Overview
Hyphema is defined as the presence of blood within the aqueous fluid of the anterior chamber. The most common cause of hyphema is trauma.
Postinjury accumulation of blood in the anterior chamber is one of the most challenging clinical problems encountered by the ophthalmologist. Even a small hyphema due to ocular injury can be a sign of major intraocular trauma with associated damage to vascular and other intraocular tissues.
Blunt trauma to the eye may result in injury to the conjunctiva, cornea, iris, pupillary sphincter, angle structures, lens, zonules, retina, vitreous, optic nerve, and other intraocular or intraorbital structures. Rapid, marked elevation in intraocular pressure with sudden distortion of intraocular structures produces the dynamic changes responsible for hyphema formation.
The lack of an ideal therapeutic program, the potential for secondary hemorrhage, and the secondary onset of glaucoma all threaten to turn an eye with an initially good visual prognosis into a complex therapeutic problem with a poor final visual result.
Classification and characteristics
Traumatic hyphema is encountered in both children and adults with the peak incidence between ages 10-20 years. [1] Hyphema usually is the result of a projectile or deliberate blow that hits the exposed portion of the eye despite the protection of the bony orbital rim. Various missiles and objects have been incriminated, including balls, rocks, projectile toys, air gun pellets, BB gun pellets, automobile airbags, hockey pucks, badminton birdies, champagne corks, bungee cords, paintballs, and the human fist. [2, 3, 4] More recently, air gun pellets and BB gun pellets have been made of plastic polymers.
There have been reported cases of hyphema due to objects larger than the orbit, such as soccer balls, and even durian fruit falling on the unlucky person napping beneath the durian tree. [5, 6] Slow-motion photography has demonstrated deformation of the soccer balls as impact occurs with the orbital rim, thereby imparting direct pressure to the globe, causing the hyphema. With the increase of child abuse, fists and belts have started to play a prominent role. Males are involved in 75% of traumatic hyphema cases. [7, 8]
Hyphema related to surgical procedures on the eye may occur intraoperatively or postoperatively. Surgical hyphema is a known complication of intraocular surgery and should be managed in a similar manner as traumatic hyphema.
Rarely, spontaneous hyphemas may occur and be confused with traumatic hyphemas. Spontaneous hyphemas are secondary to neovascularization (eg, diabetes mellitus, ischemia, cicatrix formation), ocular neoplasms (eg, retinoblastoma, iris melanomas, medulloepitheliomas, [9] uveitis, and vascular anomalies (eg, juvenile xanthogranuloma). Vascular tufts that exist at the pupillary border have been implicated in spontaneous hyphemas. [10] Anticoagulate substance use (eg, ethanol, aspirin, warfarin) have been known to cause spontaneous hyphemas. [11, 12] Spontaneous hyphemas due to iris chafing can be seen with anterior chamber intraocular lenses as in uveitis-glaucoma-hyphema (UGH) syndrome or poorly placed posterior chamber intraocular lenses. Hyphemas from UGH can occur early after cataract surgery or up to 14 years later. [13]
Finally, an idiopathic hyphema of no known cause may occur with spontaneous resolution. This is extremely rare.
The following is the clinical grading system for traumatic macrohyphemas:
-
Grade 1 - Layered blood occupying less than one third of the anterior chamber
-
Grade 2 - Layered blood filling one third to one half of the anterior chamber
-
Grade 3 - Layered blood filling one half to less than the total of the anterior chamber
-
Grade 4 - Total clotted blood, often referred to as blackball or 8-ball hyphema
Most hyphemas fill less than one third of the anterior chamber. When hyphemas are divided into 4 groups according to the amount of filling of the anterior chamber, 58% involve less than one third of the anterior chamber, 20% involve one third to one half of the anterior chamber, 14% involve one half to less than the total of the anterior chamber, and 8% are total hyphemas. Slightly fewer than one half of all hyphemas settle inferiorly to form a level; approximately 40% form a definite clot, usually adherent to the iris stroma; and 10% have a dark clot in contact with the endothelium. This last form may portend a poor outcome and corneal blood staining, particularly in the setting of elevated intraocular pressure.
An alternative method of grading hyphemas involves measuring (in millimeters) the hyphema from the inferior 6-o'clock limbus. This method may help in monitoring the progress of resolution or the occurrence of rebleeding. Digital imaging analysis is also useful and objective but is available in only a few research or academic facilities.
When there is no layering of blood, but blood cells are seen within the anterior chamber, the term microhyphema can be used and graded with the Standardization of Uveitis Nomenclature (SUN) criteria scale for grading anterior segment cells.
