Background
The ulnar nerve is an extension of the medial cord of the brachial plexus. It is a mixed nerve that supplies innervation to muscles in the forearm and hand and provides sensation over the medial half of the fourth digit and the entire fifth digit (the ulnar aspect of the palm), and the ulnar portion of the posterior aspect of the hand (dorsal ulnar cutaneous distribution). The entrapment of the ulnar nerve is the second most common entrapment neuropathy in the upper extremity (after entrapment of the median nerve). [1, 2, 3, 4]
The most common site of ulnar nerve entrapment is at or near the elbow region, especially in the region of the cubital tunnel [5] or in the epicondylar (ulnar) groove; the second most likely site is at or near the wrist, especially in the area of the anatomic structure called the canal of Guyon. [1, 6, 7, 8] Entrapment can also occur in the forearm between these two regions, below the wrist (hand) or above the elbow.
Pressure on or injury to the ulnar nerve may cause denervation and paralysis of the muscles supplied by the nerve. Affected patients often experience numbness and tingling along the little finger and the ulnar half of the ring finger. This discomfort is often accompanied by grip weakness and, rarely, intrinsic wasting. One of the most severe consequences is losing intrinsic muscle function in the hand. When the ulnar nerve is divided at the wrist, only the opponens pollicis, superficial head of the flexor pollicis brevis, and lateral two lumbricals are functioning.
Conservative nonsurgical treatment may play a beneficial role in management. However, surgical treatment is warranted if such treatment fails or the patient has severe or progressive weakness or loss of function.
Anatomy
Course of ulnar nerve
The ulnar nerve is the terminal branch of the medial cord of the brachial plexus and contains fibers from C8, T1, and, occasionally, C7. [9, 10] It enters the arm with the axillary artery and passes posterior and medial to the brachial artery, traveling between the brachial artery and the brachial vein.
At the level of the insertion of the coracobrachialis in the middle third of the arm, the ulnar nerve pierces the medial intermuscular septum to enter the posterior compartment of the arm. [11, 12] Here, the nerve lies on the anterior aspect of the medial head of the triceps, which is joined by the superior ulnar collateral artery. The medial intermuscular septum extends from the coracobrachialis proximally, where it is a thin and weak structure, to the medial humeral epicondyle, where it is a thick, distinct structure.
The arcade of Struthers is the next important site along the course of the ulnar nerve. This structure is found in 70% of patients, 8 cm proximal to the medial epicondyle, and extends from the medial intermuscular septum to the medial head of the triceps. The arcade of Struthers is formed by the attachments of the internal brachial ligament (a fascial extension of the coracobrachialis tendon), the fascia and superficial muscular fibers of the medial head of the triceps, and the medial intermuscular septum.
It is essential to distinguish the arcade of Struthers from the ligament of Struthers, which is found in 1% of the population and extends from a supracondylar bony or cartilaginous spur to the medial epicondyle. This supracondylar spur can be found on the anteromedial aspect of the humerus, 5 cm proximal to the medial epicondyle, and it can often be seen on radiographs. The ligament of Struthers may occasionally cause neurovascular compression, usually involving the median nerve or the brachial artery but sometimes affecting the ulnar nerve.
Next, the ulnar nerve passes through the cubital tunnel, which is the space bounded by the following:
-
The medial epicondyle (medial border)
-
The olecranon (lateral border)
-
The elbow capsule at the posterior aspect of the ulnar collateral ligament (floor)
-
The humeroulnar arcade (HUA), or Osborne fascia or ligament (roof)
The deep forearm investing fascia of the flexor carpi ulnaris and the arcuate ligament of Osborne, also known as the cubital tunnel retinaculum, form the roof of the cubital tunnel. The cubital tunnel retinaculum is a 4-mm-wide fibrous band that passes from the medial epicondyle to the tip of the olecranon. Its fibers are oriented perpendicularly to the flexor carpi ulnaris aponeurosis fibers, which blend with its distal margin.
The elbow capsule and the posterior and transverse portions of the medial collateral ligament form the floor of the cubital tunnel. The medial epicondyle and olecranon form the walls.
