; they have an infection rate of 0-2%
-
Type II fractures occur with moderate energy forces and cause an associated soft-tissue laceration greater than 10 cm; these injuries have an infection rate of 2-7%
-
Type III fractures result from high-energy forces and are divided into IIIA, IIIB, and IIIC, depending on degree of soft-tissue involvement, bone destruction, and vascular injury; type IIIA fractures have a 7% infection rate, type IIIB have a 10-50% rate, and type IIIC have a 25-50% rate
Early debridement and appropriate antibiotic therapy are essential for prevention of pedal infections. Patzakis et al demonstrated a rate of infection with open fractures of 13.9% in a group of patients receiving a placebo and a rate of 2.3% in patients receiving a cephalosporin. [15] Antibiotic coverage for type I open fractures is oriented toward gram-positive organisms; cefazolin is the first-line agent. Combination therapy using an aminoglycoside plus cefazolin should be used in type II and III fractures to provide additional coverage against gram-negative organisms. If Clostridium infection is suspected, coverage with penicillin should be added. [49]
The incidence of osteomyelitis following gunshot wounds to the foot is relatively low.
A retrospective study by Boucree et al demonstrated that 81 of 101 patients with gunshot wounds to the foot sustained fractures. Of these, only four had osteomyelitis and were associated with high-velocity gunshots. Contributing to the low infection rate was an aggressive treatment protocol. Low-velocity wounds demonstrated ideal uncomplicated healing following hospitalization, a minimum of 3 days of IV antibiotic therapy using a first-generation cephalosporin, and irrigation and debridement as needed. Incision and drainage (I&D) usually was performed only if the patients exhibited clinical signs of infection after the 3 days (see the images below). [32]
 Bullet entry wound. Patient was initially treated conservatively with antibiotics and no surgical debridement.
Bullet entry wound. Patient was initially treated conservatively with antibiotics and no surgical debridement.
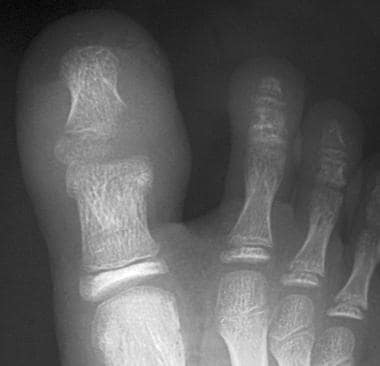
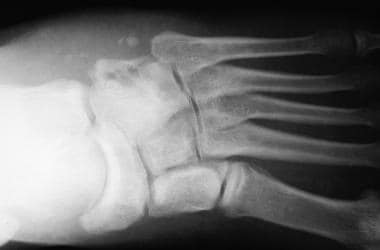 Radiographs of foot demonstrate development of osteomyelitis. Cuboid, anterior portion of calcaneus, base of fifth metatarsal, and base of fourth metatarsal were all involved and required debridement.
Radiographs of foot demonstrate development of osteomyelitis. Cuboid, anterior portion of calcaneus, base of fifth metatarsal, and base of fourth metatarsal were all involved and required debridement.
In Boucree's study, high-velocity gunshot wounds were treated with a protocol with slight variations. These patients were hospitalized, received broad-spectrum IV antibiotics, and underwent multiple debridements. Three of the four patients with osteomyelitis had I&D only once, whereas the others had I&D a minimum of three times. The authors believed that the high-velocity wounds required repeat debridements because the amount of necrotic bone was not completely evident after 48 hours. Aggressive treatment with repeat debridement is important for prevention of infection. [32]
Diabetic Foot Infections
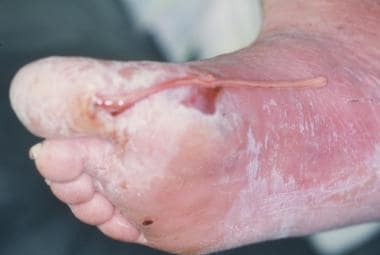
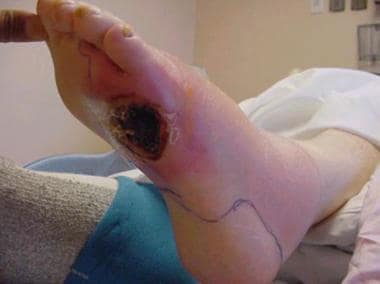
Foot infections in diabetic patients are responsible for more hospital days than any other aspect of diabetes. [50, 51] Typically, the diabetic foot infection stems from an open ulceration or wound (see the image below).
Peripheral neuropathy with or without vascular disease puts these patients at increased risk. Immunopathy, neuropathy, and vascular disease provide an environment that fails to support standard wound healing as observed in the nondiabetic population. [52, 53] Diabetic peripheral neuropathy has several components involving both somatic and autonomic fibers. Type-A sensory fibers are responsible for light touch sensation, vibratory sensation, pressure, proprioception, and motor innervation to the intrinsic muscles of the foot.
The muscles exhibiting early involvement are the flexor digitorum brevis, the lumbricals, and the interosseous muscles. These groups act to stabilize the proximal phalanx against the MT head, preventing dorsiflexion at the metatarsophalangeal (MTP) joint during midstance in the gait cycle. With progression of the neuropathy, these muscles atrophy and fail to function properly. This causes the MTP joints to become unstable, allowing the long flexors and extensors to act unchecked on the digits.
Dorsal contractures develop at the MTP joints with development of hammertoe deformity, also known as intrinsic minus disease. The deformity acts to plantarflex the MTs, making the heads more prominent and increasing the plantar pressure created beneath them. It also acts to decrease the amount of toe weightbearing during the gait cycle, which also increases pressure on the MT heads.
Normal anatomy consists of an MT fat pad located plantar to the MTP joints. This structure helps dissipate pressures on the MT heads from the ground. When the hammertoe deformity occurs, the fat pad migrates distally and becomes nonfunctional. This results in elevated plantar pressures that increase the risk of skin breakdown and ulceration due to shearing forces. [54]
Type-C sensory fibers detect painful stimuli, noxious stimuli, and temperature. When these fibers are affected, protective sensation is lost. This manifests as a distal symmetric loss of sensation described in a "stocking" distribution and proves to be the primary factor predisposing patients to ulcers and infection. [55]
Patients are unable to detect increased loads, repeated trauma, or pain from shearing forces. Consequently, injuries such as fractures, ulceration, and foot deformities go unrecognized. Repeat stress to high-pressure areas or bone prominences that would be interpreted as pain in the patient without neuropathy also go unrecognized. Gait patterns go unchanged, and the stresses eventually cause tissue breakdown and ulceration.