The cause of an anterior chamber hemorrhage in contusion injuries is thought to be related to the posterior displacement of tissue or to the resultant fluid wave in the aqueous humor and the vitreous. This sudden dynamic shift stretches the limbal vessels and displaces the iris and the lens. This displacement may result in a tear at the iris or the ciliary body, usually within the angle structures. [14] A tear at the anterior aspect of the ciliary body is the most common site of bleeding and occurs in about 71% of cases. [15] The blood exits from the anterior chamber via the trabecular meshwork and the Schlemm canal or the juxtacanalicular tissue.
The usual duration of an uncomplicated hyphema is 5-6 days. The mean duration of elevated intraocular pressure, when it accompanies hyphema, is 6 days.
Pathophysiology
Hyphema describes the condition of the aqueous humor when red blood cells form a suspension in it.
The choroid and the iris contain a rich complex of vessels. The pupil is outlined and controlled by a complex set of iridial muscles, both sphincters and dilators. These muscles can be ruptured by sharp and/or blunt trauma. This is a frequent source of intraocular hemorrhage (hyphema). In addition, the iris root and/or the ciliary spur is a common location of bleeding from blunt trauma.
Surgical intervention into the eye for anterior segment procedures is accomplished routinely through various approaches. The most commonly used approaches in modern small incision surgery are via the limbus and/or the clear cornea. Clear cornea surgery markedly reduces the risk of bleeding from limbal vessels since the cornea in its healthy state is avascular. Scleral tunnel incision is subject to unpredictable hemorrhage, and the incision must be closed carefully with sutures.
Hyphema can result from intraocular surgery, as follows:
-
Intraoperative bleeding: Ciliary body or iris injury may occur during a peripheral iridectomy, cataract extraction, cyclodialysis, canaloplasty, [16] and filtration procedure. It can also occur with laser peripheral iridectomy, more commonly with YAG laser than with argon laser, and argon laser trabeculoplasty (ALT). Hyphema is encountered during insertion of increasingly popular microstents with minimally invasive glaucoma surgeries (MIGS), as described by Hoeh et al with their experience with the CyPass Micro-Stent. [17]
-
Early postoperative bleeding: A traumatized uveal vessel that was in spasm and suddenly dilates or conjunctival bleeding that makes its way into the anterior chamber via a corneoscleral wound or sclerostomy
-
Late postoperative bleeding: New vessels growing across the corneoscleral wound that bleed when manipulated, a uveal wound that is reopened, or an intraocular lens (IOL) that causes chronic iris erosion (eg, UGH syndrome)
Frequency
In the United States, the incidence of hyphema is 17-20 per 100,000 people per year.
Differentials
Fuchs Heterochromic Uveitis [18]
Herpes Zoster Uveitis [18]
Other problems to be considered
Trauma
Intraocular surgery
Spontaneous hyphema
Iris microhemangiomas, iris varix, and pupillary microhemangiomas
Iris neovascularization
Bleeding diatheses or clotting disturbances such as idiopathic thrombocytopenic purpura (ITP) or leukemias
Following laser trabeculoplasty [19] or laser iridotomy
Anticoagulation therapy, such as warfarin (Coumadin), clopidogrel bisulfate (Plavix), or aspirin
Elevated Intraocular Pressure
Increased intraocular pressures may accompany hyphemas of any size.
Chance of elevated intraocular pressure by grade of traumatic hyphema:
-
Grade 1-2: 13.5%
-
Grade 3: 27%
-
Grade 4: 52% [20]
Elevated intraocular pressures (>22 mm Hg) may be anticipated in approximately 32% of all patients with hyphemas at some time during their course. [15] Higher, more prolonged elevations of intraocular pressure are more commonly associated with near total or total hyphemas. In the absence of elevated intraocular pressure with a total hyphema, globe rupture should be suspected. [21] Patients predisposed to glaucoma or with preexisting glaucoma and decreased facility of trabecular outflow are also more likely to develop glaucoma with a hyphema. Sickle cell disease patients can present with intraocular pressures out of proportion to expected with relatively low hyphema levels.
These highly elevated intraocular pressures occur during the acute phase of the hyphema and are separate from those related to angle recession. [22] In patients with pressure elevations, abnormal tonometric readings are frequently detected during the first 24 hours after injury. This initial period of elevated intraocular pressure is often followed by a period of normal or below normal pressure from the second day to the sixth day. Careful monitoring of the intraocular pressure is important and may determine the course of treatment. [23] 50% of cases of rebleeding have a risk of elevated intraocular pressure. [20]
The early period of elevated intraocular pressure is probably the result of trabecular plugging by erythrocytes and fibrin. The following period of reduced pressure is most likely due to reduced aqueous production and uveitis, and hypotony may increase the chance of secondary hemorrhage. This period of reduced intraocular pressure is commonly followed by a subsequent rise in intraocular pressure, probably coincidental with the recovery of the ciliary body.