O’Driscoll suggested that the roof of the cubital tunnel (ie, the Osborne ligament or fascia) is a remnant of the anconeus epitrochlearis, [13] an aberrant muscle that has been found in 3-28% of cadaver elbows and in as many as 9% of patients undergoing surgery for cubital tunnel syndrome. This muscle arises from the medial humeral condyle and inserts on the olecranon, crossing superficially to the ulnar nerve, which may cause compression. [14]
O’Driscoll also identified a retinaculum at the proximal edge of the arcuate ligament in all but 4 of 25 cadaveric specimens. [13] He classified this retinaculum into the following four types:
-
Absent retinaculum
-
Thin retinaculum that becomes tight with full flexion without compressing the nerve
-
Thick retinaculum that compresses the nerve between 90° and full flexion
-
Accessory anconeus epitrochlearis
Upon entering the cubital tunnel, the ulnar nerve gives off an articular branch to the elbow. It then passes between the humeral and ulnar heads of the flexor carpi ulnaris and descends into the forearm between the flexor carpi ulnaris and the flexor digitorum profundus. Finally, about 5 cm distal to the medial epicondyle, the ulnar nerve pierces the flexor-pronator aponeurosis, the common fibrous origin of the flexor and pronator muscles.
The ligament of the Spinner is an additional aponeurosis between the flexor digitorum superficialis of the ring finger and the humeral head of the flexor carpi ulnaris. This septum is independent of the other aponeuroses and attaches directly to the medial epicondyle and the medial surface of the coronoid process of the ulna. With the anterior transposition of the ulnar nerve, it is essential to recognize and release this structure to prevent kinking.
In the forearm, the ulnar nerve extends motor branches to the flexor carpi ulnaris, the flexor digitorum profundus of the ring, and small fingers. The ulnar nerve may extend as many as four branches to the flexor carpi ulnaris, ranging from 4 cm above to 10 cm below the medial epicondyle. Proximal dissection of the first motor branch to the flexor carpi ulnaris from the ulnar nerve may be performed up to 6.7 cm proximal to the medial epicondyle, facilitating anterior transposition of the nerve.
Posterior branches of the medial antebrachial cutaneous nerves cross the ulnar nerve from 6 cm proximal to 4 cm distal to the medial epicondyle. These branches are often cut when making the skin incision for a cubital tunnel release, creating an area of dysesthesia or resulting in potential neuroma formation.
As the ulnar nerve courses down the forearm toward the wrist, the dorsal ulnar cutaneous nerve leaves the main branch. A little further down, the palmar cutaneous branch takes off. Thus, neither of these two branches goes through the canal of Guyon. [1] The remainder of the ulnar nerve enters the canal at the proximal portion of the wrist. It is bounded proximally and distally by the pisiform bone and the hook of the hamate bone. In addition, it is covered by the volar carpal ligament and the palmaris brevis.
The following two nerve anomalies should be mentioned because they may confuse the diagnosis in the setting of ulnar neuropathy:
-
Martin-Gruber anastomosis in the forearm - In this anomaly, fibers that supply the intrinsic muscles are carried in the median nerve to the middle of the forearm, where they leave the median nerve to join the ulnar nerve; functioning intrinsic muscles could be observed with injury above this anastomosis, though the ulnar nerve dysfunction is proximal
-
Riche-Cannieu anastomosis - Median and ulnar nerves are connected in the palm; even with an injury at the wrist, there is some intrinsic function.
Blood supply
The extrinsic blood supply to the ulnar nerve is segmental and involves the following three vessels:
-
Superior ulnar collateral artery
-
Inferior ulnar collateral artery
-
Posterior ulnar recurrent artery
Typically, the inferior ulnar collateral artery (and often the posterior ulnar recurrent artery) is sacrificed with anterior transposition. At the level of the medial epicondyle, the inferior ulnar collateral artery is the sole blood supply to the ulnar nerve. In an anatomic study, no identifiable anastomosis was found between the superior ulnar collateral artery and the posterior ulnar recurrent arteries in 20 of 22 arms; instead, communication between the two arteries occurred through proximal and distal extensions of the inferior ulnar collateral artery.
The intrinsic blood supply is composed of an interconnecting network of vessels that run along the fascicular branches and along each fascicle of the ulnar nerve itself. The surface microcirculation of the ulnar nerve follows an anastomotic stepladder arrangement. The inferior ulnar collateral artery is consistently found 5 mm deep to the leading edge of the medial intermuscular septum on the surface of the triceps. [15]
Sites of nerve entrapment
As diagnostic and surgical methodologies have evolved over the past century, physicians’ ability to recognize and describe entrapment sites has improved. However, the terminology used to describe ulnar nerve entrapment has become confusing because not all clinicians use the exact words for the same things. This confusion can be illustrated by examining the terms applied to ulnar nerve entrapment in the elbow region, [16] of which the two most commonly used (and misused) are tardy ulnar palsy [17] and cubital tunnel syndrome. [18]
In 1878, Panas first described what is now often called tardy ulnar palsy, in which either prior trauma or osteoarthritis gradually caused damage to the ulnar nerve. [19] Additional cases were reported over the ensuing decades, [20, 21] usually associated with trauma (eg, fractures in the elbow region) and typically occurring in the epicondylar groove. [22, 23] Initially denoting time (ie, appearing years after trauma), the term came to have an anatomic connotation (ie, usually seen in or very near the epicondylar groove). [24]
From 1922 on, physicians began to recognize ulnar entrapments in the HUA. [25, 26] In 1958, the term cubital tunnel syndrome was coined to describe the effects of the ulnar nerve entrapment [27] at the HUA. Numerous other reports ensued.