Autonomic involvement causes an interruption of normal sweating at the epidermal level and causes arteriovenous (AV) shunting at the subcutaneous and dermal levels. Hypohidrosis leads to a noncompliant epidermis that increases the risk of cracking and fissuring. AV shunting diminishes the delivery of nutrients, oxygen, and antibiotics to a certain tissue region. Skin and subcutaneous tissues become more susceptible to infection. [56]
Vascular disease is a common manifestation of DM. Diminished perfusion to the feet decreases tissue resilience, leading to rapid tissue death and impeded wound healing. The oxygen supply, nutrients, and chemical mediators needed for the wound-healing repair process often are delayed or absent. Long-standing open wounds are prone to infection, and the lack of adequate blood flow makes fighting infection and delivery of antibiotics difficult. The vessels typically are affected by atherosclerotic obstruction, [57] with individuals who are diabetic being affected at an earlier age than persons with vasculopathy without diabetes. The larger arteries in the legs are affected, with usual sparing of the arteries in the feet. [58]
The absence of palpable pedal pulses suggests some form of PVD. Other signs of a more progressive disease state include pallor on elevation, dependent rubor, absence of digital hair, thickened toenails, and slow capillary refill time to the digits. A history of pain in the feet at rest, claudication, impotence, and coronary artery disease are also markers of vascular disease.
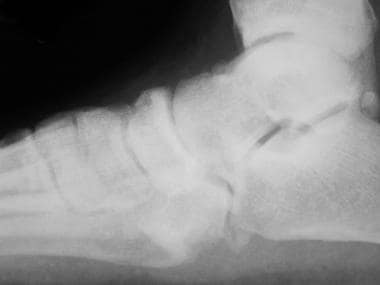
Charcot foot disease is known as a neuropathic osteoarthropathy and can be observed in other patients who are neuropathic in addition to patients with diabetes. [7] The exact etiology is still unknown; however, the most common theory involves hyperperfusion of the foot. The autonomic component of the neuropathy leads to vasodilation and hyperperfusion. The perfusion causes demineralization of the bones. Weightbearing forces cause the bones to begin to fragment and fracture, leading to collapse of the arch. The long-term sequelae of a foot with a rocker-bottom shape lead to high-pressure areas that are prone to ulceration (see the images below).
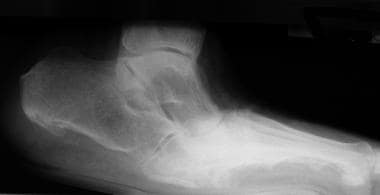 Lateral radiograph of diabetic patient with Charcot foot disease. Note midfoot collapse, leaving patient with inverted arch (rockerbottom foot deformity).
Lateral radiograph of diabetic patient with Charcot foot disease. Note midfoot collapse, leaving patient with inverted arch (rockerbottom foot deformity).
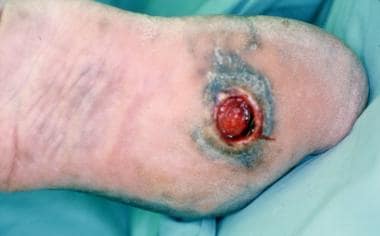 Ulceration on plantar surface of the foot secondary to high pressures created from Charcot foot deformity.
Ulceration on plantar surface of the foot secondary to high pressures created from Charcot foot deformity.
Charcot ulcerations are typically mechanical in nature but can become infected within the soft-tissue and osseous structures. A midfoot collapse with tissue loss and radiographic signs of osteomyelitis in addition to clinical signs of edema and erythema often leads to a confusing and difficult-to-diagnose condition. [59, 60] (See the image below.) Charcot osteoarthropathies often are diagnosed as osteomyelitis by plain radiographs. Scintigraphic studies help in determining the nature of these changes.
Adding to the complexities of diabetic foot infections are the multiple organisms observed in this setting. Diabetic foot infections are typically polymicrobial, with moderate-to-severe infections culturing three to five organisms. [61] Gram-positive and gram-negative organisms, as well as aerobes and anaerobes, are often noted. The most common gram-positive organisms include Enterococcus faecalis, S aureus, S epidermidis, and group B streptococci. Common gram-negative organisms include Proteus species, E coli, Klebsiella species, and Pseudomonas species. Of the anaerobes, Peptococcus species and Bacteroides fragilis are observed most often.
Although aerobic gram-negative bacilli and anaerobes are also found in addition to the gram-positive cocci, they are rarely the sole cause of the infections. Methicillin-resistant S aureus (MRSA) is more prominent in the diabetic population. [62] MRSA does not produce a more severe infection, but the antibiotic selection becomes more limited. It is also not uncommon to see vancomycin-resistant Enterococcus (VRE) infection. Strict isolation measures must be implemented when these patients are hospitalized.
Yates et al determined the microbiologic profiles of 653 specimens taken from 376 patients with diabetes-related foot infection. In 36% of the specimens, only one isolate was identified, with 77% being gram-positive (43% Staphylococcus, 13% Streptococcus). In 23%, MRSA was isolated (risk factors were inpatient management, prolonged wound duration, and chronic kidney disease). In chronic wounds, Pseudomonas organisms were more common. The authors, noting that complexity increases with inpatient care and ulcer duration, suggested that MRSA antibiotic coverage be considered in cases of prolonged duration, inpatient management, or chronic kidney disease. [63]
Presentation
History and physical examination
A complete history and thorough physical examination are mandatory first steps toward diagnosing foot infections. A good history reveals several things to the examiner, including time of onset, conditions surrounding the injury, and type of injury. This is important because it provides a good idea of what organisms to expect in the wound. Different bacteria are observed in persons with chronic cellulitis, acute cellulitis, puncture wounds, diabetic wounds, and farm-type injuries.
Constitutional symptoms of fever, chills, nausea, or vomiting can indicate bloodstream infection or bacteremia, necessitating a more aggressive treatment plan. Allergies to medications and tetanus status should be elicited if puncture wounds or acute trauma is involved.
