Practice Essentials
The term birth-related brachial plexus palsy (BRBPP) refers to injury noted in the perinatal period to all or a portion of the brachial plexus. [1, 2] The term obstetrical brachial plexus palsy (OBPP) has also been used but has negative implications; accordingly, other terms, such as birth-related brachial plexus injury (BRBPI), are often preferred, particularly in the United States. [3] Injuries associated with the upper brachial plexus are classically termed Erb palsies, and those associated with the lower plexus are traditionally termed Klumpke palsies.
In 1768, Smellie described bilateral arm paralysis in a newborn. [4] In 1872, Guillaume Duchenne coined the term obstetrical paralysis. [5] Erb described C5-C6 paralysis in 1874, [6] and in 1885, Klumpke described paralysis of the lower plexus. The first description of operative management for obstetrical brachial plexus lesions was reported in 1903. [7] Poor outcomes and reports by Sever recommending nonoperative management [8, 9] led to little interest in surgical management of OBPP until the microsurgical era brought renewed interest. In the 1980s, Gilbert popularized the most common indication for surgical reconstruction of obstetrical brachial plexus injuries. [10, 11]
Management of children with BRBPI remains challenging. Technological and surgical advancements have improved patient outcomes, but room for improvement remains. Previous controversy regarding surgical timing has been replaced with evidenced-based results. Outcomes analysis would benefit from a universal outcome measurement tool to allow comparison among institutions. Given the small number of patients who require surgical management, meta-analyses would allow better trend analysis.
Continued experience with direct nerve transfers may eventually minimize interpositional grafting procedures. Basic science research continues for methods of enhancing peripheral nerve regeneration and target muscle protection. The future of management of patients with BRBPI continues to benefit from a multidisciplinary approach for delineating optimal treatment methods.
Anatomy
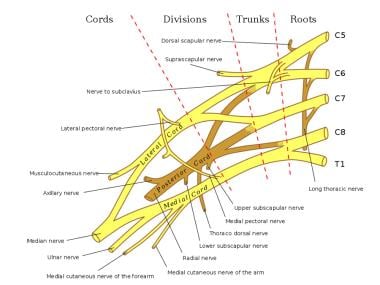
The brachial plexus provides the nerve supply to the upper extremity, from roots C5 to T1 (see the image below). [12] An anatomic study of 100 cadavers demonstrated that the suprascapular nerve most frequently originates from the posterior division of the upper trunk and the lateral pectoral nerve most commonly branches from the anterior divisions of the upper and middle trunks. [13]
Pathophysiology
BRBPI arises from an increase in the infant’s neck-shoulder angle, which results in a traction force to the brachial plexus. [14, 15] This force on the brachial plexus can cause varying degrees of nerve injury, ranging from neurapraxia to complete root avulsion. Clinically, this injury results in disruption of the sensory and motor function of the injured nerve. Seddon and Sunderland each described a classification for nerve injuries. [16, 17] The classification of nerve injury described by Seddon consisted of neurapraxia, axonotmesis, and neurotmesis. Sunderland expanded the classification system into five degrees of nerve injury, as follows.
A first-degree injury, or neurapraxia, involves a temporary conduction block with demyelination of the nerve at the site of injury. Electrodiagnostic studies elicit normal results above and below the level of injury, and no denervation changes are present within the muscle. No Tinel sign is present. Once the nerve has remyelinated at that area, complete recovery occurs. Recovery may take up to 12 weeks.
A second-degree injury, or axonotmesis, results from a more severe trauma or compression. This causes Wallerian degeneration distal to the level of injury and proximal axonal degeneration to at least the next node of Ranvier. In more severe traumatic injuries, the proximal degeneration may extend beyond the next node of Ranvier. Electrodiagnostic studies demonstrate denervation changes in the affected muscle(s), and in cases of reinnervation, motor unit potentials (MUPs) are present.
Axonal regeneration after second-degree injury occurs at a rate of 1 mm/day or 1 in./month and can be followed with an advancing Tinel sign. The endoneurial tubes remain intact, and the recovery, therefore, is complete with axons reinnervating their original motor and sensory targets.
A third-degree injury, introduced by Sunderland, is similar to second-degree injury in that Wallerian degeneration occurs; electrodiagnostic studies demonstrate denervation changes with fibrillations in affected muscles. In reinnervation, MUPs are present. Regeneration occurs at a rate of 1 mm/day, and progress may be followed with an advancing Tinel sign. With the increased severity of the injury, the endoneurial tubes are not intact, thus the regenerating axons may not reinnervate their original motor and sensory targets.
The pattern of recovery after third-degree injury is mixed and incomplete. Reinnervation occurs only if sensory fibers reach their sensory end organs and motor fibers reach their muscle targets. Even within a sensory nerve, recovery can be mismatched if sensory fibers reinnervate a different sensory area within the nerve's sensory distribution. If the muscle target is far from the site of injury, nerve regeneration may occur, but the muscle may not be reinnervated completely, due to the long period of denervation.