Although the current state of knowledge is still incomplete, it is possible to identify approximately five sites in the elbow region at which the ulnar nerve is most likely to be compressed. (Five is not a firm figure; some areas are so close together that certain authorities categorize them differently to get a different number.) Therefore, this article principally follows Posner’s classification, [28] which lists the following sites (see the image below):
-
Above the elbow in the region of the intermuscular septum
-
The medial epicondylar region
-
The epicondylar (ie, ulnar) groove
-
The region of the cubital tunnel
-
The region where the ulnar nerve exits from the flexor carpi ulnaris, at which the usual cause of compression is the deep flexor-pronator aponeurosis
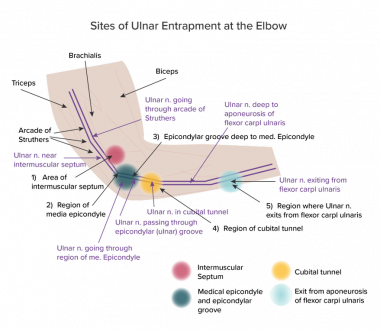 A schematic diagram of the elbow region with 5 main sites (as given by Posner) labeled 1-5; other sites and structures are also named. The main regions of interest are colored circles. Sites 2 and 3 are close together and cannot be distinguished by means of electromyography and nerve conduction studies. This location is referred to as ulnar (or epicondylar) groove.
A schematic diagram of the elbow region with 5 main sites (as given by Posner) labeled 1-5; other sites and structures are also named. The main regions of interest are colored circles. Sites 2 and 3 are close together and cannot be distinguished by means of electromyography and nerve conduction studies. This location is referred to as ulnar (or epicondylar) groove.
Region of intermuscular septum
Halikis et al [29] divided this region into 2 areas, the arcade of Struthers [30, 31] and the medial intermuscular septum. According to the standard anatomic definition, the arcade of Struthers is a thin fibrous band that usually extends from the medial head of the triceps to the medial intermuscular septum. It is often about 6-10 cm proximal to the medial epicondyle.
Considerable anatomic variation exists; in fact, there is outright controversy about the arcade of Struthers. [32] One component of the controversy is quite trivial: There is no evidence that Struthers discovered this structure or was even aware of it; his name was attached to it by Kane et al in a 1973 paper. [33]
In an autopsy study of 60 upper limbs, Siqueira found a structure reasonably approximating the definition given above in 8 limbs (13.5%). [32] Ulnar nerve entrapment occurred in none of them (but there was no clinical reason to expect that it might have).
Bartels et al could not find the arcade of Struthers in their dissections, and they expressed doubts about its existence. [34]
Wehrli and Oberlin described a different structure in the same region that might be involved in ulnar entrapment in some cases—the internal brachial ligament. [35] This structure was described by Struthers, but not in relation to ulnar nerve entrapment. Wehrli and Oberlin advocated abolishing the concept of the arcade of Struthers.
Von Schroeder and Scheker described yet another structure, a fibrous tunnel in roughly the same region. [36] They maintained that the ulnar nerve goes through this tunnel and could be trapped therein and suggested naming this structure the arcade of Struthers.
Settling this anatomic controversy is beyond the scope of this article. However, it is sufficient to note that in rare cases, the ulnar nerve may be compressed considerably above the ulnar groove and that surgeons may find it entrapped in a fibrous or ligamentous structure that may correspond to one of the previous anatomic descriptions.
Medial epicondylar region
Ulnar compression [37] in the medial epicondylar region is generally from a valgus deformity of the bone. For example, a patient is placed in a standard anatomic position with the palms rotated toward the front and the thumb away from the midline. In that case, a valgus deformity means that the elbow would be deformed away from the body’s midline.
Epicondylar groove
The epicondylar (ulnar) groove is a fibro-osseous tunnel holding the ulnar nerve and its vascular accompaniment. It is slightly distal to the medial epicondyle, or at least to its beginning.