A physical examination initially should involve ascertaining vital signs, including a fingerstick to check for DM. Constitutional signs of infection include tachycardia, febrile status, nausea, and vomiting. Leukocytosis may or may not be present in persons with diabetes in the presence of infection. Hyperglycemia with anion gap changes can indicate a bacteremia, infection, or both. (See the Anion Gap calculator.)
On examination, local signs of infection include erythema, edema, and increased temperature. Loss of skin wrinkles due to edema is a significant indication of infection and abscess. Open wounds with drainage, purulent discharge, and odor are usually indications of infection. The skin around the lesion should be "milked" toward the ulceration to see if exudate is brought from the deep spaces to the surface through the lesion (see the image below).
 Gangrene with underlying deep-space infection. "Milking" wound produced purulent exudate from the abscess.
Gangrene with underlying deep-space infection. "Milking" wound produced purulent exudate from the abscess.
Foul-smelling drainage is consistent with infections involving anaerobic organisms. Drainage from staphylococcal infections is usually odorless with a creamy yellowish color. Streptococcal infections are also odorless and have a milky yellow-white color. Proteus species infections smell like urea, and pseudomonal infections are green with a fruity putrid odor. Exudate from gram-negative organisms is typically clear. All abscesses and infections of soft tissue and bone should be considered surgical emergencies.
Infections of the lower extremities in the presence of vascular insufficiencies require that cellulitis be differentiated from dependent rubor by elevation of the extremity (see the image below). These patients often have ischemic ulcers with eschar covering them. The lesions must be palpated for fluctuance under the eschar. If eschar is present, it requires debridement. Fluctuance also can be palpated deep to normal skin; this also requires debridement.
 Dependent rubor in lower extremity of patient with peripheral vascular disease. This condition can mimic cellulitis, which must be excluded during physical examination. Erythema completely resolves when extremity is elevated.
Dependent rubor in lower extremity of patient with peripheral vascular disease. This condition can mimic cellulitis, which must be excluded during physical examination. Erythema completely resolves when extremity is elevated.
Life-threatening infections such as necrotizing fasciitis and gas gangrene are more common in hosts with vascular compromise and should be considered among the differential diagnoses with a high index of suspicion. These patients usually have systemic toxemia out of proportion to the degree of the apparent infection, with progressive necrosis of the skin and subcutaneous tissue. Local tissue changes can include minimal suppurative drainage and periwound necrosis. Palpation reveals pain out of proportion to the wound, induration, and, often, crepitus. Pain in the presence of neuropathy is a significant indicator of infection, underlying abscess formation, or both.
A thorough evaluation of foot infections associated with ulcerations includes probing of the wound. This is important for determining the depth and extent of the lesion. The area should be prepared with povidone-iodine and probed with the wooden end of a sterile cotton-tipped swab or metal probe.
If the lesion is deep, the infection should be assumed to have spread to that level. Once in the deep compartments of the foot, bacteria can migrate easily along fascial planes and tendon sheaths. If the lesion can be probed directly to bone, osteomyelitis is predicted, and the lesion should be treated as such. Studies have demonstrated that palpating bone when probing the lesion is highly specific and has a high positive predictive value for osteomyelitis. [64]
Diabetic foot infection wound score
Lipsky et al developed a 10-item diabetic foot infection wound score (range from least severe to most severe, 3-49) that takes into account semiquantitative grading of wound measurements and infection parameters. [65] The authors used data from a trial of antibiotics for foot infections in diabetic patients (SIDESTEP) and evaluated the accuracy of their wound-scoring technique in predicting outcome. They found that their wound scores had high correlation with the clinical response of 371 patients in the study. The scores had good internal consistency as well, with more severe wounds garnering higher scores.
Laboratory Tests
Laboratory analysis should include a complete blood count (CBC) with differential, chemistry panel, and coagulation studies.
Elevated WBC count in association with a left shift can be pathognomonic for a systemic infection. It is important to note that many patients with DM and limb-threatening infections fail to produce a true leukocytosis; accordingly, laboratory values can be nonpredictive in this setting.
Renal values such as blood urea nitrogen (BUN) and creatinine should be evaluated to establish a baseline prior to initiation of antibiotics, which can be nephrotoxic. Many individuals who are diabetic have chronic renal insufficiency or end-stage renal disease (ESRD) for which antibiotic dosing must be very specific. Patients with renal diabetes are often anemic and require epoetin alfa or transfusions prior to surgery because blood loss can initiate or potentiate a cardiac event, especially in this population with global vascular diseases.
Coagulation studies are important if the patient is going to surgery for irrigation and debridement. Patients with PVD often have foot infections. Coronary artery disease and atrial fibrillation necessitate anticoagulation with warfarin; therefore, prothrombin time (PT) and international normalized ratio (INR) values are important prior to surgery. Fresh frozen plasma (FFP) and vitamin K can be given prior to débridement if the INR is too high.
Other useful lab studies are C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). CRP is an inflammatory marker that is usually elevated in the presence of a bone infection and provides a good gauge of treatment efficacy. ESR is another inflammatory marker that indicates an infectious or reactive process and is useful for evaluating response to antibiotics. Albumin levels should be checked when malnutrition is suspected and can pose a problem for wound healing if too low.
Wound cultures with susceptibilities are important parts in the diagnostic process. Organisms are identified and antibiotic sensitivities noted. Initial Gram stains help with empiric antibiotic therapy. Superficial ulcerations should not be swabbed because the bacterial flora are contaminants and not true pathogens. Deep cultures taken in a controlled setting (eg, surgery) are much more accurate and truly represent the pathogens responsible for the infection. Overall, the current consensus is that superficial cultures should be avoided. [66]
Culture-based methods of identifying microorganisms have limitations, in that they may underestimate or exclude some species while being oversensitive to others. Such limitations can be countered by employing advanced molecular technologies, such as next-generation sequencing (NGS). [67]
Imaging Studies
Plain radiography
Plain films remain the initial imaging modality for most foot infections including soft tissue, bone, or combinations thereof. Plain films provide useful information rapidly and inexpensively. Soft-tissue infections such as cellulitis are observed on plain films as increased soft-tissue densities. Radiographs can reveal soft-tissue swelling, obscuration of fascial planes, and mottled opacification of subcutaneous fat.