Elevated intraocular pressure then subsides with recovery of the trabecular meshwork and disappearance of the hyphema.
Exceptions include patients with a hyphema occupying greater than 75% of the anterior chamber and those with a total hyphema, in whom pressure elevation frequently has its onset simultaneously with the initial hyphema and remains continually elevated until the hyphema has had considerable resolution. When large segments of the anterior chamber angle are irreparably damaged and/or when organization of the fibrin or clot produces extensive peripheral anterior synechiae, the intraocular hypertension continues, becoming intractable chronic glaucoma.
Ghost cell glaucoma with khaki-colored hyphema, together with vitreous hemorrhage, may cause elevated intraocular pressure 2 weeks to 3 months after the initial injury. [24] Gradual clearing of the hyphema occurs, and erythrocytes in the vitreous cavity lose hemoglobin and become so-called ghost cells. The ghost cells then circulate forward into the anterior chamber, with resultant trabecular blockage due to the distorted, bulky configuration of the crenated red blood cell. Considerable delayed elevation of intraocular pressure may occur with ghost cell glaucoma, particularly in patients with poor facility of outflow.
Secondary Hemorrhage
Rebleeding into the anterior chamber results in a markedly worse prognosis. Eventual visual recovery to a visual acuity of 20/50 (6/15) or better occurs in approximately 64% of patients with secondary hemorrhage as compared with 79.5% of patients in whom no rebleeding occurred. [7, 15] True secondary bleeding into the anterior chamber is indicated by an obvious increase in the amount of blood in the anterior chamber. Secondary hemorrhage occurs in approximately 25% (range, 7-38%) of all patients with hyphema. [7, 15] The incidence of secondary hemorrhage is higher in hyphemas classified as Grades 3 and 4. [8]
The highest incidence of secondary hemorrhages occurs on day 3-4 but can occur from day 2-7 following the traumatic event. [7, 25] A large proportion (33%) of patients younger than 6 years old have secondary hemorrhages; the likelihood of secondary hemorrhages decreases with age.
With near total to total hyphemas, in which the blood is dark and clotted, bright red blood often begins to appear at the periphery of the clot on the fourth to the sixth day. This probably results from early dissolution of the clot and does not necessarily indicate a secondary hemorrhage.
Secondary hemorrhage is probably due to lysis and retraction of the clot and fibrin aggregates that have occluded the initially traumatized vessel. [15] The secondary bleeding may result in increased intraocular pressure and corneal staining and is associated with a poorer visual prognosis. [26, 27]
Several studies have documented that secondary hemorrhage occurs more frequently in AfricanAmerican patients. In 1990, Spoor et al observed secondary hemorrhage in 24.2% of African American patients and in only 4.5% of white patients. [28] Lai et al proposed that melanin was involved in modulating the fibrinolytic activity leading to prolonged hyphema resorption and higher rates of secondary hyphema. [29] Two other studies demonstrated greater rates of secondary hemorrhage in AfricanAmerican patients that are highly significant (P < 0.05). [30, 31] In the initial systemic aminocaproic acid (ACA) study, African American patients comprised 66.2% of the population [15] ; 34% of AfricanAmerican patients in the placebo group developed secondary hemorrhage, and 20% of them had positive sickle cell trait by hemoglobin electrophoresis. There have also been studies showing a higher incidence of rebleeding in cases of hemophilia. [32]
Postoperative Hyphema
Early postsurgical hyphemas can be caused by bleeding from the ciliary body, from cut ends of the Schlemm canal, from the iris or iris root, or from the corneoscleral wounds. Wounds located more posteriorly tend to bleed more.
Iris neovascularization can also result in a hyphema due to fragile iris vessels that can bleed from intraoperative manipulation.
Late-onset post cataract surgical hyphemas occur from the fine arborizing neovascular vessels that form in the inner aspect of the cataract incision site. These vessels are fragile and bleed spontaneously after minor trauma. Hyphemas in this setting may be caused by posterior chamber intraocular lens (PCIOL) haptics eroding the ciliary sulcus. Anterior chamber intraocular lens (ACIOL) haptics also may cause bleeding by chafing the iris surface.
Rubeosis, or iris neovascularization, can also be a source of late postoperative hyphema.
Uveitis-glaucoma-hyphema (UGH) syndrome related to intraocular lenses is seen weeks to months after surgery. Postoperative hyphema may also occur after laser procedures involving the iris or anterior chamber angle.
After ALT, bleeding may occur from an inadvertent laser treatment of the iris root vessel or from reflux of blood from the Schlemm canal.
After a laser iridotomy, bleeding may occur from an inadvertent laser treatment of the iris root vessel. If promptly recognized intra-operatively, the physician should apply pressure with the focusing lens to reduce the rate of bleeding and the amount of hyphema formation.