Campbell used slightly different terminology, lumping the epicondylar groove with the medial epicondylar region and labeling the entire region as the area of the retrocondylar groove. Halikis et al. considered the medial epicondylar region and the epicondylar groove to be the medial epicondyle area. [29]
The medial epicondylar region and the epicondylar groove are generally considered the classic locations (or locations, if considered as a single area) for tardy ulnar palsy. However, in the author’s personal experience, electromyographers and orthopedic surgeons commonly refer to a tardy ulnar palsy at the retrocondylar groove, thus using the Campbell terminology.
Region of cubital tunnel
The cubital tunnel is the passage between the two heads of the flexor carpi ulnaris, which are connected by a continuation of the fibroaponeurotic covering of the epicondylar groove (Osborne ligament). During elbow flexion, the tunnel flattens as the ligament stretches, causing pressure on the ulnar nerve. [38, 39, 40]
Campbell’s classification was the same for this region, except that he preferred to call it the region of the HUA, apparently because he believed that too many clinicians loosely used the term cubital tunnel to refer to a place anywhere in the elbow.
Halikis et al divided this region into the cubital tunnel and the Osborne fascia. [29] This is an excellent example of the problems with the terminology: Different terms are used for locations that are virtually the same. For all practical purposes—indeed, concerning anything that can be distinguished on electromyography (EMG)—the Osborne ligament is equivalent to the Osborne fascia, and both are equivalent to the HUA.
Region where the ulnar nerve exits from flexor carpi ulnaris
Campbell [41] and Halikis et al [29] agreed with Posner in listing this region as the final entrapment site in the elbow area. As the nerve exits the flexor carpi ulnaris, it perforates a fascial layer between the flexor digitorum superficialis and the flexor digitorum profundus. Entrapment can occur here, also.
More distal entrapment sites
After the ulnar nerve passes distal to the elbow, [42, 43, 20] it makes several important divisions. The first branches to come off are those that go to the flexor carpi ulnaris. Further distally, the branches to the flexor digitorum profundus muscles of digits 4 and 5 arise.
Although the nerve could be injured or entrapped at any point along its course, four sites have been identified as the most common locations of entrapment in relation to the canal of Guyon (see the image below).
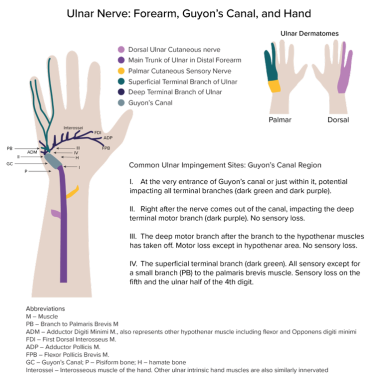 The diagram shows the ulnar nerve distal to the elbow region. The dorsal ulnar cutaneous nerve (lavender) branches off the main trunk (purple). Although the course is not followed in detail after that, the lavender region on the sensory dermatome diagram shows where this sensory nerve innervates skin. Similarly, the palmar cutaneous sensory nerve (yellow) branches off to innervate the skin area depicted in yellow. The superficial terminal branch is mostly sensory (see green-colored skin on palmar surface), though it also gives off a branch to the palmaris brevis. The deep terminal branch has no corresponding skin area, because it is solely motor-innervating the muscles shown, as well as others not explicitly depicted. A nerve could be pinched or injured anywhere, but sites labeled I-IV are more commonly involved.
The diagram shows the ulnar nerve distal to the elbow region. The dorsal ulnar cutaneous nerve (lavender) branches off the main trunk (purple). Although the course is not followed in detail after that, the lavender region on the sensory dermatome diagram shows where this sensory nerve innervates skin. Similarly, the palmar cutaneous sensory nerve (yellow) branches off to innervate the skin area depicted in yellow. The superficial terminal branch is mostly sensory (see green-colored skin on palmar surface), though it also gives off a branch to the palmaris brevis. The deep terminal branch has no corresponding skin area, because it is solely motor-innervating the muscles shown, as well as others not explicitly depicted. A nerve could be pinched or injured anywhere, but sites labeled I-IV are more commonly involved.
The canal of Guyon may be conveniently divided into three zones as follows:
-
Zone 1 (encompassing the area proximal to the bifurcation of the ulnar nerve) - Compression in zone 1 causes combined motor and sensory loss; it is most commonly caused by a fracture of the hook of the hamate or a ganglion
-
Zone 2 (encompassing the motor branch of the nerve after it has bifurcated) - Compression in zone 2 causes pure loss of motor function to all of the ulnar-innervated muscles in hand; ganglion and fracture of the hook of the hamate are the most common causes
-
Zone 3 (encompassing the superficial or sensory branch of the bifurcated nerve) - Compression in zone 3 causes sensory loss to the hypothenar eminence, the small finger, and part of the ring finger, but it does not cause motor deficits; common causes are an aneurysm of the ulnar artery, thrombosis, and synovial inflammation
Pathophysiology
As the elbow moves from extension to flexion, the distance between the medial epicondyle and the olecranon increases by 5 mm for every 45° elbow flexion. Elbow flexion places stress on the medial collateral ligament and the overlying retinaculum. In addition, the shape of the cubital tunnel in cross-section changes from round to oval, with a 2.5-mm loss of height, because the cubital tunnel rises during elbow flexion, and the epicondylar groove is not as deep on the inferior aspect of the medial epicondyle as it is posteriorly.