Puncture wounds and possible retained foreign bodies should be evaluated with plain radiography. Radiopaque objects such as metal, gravel, and glass can be identified easily on plain films. [68, 69] Radiolucent objects such as plastic, rubber, and wood are not often observed on radiographs and require identification by means of ultrasonography (US).
The most important reason for ordering plain films, especially with infected diabetic ulcers, is to exclude gas in the soft tissues. [69] Again, this represents an emergency situation and must be treated aggressively with surgical decompression.
Plain radiographs can be useful in the evaluation of osteomyelitis (see the images below). The initial manifestations of osteomyelitis occur as pathogens invade the periosteum via direct extension, causing the periosteum to be lifted and periosteal new bone formation to occur. Progression of the infectious process results in cortical disruption and medullary involvement. Simple radiographs lack sensitivity and specificity with respect to early detection of osteomyelitis. Bone destruction and periostitis are usually not detectable for up to 2 weeks after the initiation of infection.
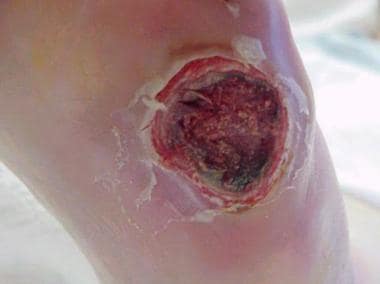
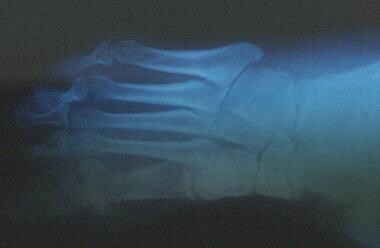 Chronic diabetic ulceration with underlying osteomyelitis. Plain film exhibits cortical disruption at medial aspect of first metatarsophalangeal joint.
Chronic diabetic ulceration with underlying osteomyelitis. Plain film exhibits cortical disruption at medial aspect of first metatarsophalangeal joint.
Plain films identify periosteal reaction, cortical disruption, Brodie metaphyseal abscess, sequestrum, and involucrum. Radiographs are often more helpful with long-term bone infection during which overt bone changes are noted.
Ultrasonography
Another useful and cost-effective radiologic modality used with foot infection is US. This modality is very helpful in visualizing retained foreign bodies not observed on plain films and in looking for soft-tissue abscesses, but its reliability tends to be highly operator-dependent.
Radiopaque foreign bodies such as wood and plastic can be identified by means of US. [19] Abscesses are observed as complex and typically cystic structures containing internal echogenicity with through transmission. The echogenicity varies, depending on the contents of the abscess. US is particularly useful in the foot because it can help identify the exact location of the abscess within the many deep compartments and fascial structures.
Scintigraphy
Bony changes can be delayed in persons with vascular compromise; therefore, plain film radiographs may not be adequate during the acute phases. Scintigraphic studies can be useful in diagnosing bone infections in the foot, distinguishing bone infection from soft-tissue infection, and differentiating bone infection from Charcot osteoarthropathy. Three common nuclear medicine studies in this group have made use of the radioactive forms of technetium, gallium, and indium. [68, 69, 70]
Technetium-99m methylene diphosphonate (MDP), when used in a three-phase bone scan, can reveal infection 24-48 hours after the clinical onset (see the images below). Three-phase bone scans assist in distinguishing cellulitis from osteomyelitis. Increased uptake of the scintigraphic tracer during the early phases of a three-phase bone scan with little or no increase in uptake during the late phase is indicative of soft-tissue infection without bone involvement. Uptake in all three phases is observed in persons with osteomyelitis.
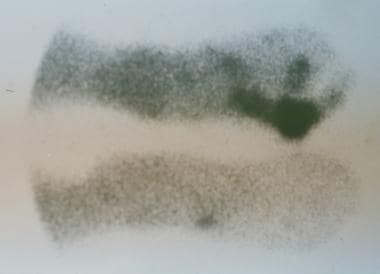 Chronic diabetic ulceration with underlying osteomyelitis. Standard technetium Tc99m methylene diphosphonate (MDP) bone scan, late-phase image.
Chronic diabetic ulceration with underlying osteomyelitis. Standard technetium Tc99m methylene diphosphonate (MDP) bone scan, late-phase image.
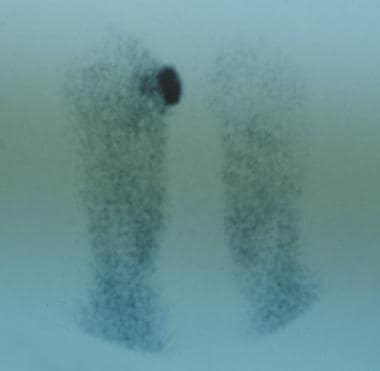 Chronic diabetic ulceration with underlying osteomyelitis. Technetium Tc99m methylene diphosphonate (MDP) hexamethylpropylamine oxime (HMPAO) leukocyte-labeled scan of same region. This demonstrates more specific uptake pattern, which is helpful in surgical planning.
Chronic diabetic ulceration with underlying osteomyelitis. Technetium Tc99m methylene diphosphonate (MDP) hexamethylpropylamine oxime (HMPAO) leukocyte-labeled scan of same region. This demonstrates more specific uptake pattern, which is helpful in surgical planning.
This test is typically very sensitive but not specific, because increased uptake of tracer can be observed with several other conditions, including bone tumors and Charcot osteoarthropathy, and after trauma or surgery. The results must be interpreted in conjunction with clinical findings and other diagnostic modalities.
Gallium-67 can be used in conjunction with technetium-99m to increase the overall specificity of the study. Gallium accumulates in infected bone, making it a useful tracer. Although specificity is increased, false-positive results still occur because it can accumulate in infected soft tissue, hematomas, some tumors, and areas of increased bone turnover (osteoarthropathy, fracture, and postsurgery). False-negative results also may occur with this tracer when patients are taking antibiotics.