A fourth-degree injury results in a large area of scar at the site of nerve injury and precludes any axons from advancing distal to the level of nerve injury. Electrodiagnostic studies reveal denervation changes in the affected muscles, and no MUPs are present. A Tinel sign is noted at the level of the injury, but it does not advance beyond that level. No improvement in function is noted, and the patient requires surgery to restore neural continuity, thus permitting axonal regeneration and motor and sensory reinnervation.
A fifth-degree injury is a complete transection of the nerve. As with a fourth-degree injury, surgery is required to restore neural continuity. Electrodiagnostic findings are the same as those for a fourth-degree injury.
Mackinnon popularized a sixth-degree injury classification to describe a mixed-nerve injury with a combination of the other degrees of injury in one patient. [18] This injury pattern commonly occurs when some fascicles of the nerve are working normally while other fascicles may be recovering. Other fascicles may require surgical intervention to permit axonal regeneration.
Spontaneous recovery may occur with Sunderland type I, II, +/- III injuries. In cases of severe nerve injury (Sunderland III, IV, or V) and /or avulsion, however, spontaneous recovery does not occur, and surgical intervention is warranted.
Glenohumeral dysplasia (GHD) occurs with BRBPP. Imbalances in muscle, other soft tissues, and bone at the shoulder may lead to asymmetric growth, contractures, and deformation at the cartilaginous joint surfaces. [19, 20] The glenoid becomes progressively retroverted and inferiorly oriented, resulting in posterior displacement or subluxation of the humeral head. [21, 22] Predictors of GHD associated with BRBPP include increasing age and muscular imbalance, but GHD may occur without restricted shoulder movement. [19] At a median age of 16 weeks, 74% of infants undergoing surgical exploration of the brachial plexus had GHD on magnetic resonance imaging (MRI). [19]
Etiology
BRBPI may result from a multitude of causes. Positioning may lead to an increase in the neck-shoulder angle, producing a stretch injury, rupture, or avulsion if forces exceed neural tensile strength. Injury may also result from compression due to hematoma, clavicular fracture, uterine contraction, or instrumentation or manipulation.
Factors associated with BRBPI include large birth weight (>4000 g), [23, 24] large fetal weight deviation at 32 weeks' estimated gestational age or large birth weight, [25, 26, 27] long or difficult labor or delivery, breech delivery, and shoulder dystocia. [28, 29, 30, 31, 32, 33] Shoulder dystocia is associated with a 100-fold increased risk of brachial plexus injury. [23] Brachial plexus injury was noted in 5-11% of shoulder dystocia cases in retrospective studies. [34]
Persistent neonatal brachial plexus injury is possible after cesarian or vaginal delivery. [35] Persistence of brachial plexus injury beyond 1 year occurred in 85% of infants with BRBPI at one institution, and persistence of symptoms was more likely in patients with cephalic presentation, induction or augmentation of labor, birth weight greater than 9 lb (4.1 kg), and Horner syndrome. [36]
Some consider intrauterine pressure neuropathy to be a cause of BRBPI, on the basis of the high pressures exerted between the brachial plexus and the mother's pelvis with labor and on the occurrence of BRBPI in uneventful cesarean deliveries and in vaginal delivery without significant mechanical difficulty. Gonik et al suggested that spontaneous endogenous uterine and maternal expulsive forces are four to nine times greater than the force calculated for clinician-applied forces. [37]
Vertex presentation accounts for most BRBPI cases (94-97%); breech presentations account for 1-2% of cases; and cesarean deliveries account for 1% of cases. Mothers with diabetes, obesity, or preeclampsia, as well as mothers who are multiparous and previously had large babies, are also considered to be at higher risk for their children to have BRBPI.
Epidemiology
BRBPIs occur in 0.5-5 infants per 1000 live births. [26, 25, 32] The incidence ranges globally from 0.2% to 4% of live births. According to the World Health Organization, prevalence is generally 1-2% worldwide, with the higher numbers being in underdeveloped countries.
A retrospective study of 199 BRBPI patients referred to the Royal National Orthopaedic Hospital in the United Kingdom found that the risk of this condition was six times higher for Black patients than for white patients and 2.7 times higher for Asian patients than for white patients. [38] There also seemed to be a trend toward greater risk for individuals from lower socioeconomic groups. These changes were consistent across the three years studied (2004, 2014, and 2017).
Prognosis
Most patients with BRBPI recover spontaneously and do not require surgical intervention. For those patients with more severe neural injuries requiring surgical management, outcomes are hopeful. The assessment of clinical functional outcomes, however, is limited by the lack of a universal outcome measure in the evaluation of patients with BRBPI.
The Brachial Plexus Outcome Measure has been proposed as an effective and consistent outcome assessment tool. [39] Different centers use different scales for evaluation of motor function, change scales depending on patient age, use different scales for different body regions, use two scales to describe motion and strength, or describe range of motion as degrees of joint angle, limiting the capacity for comparison studies or meta-analyses.