The cubital tunnel’s loss in height with flexion leads to a 55% volume decrease in the canal, which causes the mean ulnar intraneural pressure to increase from 7 mm Hg to 14 mm Hg. [44, 45] A combination of shoulder abduction, elbow flexion, and wrist extension results in the greatest increase in cubital tunnel pressure, with ulnar intraneural pressure increasing to about six times normal. [46, 47, 48, 49, 37]
Traction and excursion of the ulnar nerve also occur during elbow flexion, as the ulnar nerve passes behind the axis of rotation of the elbow. With a complete range of motion of the elbow, the ulnar nerve undergoes 9-10 mm of longitudinal excursion proximal to the medial epicondyle and 3-6 mm of excursion distal to the epicondyle. [50] The ulnar nerve elongates by 5-8 mm with elbow flexion.
In addition to prior cadaver and surgical studies of ulnar nerve motion, recently developed sonographic methods facilitate monitoring the movement in the intact arm. [51] Interestingly, the nerve is somewhat more motile in patients with ulnar neuropathies than in individuals with normal ulnar nerves.
Within the cubital tunnel, the measured mean intraneural pressure is significantly greater than the mean extraneural pressure at elbow flexion of 90° or more. [52] With the elbow flexed 130°, the mean intraneural pressure is 45% higher than the mean extraneural pressure. With this degree of flexion, significant flattening of the ulnar nerve occurs; however, with full elbow flexion, there is no evidence for direct focal compression, which suggests that traction on the nerve in association with elbow flexion is responsible for the increased intraneural pressure.
In addition, studies have shown that the intraneural and extraneural pressures within the cubital tunnel are lowest at 45° of flexion. Therefore, a 45° flexion is considered the optimum position for immobilization of the elbow to decrease the pressure on the ulnar nerve.
Subluxation of the ulnar nerve is common. Childress, in a study of 2000 asymptomatic elbows, found that although none of the patients were aware of ulnar nerve subluxation, 16.2% had this condition after flexion past 90°. [53] Of the 325 patients with ulnar nerve subluxation, only 14 had unilateral subluxation. Subluxation does not appear to cause cubital tunnel syndrome. Still, the friction generated with repeated subluxation may cause intraneural inflammation, and the subluxed position may render the nerve more susceptible to accidental trauma.
Sunderland described the internal topography of the ulnar nerve at the medial epicondyle. [54] The sensory and intrinsic muscle nerve fibers are located superficially. In contrast, the motor fibers to the flexor carpi ulnaris and the flexor digitorum profundus are located deep within the nerve. [55, 56, 57] The central location protects the motor fibers and explains why weakness of these two muscles is not typically seen in ulnar neuropathy. [58, 59, 28, 60]
Proximal compression of a nerve trunk, such as with cervical radiculopathy, may lead to increased vulnerability to nerve compression in a distal segment. This “double crush” condition can affect the ulnar nerve and results from the disruption of normal axonal transport. [61]
The nerve, axon, and myelin can be affected. Within the axon, fascicles to individual muscles may be involved selectively. Axonal involvement leads to motor unit loss and amplitude/area reduction. Conduction block implies impaired transmission through a segment of the nerve. In the absence of changes indicating axonal damage, conduction block means myelin damage to the involved segment. Significant slowing of conduction or spreading out of the temporal profile of the recorded response with preserved axonal integrity suggests demyelination.