Leukocyte-labeled indium-111 reveals infection at an earlier stage than technetium-99m MDP alone and is both sensitive and specific. It is often combined with technetium-99m MDP to increase the sensitivity and specificity further. Although this scan has many attributes, several problems also exist. Indium-111 uses PMNs and not lymphocytes; accordingly, it is not as sensitive for detecting chronic osteomyelitis. Because it has a low spatial resolution, differentiation from other soft-tissue infections is difficult. It is a time-consuming examination, requiring 3 days to complete. Finally, it is more expensive than magnetic resonance imaging (MRI).
Another nuclear medicine study uses leukocyte-labeled technetium-99m hexamethylpropylamine oxime (HMPAO). [70] This offers a more cost-effective alternative to the indium-111 labeled scan. The biodistribution and function are very similar to those of indium-111, with the benefit of a more favorable radiation dosimetry, allowing a larger dose of radioactivity to be administered with less systemic absorption. This provides a larger amount of local activity and facilitates interpretation of the structures involved. The test is completed in 3-4 hours rather than 3 days.
HMPAO has many uses but proves to be invaluable in patients in two specific situations: the postoperative patient and the patient with recent fracture in whom osteomyelitis is suspected. Healing bone following surgery or fracture lights up on technetium-99m MDP 1-2 years after the incident. If osteomyelitis is suspected at a recent surgical site, differentiating the two with conventional technetium-99m MDP scintigraphy is not possible. However, technetium-99m HMPAO scans are more specific for the infectious process.
Another difficult situation involves the patient with a chronic ulceration secondary to a rocker-bottom foot deformity from Charcot osteoarthropathy. The physician cannot determine definitively if bone infection is present with technetium-99m MDP scintigraphy because both the osteoarthropathy and the infection cause it to light up. HMPAO has demonstrated promise in differentiating between the two, but larger studies must be performed. [70]
Computed tomography
Computed tomography (CT) is beneficial for diagnosing both soft-tissue and bone infections in the foot. It is very useful for detecting small areas of osteolysis in cortical bone, small foci of gas, and small foreign bodies that may be associated with infection. Abscesses are observed as fluid collections displaying walls of various thicknesses. Edema and cellulitis are observed as mild enhancements in the soft tissue with thickening of the fascia. Gas in the deep tissues associated with gas gangrene or necrotizing fascitis is displayed readily on CT, exhibiting the seriousness of the infection.
CT posseses high spatial resolution and yields great detail of cortical bone in a cross-sectional display. For this reason, it is excellent for picking up cortical disruptions or changes in bone associated with osteomyelitis. On CT, sequestra appear as fragments of dense bone often surrounded by increased soft-tissue or fluid density. It can depict increased intraosseous density, reflecting the accumulation of pus that replaces the fat in the marrow. Involucra and sequestra are also readily detectable with CT. Because of its great detail, CT works well for identifying subtle changes within the intricate soft-tissue layers and bones of the foot.
Single positron emission computed tomography
In a study by Filippi et al, single-photon emission CT (SPECT)/CT was performed to diagnose diabetic foot infections by labeled leukocytes in 17 patients with 19 sites suspected of clinical infection. After leukocyte labeling and administration, planar scans were acquired at 30 minutes, 4 hours, and 24 hours; and SPECT/CT was done at 6 hours. The final diagnosis was then established by clinical follow-up at 24 months for all cases and by bone biopsy for 14 of the sites. [71]
Leukocyte scanning showed positive results in 16 of 19 lesions and negative results in three. SPECT/CT changed the interpretation of the planar and SPECT images for 10 of 19 suspected sites (52.6%) by excluding osteomyelitis in six of the cases, revealing a bone infection in one case, and identifying bone and soft-tissue infection in three cases. [71]
Magnetic resonance imaging
The most advanced radiologic study used in foot infections is MRI. This is ideal for identifying lesions within soft tissues and works well for detecting changes taking place within bones. [72] MRI is highly sensitive in determining the presence and extent of inflammation. Accordingly, it is helpful in delineating whether an infection is limited to soft tissue, joints, or bones or whether several structures are involved. This is beneficial for treatment, in view of the complex anatomy of the foot. MRI also readily differentiates viable from necrotic tissue; this aids in surgical planning and helps preserve the viability of adjacent healthy tissues during debridement.
It should be understood, however, that MRI tends to overrepresent the amount of involved tissue, especially bone. This must be taken into account when MRI is used to help plan surgical debridement.
Because MRI provides an accurate visual image of soft tissue, joint spaces, tendon sheaths, and fascial planes, it is ideal for use in diagnosing or studying the extent of infectious processes or abscesses within these structures. MRI has the advantage of being able to demonstrate an abscess in any plane and can better delineate the extent of adjacent soft-tissue changes when compared with other studies such as CT.
By determining the extent of necrotizing fascitis infections, MRI, again, can aid in surgical planning. The image demonstrates thickening and tracking of abnormally high signal intensity along deep fascial planes with the presence of small gas bubbles. The general consensus is that MRI is superior to CT in assessing for nonviable tissue or drainable fluid collections. [73, 74]
The use of MRI to assist in diagnosing osteomyelitis continues to gain popularity. One study demonstrated MRI to be significantly more sensitive and accurate and equally specific as compared with plain radiography, technetium-99m MDP, and gallium-67 scans. [75] The sensitivity of MRI for diagnosing early osteomyelitis stems from its ability to differentiate normal bone marrow from abnormal bone marrow. With bone infection, marrow is replaced by fluid and inflammatory cells, which are displayed as regions of reduced signal intensity on T1-weighted images and regions of increased signal intensity on T2-weighted images and short tau inversion recovery (STIR) sequences.
Other changes displayed include erosions and perforations of the cortex, periosteal new bone formation, and soft-tissue edema. Although highly sensitive, MRI remains less specific because several disorders, including diabetic osteoarthropathy, can produce similar abnormalities in signal intensity.
When present, secondary signs of infection, such as cellulitis, sinus tract formation from skin to bone, cortical disruption, and sequestrum formation, must be looked for to rule in osteomyelitis. Overall, however, MRI is an excellent study for evaluating bone infections.