The Canadian OBPI Working Group published a meta-analysis, however, comparing outcomes of children younger than 2 years who underwent either operative nerve repair or nonoperative management of BRBPI. [40] Nerve repair reduced functional impairment significantly as compared with nonoperative management. In 222 patients, there were no deaths, major adverse events occurred in 1.5%, and minor adverse events occurred in 5%. With nonoperative management, 27% of patients exhibited residual impairment.
With neuroma excision and grafting, reported outcomes demonstrate a relationship with the extent of the brachial plexus lesion.
O’Brien et al [41] reviewed their series of 52 patients with BRBPI treated with brachial plexus neuroma resection and interpositional sural nerve grafting at an average age of 9.8 months. A Medical Research Council score of greater than or equal to 3/5 was demonstrated in the setting of C5-C6 injury in biceps function in 92% of children, triceps function in 92%, and deltoid function in 83%. In the setting of C5-C7 injuries, children scored greater than or equal to 3/5 on the Medical Research Council scale in the biceps in 76%, triceps in 76%, and deltoid in 72%. Children with a C5-C8 and T1 lesion achieved and Medical Research Council score greater than 3/5 in the biceps in 73%, triceps in 53%, and deltoid in 67%.
Gilbert et al [42] reported their 20-year experience with neuroma excision and grafting in patients with no biceps function by age 3 months. They reported “good or excellent” shoulder function after 4-year follow-up in 80% of children that had C5-C6 lesions. Only 61% of patients achieved this function with C5-C7 injuries. Children with complete brachial plexus lesions achieved “average, good, or excellent results” in 77% of cases at 8 years postoperatively. They reported better outcomes in elbow function, with 81% of children achieving “good or excellent” results at 8 years. Hand function was reported as “useful” in 76% of children.
After reconstruction with neurolysis, neuroma excision and grafting, and/or nerve transfers, El-Gammal et al [43] reported good or excellent outcomes for elbow flexion and extension in 77% of children, but they reported achieving this same level of function for thumb extension in only 28% of patients and for wrist extension in only 31% of patients.
Transfer of the spinal accessory nerve to the suprascapular nerve has shown similar functional outcomes compared to nerve grafting from C5 to the suprascapular nerve. A retrospective review of suprascapular nerve reconstruction with either spinal accessory nerve transfer or C5 nerve root grafting found no difference in external shoulder rotation after 3 years of follow-up. [44]
Similarly, Terzis and Kostas [45] saw no significant differences in outcome in patients undergoing suprascapular nerve reconstruction via either direct neurotization (spinal accessory or intraplexus) or nerve graft. They reported good or excellent results in supraspinatus function in 96% of patients and in infraspinatus function in 75%. Forty-one of 50 patients achieved Mallet grade III or IV external shoulder rotation, and the authors described a trend towards increased range of shoulder abduction in patients with direct nerve transfers.
Pondaag et al [46] also found no differences in glenohumeral rotation, passive external rotation, and Mallet hand-to-head movement after nerve transfer versus grafting.
Nerve transfers have demonstrated functional improvements. In 54 patients with no external shoulder rotation, but otherwise spontaneous recovery, the spinal accessory–to–suprascapular nerve transfer resulted in Mallet score IV external rotation in 40 patients, Mallet grade III in 10 patients, and unchanged function in 4 patients. [47]
Intercostal nerve transfers to the musculocutaneous nerve resulted in Medical Research Council grade M4 biceps function in 84% [48] and good or excellent elbow flexion in 93.5%. [49] Oberlin transfer was reported to achieve Medical Research Council grade M4 or M5 biceps function in 4 of 7 patients [50] and Medical Research Council grade M5 in two of two patients [51] in separate studies.
Secondary surgical procedures involving tendon and muscle transfers and releases are available to patients with functional limitations. [52, 53] Limited shoulder function can be enhanced at a later age with procedures to release the subscapularis muscle or transfer the teres major and latissimus dorsi muscles. [54, 55, 56, 57] Recovery of elbow flexion can be augmented at a later date, if necessary, with muscle or tendon transfers.
Limb-length discrepancy between the unaffected and affected arms is a consequence of BRBPI. [58] Delayed bone age in the affected upper extremity may account for the decreased growth. [59]
Children with BRBPI score lower on health-related quality-of-life scales and have higher problem scores and maternal distress as compared with age-matched controls. [60, 61] Children who have undergone surgical repair of BRBPI commonly report pain, which is often low in intensity and episodic. [62]
Children affected with BRBPI demonstrate lower scores for global and upper-extremity function compared with pediatric norms, but they participate in organized sports at the same rate as their unaffected peers. As many as 42% of these children perceive a disability related to their sport, but they do not experience higher injury rates and do participate in multiple sports compared with their unaffected peers. [63]
The ability of children with BRBPP to perform self-care activities without significant limitation compared with their peers is determined by the presence of impairment of hand function, as assessed by the Pediatric Evaluation of Disability Inventory (PEDI). [64]
-
Schema of the brachial plexus.