Various systems have been proposed for classifying nerve injuries. Seddon in 1972 and Sunderland in 1978 took similar approaches to this classification. Seddon stratified nerve injuries into the following three levels: [62]
-
Neurapraxia - This is a transient episode of complete motor paralysis with little sensory or autonomic involvement, usually occurring secondary to transitory mechanical pressure; once the pressure is relieved, a total return of function follows
-
Axonotmesis - This is a more severe injury involving loss of continuity of the axon with the maintenance of continuity of the Schwann sheath; motor, sensory, and autonomic paralysis is complete, and denervated muscle atrophy can be progressive; recovery can be full but depends on several factors, including timely removal of the compression and axon regeneration; the time necessary to recover function depends on the distance between the denervated muscle and the proximal regenerating axon
-
Neurotmesis - This is the most severe level of injury, entailing complete loss of continuity both of the axon and the Schwann sheath; recovery rarely is full, and the amount of loss can only be determined over time; regenerating axons without intact neural tubes reinnervate muscle fibers that were not part of their original network
Sunderland’s classification specifies five degrees of nerve damage. [63] The first degree corresponds to neurapraxia in Seddon’s schema; the second corresponds to axonotmesis; and the third, fourth, and fifth correspond to increasingly severe levels of neurotmesis. In a Sunderland third-degree injury, axons and Schwann sheaths are disrupted within intact nerve fascicles. In a fourth-degree injury, the perineurium surrounding the fascicles is damaged, as is the endoneurium. Finally, in a fifth-degree injury, the nerve trunk is severed.
McGowan established the following classification system for ulnar nerve injuries [64] :
-
Grade I - Mild lesions with paresthesias in the ulnar nerve distribution and a feeling of clumsiness in the affected hand; no wasting or weakness of the intrinsic muscles
-
Grade II - Intermediate lesions with weak interosseous muscles and muscle wasting
-
Grade III - Severe lesions with paralysis of the interosseous muscles and marked weakness of the hand
In a study of the validity of the Disabilities of Arm, Shoulder, and Hand (DASH) questionnaire for elbow ulnar neuropathy, Zimmerman et al. found that the DASH questionnaire accurately reflected the clinical staging of ulnar neuropathy. [65] There was a high correlation between DASH scores, the severity of symptoms, and functional status. Correlations were identified as significant between DASH and biomechanical measures, but correlation coefficients were lower. All measurements showed significant improvement postoperatively.
Etiology
Cubital tunnel syndrome may be caused by constricting fascial bands, subluxation of the ulnar nerve over the medial epicondyle, cubitus valgus, bony spurs, hypertrophied synovium, tumors, ganglia, or direct compression of. Occupational activities may aggravate cubital tunnel syndrome secondary to repetitive elbow flexion and extension. Certain occupations are associated with the development of cubital tunnel syndrome; however, a definite relation to occupational activities is not well defined. [66, 67, 68]
Factors that may cause ulnar neuropathy at or near the elbow include the following:
-
Compression during general anesthesia
-
Blunt trauma
-
Deformities (eg, rheumatoid arthritis)
-
Metabolic derangements (eg, diabetes)
-
Transient occlusion of the brachial artery during surgery [69]
-
Subdermal contraceptive implant [70]
-
Venipuncture [71]
-
Hemophilia [72] leading to hematomas
-
Malnutrition leading to muscle atrophy and loss of fatty protection across the elbow and other joints
-
Cigarette smoking [73]
Factors that may cause ulnar neuropathy at or distal to the wrist (ie, at the canal of Guyon) include the following:
-
Ganglionic cysts
-
Tumors
-
Blunt injuries, with or without fracture
-
Aberrant artery
-
Idiopathic
Epidemiology
United States statistics
The elbow is the second most common site of nerve entrapment in the upper extremity, the first being the wrist (ie, carpal tunnel syndrome). In the general population, abnormalities in the ulnar nerve at the elbow in asymptomatic subjects are common (about 40%).
Age-related demographics
The older literature indicated that most cases of ulnar compression neuropathy occur in patients older than 35 years. [74] This is consistent with an independent anatomic study of 200 cadavers from 1963, which showed that the ulnar nerve is the largest at the entrance to the cubital tunnel and that this enlargement is a maximal size in males older than 35 years. [75] A prospective study of 76 patients published in 2006 showed that increased age correlates with a greater tendency toward ulnar neuropathy. [76]
Sex-related demographics
No gross anatomic differences in the course of the nerve are noted between the sexes. However, the following have been noted: [77]
-
Women have 2-19 times more fat content in the medial elbow overlying the tubercle of the ulnar coronoid process
-
The tubercle of the coronoid process is 1.5 times larger in men
Contreras et al suggested that the coronoid process may be a potential site for ulnar nerve compression in men and that the increased subcutaneous fat around the ulnar nerve in women may provide a protective advantage against acute ulnar neuropathy. [77]
Prognosis
A favorable surgical outcome is more likely for sensory function than motor function. However, a favorable outcome occurs in 85-95% of cases.