Other Diagnostic Tests and Procedures
Tissue biopsy and quantitative counts are other means of identifying bacteria and diagnosing infections. Soft-tissue culture swabs are preferred over biopsy for identifying pathogens. Biopsy is important in determining quantitative bacteria counts. Biopsy is also very useful for diagnosing bone infections. Bone biopsy remains the criterion standard in the diagnosis of osteomyelitis. [76]
Noninvasive Therapy
Noninvasive treatment options vary, depending on the type of foot infection present. Mild superficial infections and simple cellulitis usually are treated on an outpatient basis with oral antibiotics. The patient is encouraged to decrease activities and keep the extremity elevated until the infection resolves.
Antibiotics should be administered for 7-10 days and are directed toward the most common organisms, including S aureus, S epidermidis, and Streptococcus species. The most frequently used medications for foot infections are first-generation cephalosporins, clindamycin (for patients allergic to penicillin), amoxicillin-clavulanate, and levofloxacin. Antibiotics active against MRSA should be considered in those with increased risk factors.
Patients are reevaluated after 3 days to ensure that the infection is responding to therapy. If no response occurs, the antibiotic should be changed. If the condition becomes significantly worse, IV therapy should be considered along with further treatment. [1, 3, 13, 37, 47, 77, 78, 79, 80]
In the setting of mild foot infections associated with open lesions or ulcerations, local wound care is used in addition to antimicrobial medications. Various dressing types are available and should be chosen according to the characteristics of the lesion. The goals are to provide a moist wound environment, encourage drainage, avoid build-up of necrotic tissue, and keep the bacterial count low.
The most commonly used dressing is the wet-to-dry dressing. Gauze moistened with isotonic sodium chloride solution is placed in the wound and covered by dry gauze. The dressing maintains a moist environment, wicks away drainage, and debrides necrotic tissue. It works well in stable wounds and encourages granulation. For treating more aggressive wounds, a topical antimicrobial agent can be used.
Agents such as povidone-iodine, hydrogen peroxide, Dakin solution, and chlorhexidine are bactericidal; however, they are also unhealthy for normal tissue. They should be avoided unless gangrene or a large amount of necrotic tissue are present in the lesion.
Silver sulfadiazine (SSD) works well as a topical agent because it controls both gram-negative and gram-positive bacteria while encouraging wound healing and minimizing damage to healthy tissue. [81] Cadexomer iodine is an iodine formulation that releases low levels of iodine over a sustained period. It is safe for healthy tissue, is highly effective against wound pathogens, and does not impair wound healing. [82]
Several newer dressings work very well for wounds with large amounts of drainage. Different foam dressings exist that absorb large amounts of exudate without macerating the surrounding tissues. Occlusive dressings work well to create a moist environment, but they are not recommended unless the wound is very clean, because they can become an excellent medium for bacteria to proliferate.
Another option is the vacuum-assisted closure (VAC) device, which is a continuous suction device placed directly on the wound. An open cell sponge with a vacuum hose attached is placed within the wound and covered with an occlusive dressing. Fluid from the wound is removed immediately, and surrounding edema is diminished. This approach decreases the bacterial count significantly and encourages the development of granulation tissue. The VAC device works well as an adjunct after debridement of a wound.
Severe limb-threatening foot infections require aggressive treatment with a combination of local wound care, IV antibiotics, and surgical debridement. Infections associated with open wounds are usually inoculated with a combination of gram-positive and gram-negative aerobes and anaerobes. These should be treated with broad-spectrum antimicrobials such as ampicillin-sulbactam, ticarcillin-clavulanate, or piperacillin-tazobactam. [77]
In penicillin-allergic patients, IV clindamycin in combination with oral ciprofloxacin, levofloxacin, or gatifloxacin may be used. These oral medications are mentioned because they are more cost-effective and have the same absorption into bone as the IV versions. Wounds that are suspected of containing very aggressive gram-negative organisms should be covered additionally with an aminoglycoside.
Once culture and sensitivities from a deep intraoperative swab return, antimicrobial therapy can be changed to a more specific medication. However, if the patient is responding well to the current regimen, a change of agent may not be necessary. In situations where a patient is not responding, an infectious disease consultation is highly recommended.
MRSA is common, especially in persons with chronic wounds and in patients who are diabetic. MRSA is present in as many as 25% of diabetic foot infections involving S aureus. [78] It is more common in patients who have been on prolonged ciprofloxacin therapy, have recently stayed in a healthcare facility, or have been colonized in the past. [83] Often, MRSA only colonizes the superficial surface of the ulceration and should not be treated as the infectious agent; however, when it does become the infectious agent, it must be treated aggressively.
A limited number of antibiotics are indicated for the treatment of MRSA. The drug of choice is IV vancomycin. Additional medications for MRSA and other resistant bacteria include quinupristin-dalfopristin and linezolid [84, 85] ; these have typically been reserved for strongly resistant strains.
The growth of antibiotic resistance can make diabetic foot infections difficult to treat. Phage therapy is being investigated as a potential alternative treatment in such cases. [86]
Surgical Therapy
Excision of nonviable tissue
Appropriate wound care and antibiotic therapy are no substitute for adequate surgical debridement in the treatment of deep-space infections in the foot. Some physicians believe that the best antibacterial agent for treating these infections is a No. 15 blade. Moderate-to-severe infections should receive aggressive irrigation and debridement with removal of all nonviable skin, soft tissue, and bone. [87, 88, 89]
These wounds can be thought of as tumors and excised completely until only normal tissue remains. [3] To accomplish this, serial debridements are often required because determining the viability of tissue early in the infection is difficult.
The surgical site is left open, and local wound care is provided along with appropriate antibiotics. Lines of demarcation are allowed to form between healthy and nonviable tissues for 2-3 days before the patient is taken back to surgery for further debridement. This procedure may have to be repeated several times before the wound is converted to a healthy one that is free of necrotic material (see the images below).
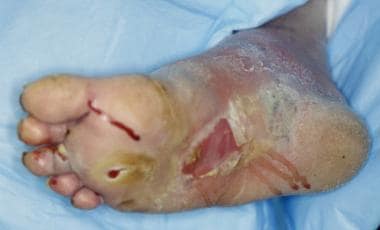 Infected diabetic ulceration prior to debridement. Note cellulitis extending up to ankle and edema within arch.
Infected diabetic ulceration prior to debridement. Note cellulitis extending up to ankle and edema within arch.