Complications of Hyphema
Complications of traumatic hyphema may be directly attributed to the retention of blood in the anterior chamber. In addition to glaucoma, the four most significant complications include posterior synechiae, peripheral anterior synechiae, corneal blood staining, and optic atrophy. [15, 33]
Posterior synechiae
Posterior synechiae may form in patients with traumatic hyphema. This complication is secondary to iritis or iridocyclitis. However, they are relatively rare complications in patients who are medically treated. Posterior synechiae occur more frequently in patients who have had surgical evacuation of the hyphema.
Peripheral anterior synechiae
Peripheral anterior synechiae occur frequently in medically treated patients in whom the hyphema has remained in the anterior chamber for a prolonged period, typically 9 or more days. The risk of peripheral anterior syndrome increases with the size and duration of the hyphema. [15] The pathogenesis of peripheral anterior synechiae may be due to a prolonged iritis associated with the initial trauma and/or chemical iritis resulting from blood in the anterior chamber. Alternately, the clot in the chamber angle may subsequently organize, producing trabecular meshwork fibrosis that closes the angle. Both mechanisms are likely to be involved. [7, 15]
Corneal blood staining
Corneal blood staining primarily occurs in patients with a total hyphema and associated elevation of intraocular pressure. The following factors may increase the likelihood of corneal blood staining; all of these factors affect endothelial integrity:
-
Initial state of the corneal endothelium; decreased viability resulting from trauma or advanced age (eg, cornea guttata)
-
Surgical trauma to the endothelium
-
Large amount of formed clot in contact with the endothelium
-
Prolonged elevation of intraocular pressure
-
Size of hyphema
-
Prolonged rebleeding
Corneal blood staining may occur with low or normal intraocular pressure; rarely, it may also occur in less than total hyphemas. However, these two instances can probably be anticipated only in eyes with a severely damaged or compromised endothelium. Corneal blood staining is more likely to occur in patients who have a total hyphema that remains for at least 6 days with concomitant, continuous intraocular pressures of greater than 25 mm Hg. [7]
Clearing of the corneal blood staining may require several or many months. Generally, the corneal blood stains form centrally and then spread to the periphery of the corneal endothelium. In children and infants, prolonged corneal blood staining can lead to deprivation amblyopia from decreased visual acuity. [34] During resolution, corneal blood staining reverses the sequence of the initial staining process, clearings peripherally and then centrally.
Optic atrophy
Optic atrophy may result from either acute, transiently elevated intraocular pressure, chronically elevated intraocular pressure, or optic nerve contusion; each occurrence was studied in a series of patients with hyphema in an attempt to identify predisposing factors. [15, 21] Nonglaucomatous optic atrophy in patients with hyphema may be due to either the initial trauma or the transient periods of markedly elevated intraocular pressure. Diffuse optic pallor (and not glaucomatous cupping) is the result of transient periods of markedly elevated intraocular pressure. Pallor occurs with constant pressure of 50 mm Hg or higher for 5 days or 35 mm Hg or higher for 7 days. [7, 15] In the absence of increased intraocular pressure, optic neuropathy could be due to damage of the short ciliary artery from an optic nerve contusion. [15]
The authors have observed numerous patients with sickle cell trait who developed a nonglaucomatous optic atrophy with relatively low elevations of intraocular pressure of 35-39 mm Hg for 2-4 days. [7] In spite of maximum medical therapy, final visual acuity was less than 20/400 in all patients. The authors continue to observe optic atrophy in patients with sickle cell trait who are referred to their institution and who have not had vigorous control of intraocular pressure and/or delay in paracentesis.
Other studies indicate that patients with sickle cell hemoglobinopathies and anterior chamber hyphemas have more sickled erythrocytes in their anterior chambers than in their circulating venous blood. [35] The sickled erythrocytes obstruct the trabecular meshwork more profoundly than healthy red blood cells, and a consequent elevation of intraocular pressure occurs with lesser amounts of hyphema.
Systemic ocular hypotensive agents, such as acetazolamide and methazolamide, may not always be successful in reducing the intraocular pressure. In fact, they may be contraindicated in high or repeated dose regimens because of their possible contribution to intravascular hemoconcentration and increased microvascular sludging, both of which are detrimental in sickle cell hemoglobinopathy.
The increased intraocular pressure may not be tolerated well in these patients because of the increased susceptibility to impaired vascular perfusion within the optic nerve and the retina. Indeed, moderate elevation of intraocular pressure in patients with sickle cell hemoglobinopathy may produce rapid deterioration of visual function because of profound reduction of central retinal artery and posterior ciliary artery perfusion. [36, 37] For African American patients, the prevention of secondary hemorrhage is an especially critical factor.