The following factors are relevant to the prognosis:
-
A motor amplitude of 10% of normal or a significantly reduced recruitment of motor units indicates a low likelihood of significant or full recovery
-
In some cases, nerve regeneration is accompanied by pain and paresthesias, which are thought to be secondary to random ectopic impulse generation of affected nerves
-
A diameter greater than 3.5 mm on the initial sonogram of the ulnar nerve at the elbow is associated with persistent symptoms or signs, regardless of whether conservative treatment or surgical treatment is provided [79]
-
The outcome is not correlated with clinical features noted at baseline or with the duration of symptoms before treatment
-
The presence of motor conduction velocity slowing or pure conduction block across the elbow signifies a favorable outcome; these are considered independent prognostic factors [80]
Unfavorable or poor surgical outcome is associated with the following:
-
Age older than 50 years
-
Coexisting diabetes or other causes of peripheral polyneuropathy
-
Atrophy and ongoing denervation of ulnar-derived muscles
-
Absent ulnar sensory responses
Bartels et al performed a meta-analysis of the literature from 1970 to 1997, which included 3024 patients. [84] Irrespective of preoperative status, simple decompression had the best outcomes, and subcutaneous and submuscular transposition had the worst. For severe compression (McGowan grade III), anterior intramuscular transposition had the best outcome, and simple decompression and submuscular transposition had the following best outcomes.
Heithoff reviewed 14 clinical studies covering 516 patients in which a simple decompression was performed for cubital tunnel syndrome. Results were satisfactory in 75-92% of the patients. [85]
Steiner et al. monitored 41 patients who underwent simple ulnar nerve decompression for an average follow-up period of 2 years. [86] Results were good or excellent in 89% of the patients; 8% had no improvement.
Lluch studied 20 patients who underwent decompression in situ through a transverse incision. [87] A retrospective review of 22 patients noted a 24% incidence of complications from unsightly scarring and injury to the posterior branches of the medial antebrachial cutaneous nerve. To avoid this complication, a transverse incision was used for decompression in 20 patients, allowing easier identification and protection of the nerve branches. No problems with dysesthesia or amputation neuromas occurred, and an excellent cosmetic result was obtained.
Heithoff and Millender reviewed 12 clinical studies involving 350 patients in which a medial epicondylectomy was performed for cubital tunnel syndrome. Results were satisfactory in 72-94% of the patients. [88]
Kaempffe and Farbach reviewed 27 patients who underwent partial medial epicondylectomies and were monitored for an average of 13 months. [89] Subjective improvement was noted in 93% of cases. Results were excellent in 8 patients, good in 10, and fair in 8; 1 had a poor outcome.
To assess factors influencing outcome after medial epicondylectomy, Seradge and Owen studied 160 patients over ten years and monitored them for three years postoperatively. [90] In all, 21 patients had a recurrence—a return of symptoms three months or longer after surgery—and 44% of these recurrences occurred in the fourth decade of life. The recurrence rate was 18% in females and 10% in males. In addition, the recurrence rate was twice as high in patients who did not return to work within three months.
When concomitant ipsilateral carpal tunnel syndrome was present, the recurrence rate was 17%, compared with 9% when this syndrome was absent. [90] When concurrent thoracic outlet syndrome was present, the recurrence rate was 20%, compared with 9% when this syndrome was absent. In conclusion, Seradge and Owen noted a high recurrence rate after medial epicondylectomy in middle-aged women with ipsilateral carpal tunnel syndrome or thoracic outlet syndrome who did not return to work within three months postoperatively.
Seradge also examined the results of medial epicondylectomy in patients on workers’ compensation. [91] These patients stayed out of work longer, used a long period of conservative treatment without a positive impact on surgical outcome, had a less favorable surgical result, and had a higher recurrence rate.
Glowacki and Weiss reviewed the results of anterior intramuscular transpositions in 45 patients monitored for an average of 15 months. [92] In 87% of patients, symptoms resolved or improved. In addition, the 24 patients receiving workers’ compensation had a 33% rate of complete symptom resolution, whereas those not receiving workers’ compensation had a 57% rate of full symptom resolution.
Geutjens et al conducted a prospective study of 52 patients, comparing medial epicondylectomy with anterior transposition. [93] Results were better with medial epicondylectomy: More patients were satisfied, more stated that they would have the operation, and fewer complained of mild pain in their hand postoperatively. However, no follow-up visits or significant differences were present in motor power or nerve conduction rates.
Kleinman and Bishop monitored 47 patients after anterior intramuscular transposition for an average of 28 months. [94] Results were good or excellent in 87%, with the return of normal grip strength and two-point discrimination. None of the patients required a repeat operation.
Asami et al monitored 35 patients for an average of 70-72 months after anterior intramuscular transposition was performed with or without preservation of the extrinsic vasculature. [95] Nerve conduction velocities and clinical results were better in the group whose extrinsic vessels were preserved. When the extrinsic vessels were sacrificed, 3 excellent, 3 good, 4fair, and no poor results were obtained; when they were preserved, 16 excellent, 12 good, 3 fair, and no poor results were obtained.