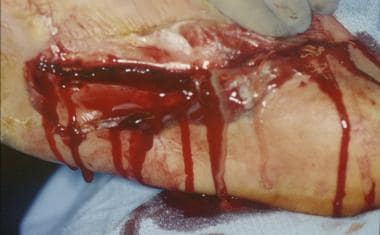 Infected diabetic ulceration. Intraoperative view demonstrates extensive myonecrosis and purulent drainage.
Infected diabetic ulceration. Intraoperative view demonstrates extensive myonecrosis and purulent drainage.
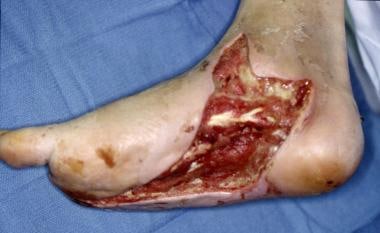 Infected diabetic ulceration. Shown is clean wound bed after several debridements, intravenous antibiotics, and local wound care. Surgical reconstruction of foot can now begin.
Infected diabetic ulceration. Shown is clean wound bed after several debridements, intravenous antibiotics, and local wound care. Surgical reconstruction of foot can now begin.
Necrotic and devitalized tissues must be removed because they provide a favorable environment for bacterial growth. [3] More aggressive infections (eg, necrotizing fasciitis) may require debridements every 1-2 days. Once the infection resolves, reconstruction of the foot can begin.
The first layer of tissue encountered during surgery is the skin. An incision is made at the border where healthy tissue meets nonviable tissue. This border can be clearly demarcated, as in individuals with dry gangrene (see the image below), or it can be more obscure, which is the more common scenario.
 Dry gangrene of digits 1-5. Digits are stable and have no signs of infection. Note line of demarcation between healthy and nonviable tissue. Treatment options include autoamputation and surgical amputation. This does not represent urgent surgical situation unless it becomes infected.
Dry gangrene of digits 1-5. Digits are stable and have no signs of infection. Note line of demarcation between healthy and nonviable tissue. Treatment options include autoamputation and surgical amputation. This does not represent urgent surgical situation unless it becomes infected.
If the lines of demarcation are not obvious, the best way to differentiate between viable and nonviable tissue is to look for bleeding margins. The margins of healthy tissue actively bleed when an incision is made. The incision is created in the center of the questionable area, and the skin is removed in concentric circles until bleeding edges are encountered.
Only when normal bleeding is encountered at the fresh skin edges can the physician be certain that the nonviable tissue has been removed. [87] The subcutaneous tissue layer consists mostly of fat but also contains nerves and blood vessels. Because fat is relatively avascular as compared with surrounding tissues, active bleeding is not the best indicator for determining its viability. Healthy fat feels soft, is resilient, and has a shiny yellow color. In contrast, nonviable fat feels hard, is nonpliable, and has a gray-white coloration.
Healthy nerves are white and shiny and should be spared if possible. Caution should be used to ensure that retained nerves do not become entrapped in granulation or scar tissue and that adequate soft-tissue coverage is created during reconstruction to avoid painful neurapraxia. When nerves must be sacrificed, neuroma formation can be minimized by sewing the epineurium at the cut end together with 8-0 sutures or by implanting the cut end of the nerve into muscle or bone.
Blood vessels that are to be removed should be cauterized at the open ends or tied off when appropriate.
Deep structures in the foot include tendons, tendon sheaths, muscles, bones, and the fascia that divides them into different planes. When debridement reaches these deep structures, careful planning is needed because foot function is altered. The surgeon should concentrate not only on removing all nonviable tissue but also on developing a medical care plan for the reconstruction and eventual long-term functionality of the foot.
Healthy tendon is white and shiny, whereas infected tendon is dull, soft, and stringy and can be in the process of liquefying. Normal muscle is beefy red and bleeds when cut. Dead muscle tends to be dull, dark, and falls apart easily when handled. An effective test for determining muscle viability is to pinch it with an instrument or stimulate it with a Bovie electrocautery device; muscle that is still viable contracts. Infected bone is soft and does not bleed when cut.
As with the other tissues, nonviable bone should be excised until a healthy bleeding margin is obtained. An additional small portion of what is deemed to be healthy bone should be removed and sent for culture and pathologic examination to ensure that all osteomyelitic tissue has been removed. The use of a gentamicin-eluting calcium sulfate/hydroxyapatite bone graft substitute to fill residual bone voids after debridement has been described. [90]
Reconstruction
Once the infection completely resolves and the wound is converted to healthy tissue, the often-difficult task of foot reconstruction begins. The goal is to create a functional foot that resists future breakdown and infection (see the images below). A multidisciplinary team approach is used to achieve the best results. Peripheral vascular, podiatric, plastic, and orthopedic surgeons should be involved along with nonsurgical specialties for management of the complex nutritional, cardiovascular, endocrine, renal, and pulmonary issues that often arise in these cases.
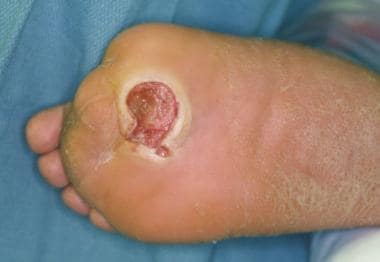 Open ulceration in diabetic foot after infection has resolved. Reconstructive surgery can now begin. Patient underwent previous hallux amputation for separate infection.
Open ulceration in diabetic foot after infection has resolved. Reconstructive surgery can now begin. Patient underwent previous hallux amputation for separate infection.
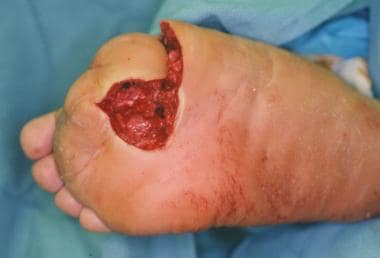 Reconstructive surgery of diabetic foot after infection has resolved. Patient underwent previous hallux amputation for separate infection. Delayed primary closure via creation of local rotational flap of tissue for coverage.
Reconstructive surgery of diabetic foot after infection has resolved. Patient underwent previous hallux amputation for separate infection. Delayed primary closure via creation of local rotational flap of tissue for coverage.