Other complications associated with hyphema involve disruption of the posterior segment. These complications include, but are not limited to, choroidal rupture, macular scarring, retinal detachment, vitreous hemorrhage, and zonular dialysis. Even a case of sympathetic ophthalmia following hyphema has been reported. [38]
Prognosis and Treatment
It is important to recognize that the prognosis of visual recovery is directly related to the following 3 factors, as follows:
-
Amount of associated damage to other ocular structures (ie, choroidal rupture, macular scarring)
-
Whether secondary hemorrhage occurs
-
Whether complications of glaucoma, corneal blood staining, or optic atrophy occur
Treatment modalities should be directed at reducing both the incidence of secondary hemorrhage and the risk of corneal blood staining and optic atrophy.
The success of hyphema treatment, as judged by the recovery of visual acuity, is good in approximately 75% of patients. Approximately 80% of those with less than one third filling of the anterior chamber regain visual acuity of 20/40 (6/12) or better. Approximately 60% of those with a hyphema occupying greater than one half but less than total filling of the anterior chamber regain visual acuity of 20/40 (6/12) or better, whereas only approximately 35% of those with an initial total hyphema or a Grade 4 hyphema have good visual results. Approximately 60% of patients younger than 6 years have good visual results; older age groups have progressively higher percentages of good visual recovery.
The severity of the trauma is frequently related to the final visual outcome. Lens opacities, choroidal rupture, vitreous hemorrhage, angle-recession glaucoma, secondary macular edema, and retinal detachment are commonly associated with traumatic hyphema, compromising the final visual result. Hyphema related to any penetrating injury of the eye has a worse visual prognosis than hyphema associated with blunt trauma.
Of patients with hyphema, 14% have poor visual results from associated trauma, including such complications as glaucoma, vitreous hemorrhage, retinal detachment, choroidal rupture, or scleral rupture. Poor visual outcome in traumatic hyphema can be directly attributed to the hyphema in 11% of patients [21, 15] ; the poor visual outcome is usually the result of secondary hemorrhage associated with optic atrophy or corneal blood staining.
For excellent patient education resources, visit eMedicineHealth's Eye and Vision Center. Also, see eMedicineHealth's patient education articles Hyphema (Bleeding in Eye) and Eye Injuries.
Workup
Lab studies
In African American patients, a sickle cell prep should be ordered if a hyphema is seen because the presence of a hyphema in patients with sickle cell trait or disease can produce significant ocular complications and change medical management. Sickled red blood cells can more easily obstruct the trabecular meshwork and result in a high IOP, even in the presence of a relatively small hyphema. This could be aggravated by the addition of a carbonic anhydrase inhibitor eye drop (eg, dorzolamide) which can cause systemic acidosis. [39] In addition, ischemic complications of the retina and the optic nerve are greater in patients with sickle cell trait and disease. A hemoglobin electrophoresis is also helpful. It helps distinguish sickle cell trait from disease once the sickle cell prep is positive.
In idiopathic hyphemas in patients with easy bruising, pallor, or complaints of fatigue, hematological evaluation should be considered.
Imaging studies
Infrequently, a B-scan and/or a CT scan may be necessary to rule out an intraocular tumor or a foreign body if a thorough examination is not possible and the reasons for postoperative hyphema are not clear.
Other tests
Rarely, an iris fluorescein angiogram may be needed if early iris neovascularization is suspected as an underlying cause of the hyphema.
Gonioscopy
Examination of the angle structures is critical to understanding the extent of the blunt trauma precipitating a hyphema. This can be delayed until after the critical 5-day, high-risk, re-bleed period. In particular, dynamic gonioscopy should be avoided in the acute phase. Angle abnormalities, synechiae, and recession may commonly be found. Rarely, a focus of bleeding can be photocoagulated with the argon laser on low-power settings, up to 300 mW with a 200-µm spot size. All patients with hyphema due to blunt trauma should undergo gonioscopy once the hyphema has cleared to rule out angle recession.