Nouhan and Kleinert monitored 33 limbs in 31 patients who underwent anterior submuscular transposition for an average of 49 months. [96] A flexor-pronator Z-lengthening technique was performed without internal neurolysis and yielded 36% excellent, 61% good, and 3% poor results.
Tsujino et al followed 16 patients after cubital tunnel reconstruction for ulnar nerve neuropathy in osteoarthritic elbows. [97] A simple decompression with resection of the osteophytes from the epicondylar groove was performed. Patients were monitored for an average of 36 months. All patients were relieved of their preoperative discomfort and recovered all or some parts of their motor and sensory functions.
In a 2011 Cochrane review, Caliandro et al found no difference in clinical outcomes between simple decompression and transposition of the ulnar nerve in terms of both clinical and neurophysiologic improvement. Transposition was associated with a higher incidence of wound infections. [98]
-
A schematic diagram of the elbow region with 5 main sites (as given by Posner) labeled 1-5; other sites and structures are also named. The main regions of interest are colored circles. Sites 2 and 3 are close together and cannot be distinguished by means of electromyography and nerve conduction studies. This location is referred to as ulnar (or epicondylar) groove.
-
The diagram shows the ulnar nerve distal to the elbow region. The dorsal ulnar cutaneous nerve (lavender) branches off the main trunk (purple). Although the course is not followed in detail after that, the lavender region on the sensory dermatome diagram shows where this sensory nerve innervates skin. Similarly, the palmar cutaneous sensory nerve (yellow) branches off to innervate the skin area depicted in yellow. The superficial terminal branch is mostly sensory (see green-colored skin on palmar surface), though it also gives off a branch to the palmaris brevis. The deep terminal branch has no corresponding skin area, because it is solely motor-innervating the muscles shown, as well as others not explicitly depicted. A nerve could be pinched or injured anywhere, but sites labeled I-IV are more commonly involved.
-
Inching technique used to isolate conduction block in left ulnar nerve. Note significant amplitude drop at 305 mm, which correlates with position 2 cm above medial epicondyle. This is example of supracondylar block. Image courtesy of A S Lorenzo, MD.
-
Normal median and ulnar patterns are compared with those of 3 commonly recognized types of Martin-Gruber anomaly.
-
First 3 traces correspond to ulnar compound muscle action potential (CMAP) amplitude during recording at abductor digiti quinti (ADQ) and stimulating at wrist, below elbow, and above elbow, respectively. Fourth trace corresponds to stimulation of median nerve at elbow during recording at ADQ. Although CMAP amplitude is reduced markedly above elbow, this is compensated for by adding response seen after stimulation of median nerve; this represents Martin-Gruber anastomosis.
-
First 3 traces correspond to stimulation of ulnar nerve during recording at first dorsal interosseous (FDI) muscle at wrist, below elbow, and above elbow, respectively. Fourth trace corresponds to stimulation of median nerve at elbow during recording at FDI muscle; this represents Martin-Gruber anastomosis.
-
In those with Martin-Gruber anomaly who have no other significant neuropathy or nerve compression, stimulation of specific nerves at different sites yields differing results. With the median nerve, stimulation at the elbow yields larger compound muscle action potential (CMAP) at hypothenar muscles, first dorsal interosseous (FDI) muscle, or thenar muscles (or combination thereof) than does stimulation at the wrist. With the ulnar nerve, stimulation at the wrist yields larger CMAP at hypothenar muscles, FDI muscle, or thenar muscles (or combination thereof) than does stimulation at the elbow. In this context, "larger" and "smaller" generally refer to amplitude differences ≥1.0 mV.
-
Riche-Cannieu anastomosis is communication between the recurrent branch of the median nerve and the deep branch of the ulnar nerve in the hand. Although it is present in 77% of hands, it yields highly variable degrees of detectable physiologic difference. In many hands, it contributes little and does not affect diagnostic findings at all. The most common effect is probably to give ulnar innervation to some muscles usually innervated by the median nerve, median innervation to muscles usually innervated by the ulnar nerve, or both. The most extreme version is the so-called all-ulnar hand (very rare). Two examples of confusion this might cause are as follows: (1) a median lesion could cause denervation in typically ulnar muscle, such as adductor digiti minimi (adductor digiti quinti) or first dorsal interosseous muscle, and (2) an ulnar lesion could cause denervation in typically median muscle, such as flexor pollicis brevis or abductor pollicis brevis.