Outcomes
Bravo-Molina et al prospectively studied 250 patients with diabetic foot syndrome to determine whether the microbiology and the TEXAS, PEDIS, and Wagner wound classifications predicted amputation. [91] They found that the three wound scales were associated with minor amputation but did not predict this outcome. In the multivariate analysis, the presence of osteomyelitis and the location of the wound in the forefoot were predictive of minor amputation and elevated CRP levels were predictive of major amputation. In the follow-up, a low ABI predicted major amputation.
Dalla Paola et al prospectively evaluated a therapeutic protocol for 18 consecutive patients affected by heel ulcer complicated by osteomyelitis. [92] All patients underwent open partial calcanectomy associated with the positioning of a circular external frame designed for hindfoot stabilization and offloading. Reconstruction was implemented, starting with negative-pressure wound therapy (NPWT) and coverage with dermal substitute and split-thickness skin grafting. Healing was achieved in all patients (mean healing time, 69.0 ± 64.0 d). No major amputation was performed during follow-up. This approach was as efficient as limb salvage in these patients.
Ricci et al assessed all-cause and cardiovascular mortality and predictors of mortality in 194 consecutive patients with diabetic foot osteomyelitis (DFO) over a mean observation period of 2.8 ± 1.4 years. [93] Of these, 73 (37.6%) died (yearly rate, 13.2%), and 59 of the 73 deaths were attributable to cardiovascular causes. The site of osteomyelitis (hindfoot as opposed to midfoot or forefoot) was associated with a higher mortality, and surgical treatment was associated with a lower mortality.
-
Cellulitis extending to level of ankle joint secondary to infected ulceration plantar to fifth metatarsal head. Permanent marker is used to define proximal extent of cellulitis to determine if condition is improving or worsening with therapy.
-
Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
-
Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
-
Infected ulceration plantar to fifth metatarsal head. This ulceration could be probed to bone, and deep-space infection was present. Patient was taken to surgery for drainage of underlying abscess. Cellulitis completely resolved less than 24 hours postoperatively.
-
Onychocryptosis with paronychia of medial and lateral nail folds. Treatment consists of oral antibiotic therapy and removal of involved nail borders. Nail borders puncture skin and act as foreign bodies. These must be removed in order for infection to completely resolve.
-
Onychocryptosis in 13-year-old boy that has gone untreated for several months. Removal of ingrown nail has not resolved infection in this case, because underlying osteomyelitis has developed. Note remaining erythema and edema of hallux.
-
Osteomyelitis of the distal phalanx secondary to untreated onychocryptosis. This ia rare situation demonstrates importance of prompt treatment. Patient underwent partial hallux amputation.
-
Dependent rubor in lower extremity of patient with peripheral vascular disease. This condition can mimic cellulitis, which must be excluded during physical examination. Erythema completely resolves when extremity is elevated.
-
Dry gangrene of digits 1-5. Digits are stable and have no signs of infection. Note line of demarcation between healthy and nonviable tissue. Treatment options include autoamputation and surgical amputation. This does not represent urgent surgical situation unless it becomes infected.
-
Gangrene with underlying deep-space infection. "Milking" wound produced purulent exudate from the abscess.
-
Early wet gangrene of hallux.
-
Early wet gangrene of hallux. This began secondary to embolic event with showering to hallux. Patient has history of peripheral vascular disease and previously lost her fourth toe because of it.
-
Wet gangrene is surgical emergency. Intraoperative photo was taken after irrigation and debridement with partial first-ray resection. Note healthy tissue margins and high level of perfusion once necrotic tissue has been removed.
-
Bullet entry wound. Patient was initially treated conservatively with antibiotics and no surgical debridement.
-
Bullet exit wound 6 months after injury. Chronic drainage from exit site was still occurring.
-
Radiographs of foot demonstrate development of osteomyelitis. Cuboid, anterior portion of calcaneus, base of fifth metatarsal, and base of fourth metatarsal were all involved and required debridement.
-
Lateral view of foot demonstrating osteomyelitis.
-
Foot infection with purulent discharge secondary to open ulceration in diabetic foot.
-
Lateral radiograph of diabetic patient with Charcot foot disease. Note midfoot collapse, leaving patient with inverted arch (rockerbottom foot deformity).
-
Ulceration on plantar surface of the foot secondary to high pressures created from Charcot foot deformity.
-
Chronic diabetic ulceration with underlying osteomyelitis.
-
Chronic diabetic ulceration with underlying osteomyelitis. Plain film exhibits cortical disruption at medial aspect of first metatarsophalangeal joint.
-
Chronic diabetic ulceration with underlying osteomyelitis. Standard technetium Tc99m methylene diphosphonate (MDP) bone scan, late-phase image.
-
Chronic diabetic ulceration with underlying osteomyelitis. Technetium Tc99m methylene diphosphonate (MDP) hexamethylpropylamine oxime (HMPAO) leukocyte-labeled scan of same region. This demonstrates more specific uptake pattern, which is helpful in surgical planning.
-
Infected diabetic ulceration prior to debridement. Note cellulitis extending up to ankle and edema within arch.
-
Infected diabetic ulceration. Intraoperative view demonstrates extensive myonecrosis and purulent drainage.
-
Infected diabetic ulceration. Shown is clean wound bed after several debridements, intravenous antibiotics, and local wound care. Surgical reconstruction of foot can now begin.
-
Open ulceration in diabetic foot after infection has resolved. Reconstructive surgery can now begin. Patient underwent previous hallux amputation for separate infection.
-
Reconstructive surgery of diabetic foot after infection has resolved. Patient underwent previous hallux amputation for separate infection. Delayed primary closure via creation of local rotational flap of tissue for coverage.
-
Reconstructive surgery of diabetic foot after infection has resolved. Patient underwent previous hallux amputation for separate infection. Local rotational flap of tissue was retained for coverage. More than 2 months postoperatively, lesion is completely closed without complication.
Tables
What would you like to print?
- Background
- Soft-Tissue Infections in Foot
- Surgical Emergencies in Soft-Tissue Foot Infection
- Bone Infections in Foot
- Diabetic Foot Infections
- Presentation
- Laboratory Tests
- Imaging Studies
- Other Diagnostic Tests and Procedures
- Noninvasive Therapy
- Surgical Therapy
- Outcomes
- Show All
- Media Gallery
- References