Medical Management
The customary treatment of patients with traumatic hyphema is not widely supported by randomized control trials and is quite variable between providers. Treatment oftentimes includes bed rest with head-of-bed elevation, eye protection, topical cycloplegics, topical steroids, and pressure-lowering topical or oral medication. It less commonly includes systemic steroids, topical aminocaproric acid or tranexamic acid, intracameral tissue plasminogen activator (t-PA), hospitalization and sedation. [40]
The authors recommend bedrest with head-of bed- elevation and a shield for the injured eye. However, studies have not indicated that rigidly following this regimen is necessary to achieve acceptable therapeutic results. These studies provide evidence that no statistically significant difference exists in most areas of comparison between patients treated with bed rest, bilateral patches, and sedation and those treated with ambulation, a patch and shield on the injured eye only, and no sedation. [15, 41, 42, 43]
Sedation is recommended only in the extremely apprehensive individual. Hospitalization may be warranted in cases of severe trauma and rebleeding, when abuse is suspected, or when noncompliance to medical regimens or limitation of vigorous activity is a concern. In children, admission is recommended in incidences where the hyphema is greater than 50% of the anterior chamber volume, there is sustained elevated intraocular pressure, the patient has sickle cell trait or disease, there is concern for medication compliance at home, or concern for follow up. Traditionally, children are admitted for 5 to 7 days after the initial injury to watch for rebleeding. [44]
If analgesics are required for pain relief, acetaminophen (Tylenol) with or without codeine, depending on the severity of the pain, is preferred. The antiplatelet effect of aspirin tends to increase the incidence of rebleeding in patients with traumatic hyphema and should be strictly avoided. [26] Nonsteroidal anti-inflammatory drugs (NSAIDs) with analgesic activity, such as mefenamic acid (Ponstel) or naproxen (Aleve), share this deleterious antiplatelet effect.
In any therapeutic regimen, the injured globe requires adequate protection with a shield. [45] Elevating the head of the bed 30-45° when sleeping or resting facilitates settling of the hyphema in the inferior anterior chamber and aids in classifying the hyphema. Inferior settling facilitates more rapid improvement of visual acuity, earlier evaluation of the posterior pole, and greater clearing of the anterior chamber angle. A better estimate of the decrease or increase in the amount of blood in the anterior chamber is also possible during subsequent biomicroscope examinations.
Various topical medications have been recommended for treating patients with traumatic hyphema, including cycloplegics for traumatic iridocyclitis. [27, 46, 47] Topical corticosteroids have been recommended with contradictory results. [48] One recommendation regarding topical medication is that the topical use of steroids after the third day or the fourth day of retained hyphema may be advantageous to decrease the associated iridocyclitis and to prevent or deter the development of peripheral anterior synechiae or posterior synechiae. Secondly, topical atropine (1%) is indicated in hyphemas occupying more than 50% of the anterior chamber to reduce the incidence of posterior synechiae formation and avoid pupillary block.
In a 2019 Cochrane database systematic review which included 20 randomized and 7 quasi-randomized control trials studying 2643 patients, there was decreased rebleeding in patients treated with topical aminocaproic acid or tranexamic acid. With the administration of aminocaproic acid, the hyphema took longer to clear. The authors found no intervention that significantly affected visual acuity. Additionally, corticosteroids, cycloplegics, binocular patching, bed rest, and head elevation failed to show evidence to support their use, but the authors noted that their sample size was limited. [49]
Some studies have investigated the application of intracameral tissue plasminogen activator (t-PA) in the management of traumatic hyphema. [50] However, these studies have been neither large nor randomized. A potential problem with t-PA is the associated risk of rebleeding of the initial wound. Application of t-PA has been considered in resolving hyphemas that either fail to clear spontaneously or are associated with malignant intraocular pressure, [51] although the actual timing of t-PA administration from the initial injury has yet to be determined.
A 2022 clinical trial comparing the effects of topical tranexamic acid to systemic prednisolone approached significance (p = 0.081) in decreasing the chances of rebleeding in traumatic hyphema with topical tranexamic acid. Hyphema reabsorption rate was decreased (p < 0.001) with the application of topical tranexamic acid. [52]
Topical antiglaucoma medications usually lower intraocular pressure. With the advent of newer glaucoma modalities, initiating therapy incrementally with brimonidine tartrate (Alphagan, Allergan), followed by latanoprost (Xalatan, Pharmacia) and timolol maleate (Timoptic, Merck), is recommended. If intraocular pressure is still elevated, a topical carbonic anhydrase inhibitor should be added. In patients with sickle cell trait or sickle cell disease, methazolamide and topical beta-blockers should be substituted. [14, 53]
If intraocular pressure is still uncontrolled, systemic medication should be given during the acute phase of the hyphema. Acetazolamide (20 mg/kg/d) may be administered in 4 divided doses for intraocular pressure of greater than 22 mm Hg. However, acetazolamide can increase the concentration of anterior chamber ascorbate, lower the pH of human plasma, and exacerbate sickling of erythrocytes. Therefore, methazolamide (10 mg/kg/d), administered in 4 divided doses, is preferred in pediatric patients with sickle cell trait or sickle cell disease. [7, 35]
Osmotic agents (preferably mannitol) should be considered for intraocular pressure above 35 mm Hg despite topical medications. Orally administered glycerol is effective; however, nausea and vomiting are often associated with its administration in patients with elevated intraocular pressure. Mannitol is administered intravenously, 1.5 g/kg (usually in a 10% solution), over a period of approximately 45 minutes. This agent may be given 2 times a day (or every 8 hours in patients with extremely high pressure) in an attempt to keep the intraocular pressure below 35 mm Hg. Renal output, blood urea nitrogen, and electrolyte values should be monitored in all patients in whom such therapy is continued for several days.
In summary:
● Evaluate & treat systemic diseases (eg, sickle cell, hemophilia)
● Eye shield over the affected eye
● Limit strenuous activity and straining
● Elevate head of bed to 30°
● Pain control
● Dilating eye drops to reduce pupillary block
● Steroid eye drops to reduce inflammation
● Beta blockers or carbonic anhydrase inhibitors to decrease intraocular pressure
Outpatient Versus Hospitalization
With increasing emphasis on cost containment, outpatient management of hyphema has become more popular. Several studies have demonstrated no significant difference in final visual acuities in patients with smaller hyphemas treated at home or those treated in hospitals. [54, 55, 56, 57, 58, 59]
Microhyphemas can be treated on an outpatient basis, unless secondary hemorrhage occurs or elevated intraocular pressure is uncontrolled. Patients with traumatic hyphema occupying less than one third of the anterior chamber can be treated on an outpatient basis. If the hyphema occupies more than one third of the anterior chamber, intraocular pressure is elevated beyond 30 mm Hg, or both, hospitalization is sometimes recommended. The decision to hospitalize also depends on the cooperation of the patient, family members, and the extent of ocular injury. A baseline comprehensive eye exam including intraocular pressure and hyphema grading should be performed to monitor progress. Immediate threats to vision such as an open globe or ocular compartment syndrome should be ruled out.
For outpatients, daily ocular examinations, including an evaluation of the amount of hyphema and intraocular pressure, should be performed. Daily ophthalmic sketches are helpful in estimating the amount and the rate of resolution or rebleeding. Applanation tonometry must be performed at least once daily and twice daily in patients with elevated intraocular pressures.
Minimal blood staining is often difficult to detect against a background of blood in the anterior chamber. Under such circumstances, the cornea often assumes a yellowish cast, which is reflected from the yellowish fibrinous coagulum in the anterior chamber. The most typical early sign of corneal blood staining is the presence of tiny yellowish granules that initially appear in the posterior third of the corneal stroma. An additional finding is a lack of definition or a blurred appearance of the ordinarily sharply defined fibrillar structure of the involved corneal stroma. The latter is independent of the yellowish color transmitted to the stroma by the contents of the anterior chamber.
The authors have found this sign to be useful in recognizing the very early stages of corneal blood staining. These biomicroscopic signs of corneal blood staining usually precede gross staining by only 24-36 hours. Surgical treatment in this early stage may prevent gross staining, and the cornea may clear more quickly. However, once grossly visible staining develops, many months may elapse before clearing is complete.
Surgical Intervention
Approximately 5% of patients with traumatic hyphema require surgery with increased risk of surgical intervention for patients with sickle cell trait or disease. [60] Generally, medical management seems to produce the best visual results for patients with less than total hyphemas. Certainly, other causes of inflammation or bleeding should be ruled out, particularly when the history of trauma is questionable. [61]
For several reasons, surgical management is fraught with complication. [58] First, surgery is chosen for the most severe presentations of hyphema, thus selecting out the most difficult cases. Surgical intervention is rarely indicated for hyphemas that occupy less than one half of the anterior chamber; these lesser hyphemas (either primary or secondary) usually resolve spontaneously under any medical regimen and require no surgical intervention.
In 2 prospective series totaling 196 patients, no corneal blood staining or optic atrophy was noted in hyphemas of 50% or less. [7, 15] Corneal blood staining, with rare exceptions, only occurs in patients with hyphemas that are total at some time during their course. The results of surgical evacuation to improve secondary glaucoma in small hyphemas (75% or less) are disappointing. The ocular hypertension in these instances results more frequently from damage to the trabecular structures than from plugging by red cells and fibrin. Surgical evacuation in these instances may produce only temporary postsurgical hypotony, with a rapid return to preoperative intraocular pressure.
The authors believe that most hyphemas, including total hyphemas, should be medically treated for the first 4 days. Spontaneous resolution of the hyphema occurs quite rapidly during this period, and these cases have the best prognosis. In one series of 20 eyes with total hyphemas, 4 of these 20 eyes (20%) cleared sufficiently by day 4 to rule out surgery. [21] An additional 4 eyes resolved spontaneously on medical treatment over a longer period.
Surgical intervention is usually indicated on or after the fourth day. Overall, indications for surgical intervention are outlined below. [7, 21]
-
Four days since onset of total hyphema and the hyphema has not cleared
-
Microscopic corneal blood staining (at any time)
-
Total hyphema with intraocular pressures of 50 mm Hg or more for 4 days (to prevent optic atrophy)
-
Total hyphemas or hyphemas filling greater than 75% of the anterior chamber present for 6 days with pressures of 25 mm Hg or more (to prevent corneal blood staining)
-
Hyphemas filling greater than 50% of the anterior chamber retained longer than 8-9 days (to prevent peripheral anterior synechiae)
-
In patients with sickle cell trait or sickle cell disease who have hyphemas of any size that are associated with intraocular pressures of greater than 35 mm Hg for more than 24 hours
If intraocular pressure remains elevated at 50 mm Hg or more for 4 days, surgery should not be delayed. One study noted optic atrophy in 50% of patients with total hyphemas when surgery was delayed. Corneal blood staining occurred in 43% of patients. [62] A 2013 study of 138 pediatric traumatic hyphemas found an increased likelihood of required surgical intervention if patients presented with increased intraocular pressure. [4]
Patients with sickle cell hemoglobinopathies and even those with sickle cell trait require surgical intervention if intraocular pressure is not controlled within 24 hours. [7, 35] An interesting study of hyphema in rabbits measured partial oxygen pressure in the aqueous humor after injection of blood from a patient with sickle cell versus injection of an air or oxygen bubble with the blood from the patient. After 10 hours, the partial pressure of oxygen was 123.35 mm Hg in the blood plus air bubble group and 306.47 mm Hg in the blood plus oxygen group, compared to 78.45 mm Hg and 73.97 mm Hg for the placebo (no injection) and blood only injection groups, respectively. The authors recommended leaving an air or oxygen bubble in the anterior chamber after a washout in patients with sickle cell disease or trait. [63]
MomPremier et al described a two-needle, office-based technique for performing an air-fluid exchange (see image below) in several patients without sickle cell. [64] The authors of this article have not attempted this technique.
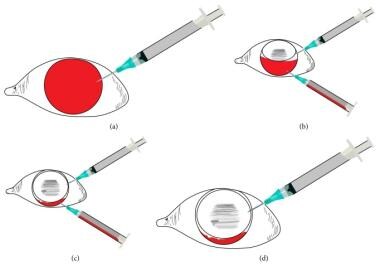 Air-fluid exchange two-needle technique: (a) Entry into the anterior chamber superiorly with gas-filled syringe. (b) After partial gas injection, entry into the deepened anterior chamber inferiorly with evacuation syringe, plunger removed. (c) Evacuation of hyphema with complete or near complete anterior chamber fluid-gas exchange. (d) Inferior needle is removed while superior gas-filled syringe is used to equilibrate intraocular pressure. Courtesy of Hindawi Publishing Corp under Creative Commons Attribution License [MomPremier M, Sadhwani D, Shaikh S. An Office-Based Procedure for Hyphema Treatment. Case Reports in Ophthalmological Medicine. Vol 2015; Article 321076; https://read.qxmd.com/doi/10.1155/2015/321076].
Air-fluid exchange two-needle technique: (a) Entry into the anterior chamber superiorly with gas-filled syringe. (b) After partial gas injection, entry into the deepened anterior chamber inferiorly with evacuation syringe, plunger removed. (c) Evacuation of hyphema with complete or near complete anterior chamber fluid-gas exchange. (d) Inferior needle is removed while superior gas-filled syringe is used to equilibrate intraocular pressure. Courtesy of Hindawi Publishing Corp under Creative Commons Attribution License [MomPremier M, Sadhwani D, Shaikh S. An Office-Based Procedure for Hyphema Treatment. Case Reports in Ophthalmological Medicine. Vol 2015; Article 321076; https://read.qxmd.com/doi/10.1155/2015/321076].
Surgery for patients with hyphema should be cautiously approached. In 2 series involving 196 patients, surgery was performed in only 14 patients (7.1%). [7, 15] Risks of surgery include damage to the corneal endothelium, the lens, and/or the iris; prolapse of the intraocular contents; rebleeding; and increased synechiae formation. Except for patients with sickle cell trait, no patients in these series required surgery if the hyphema occupied less than 50% of the anterior chamber. Total hyphema evacuation by vitrectomy instrumentation, peripheral iridectomy, and trabeculectomy has been recommended.
Generally, the authors recommend the type of surgical intervention with which the surgeon is most familiar. Hyphema surgery should be preceded by intravenous acetazolamide and mannitol if the intraocular pressure is elevated. The operation should be performed under general anesthesia in all patients. The operating microscope should be used in all instances. Presently, the 4 major approaches include the following:
-
Hyphema evacuation with closed vitrectomy instrumentation
-
Paracentesis
-
Irrigation and aspiration through a small incision
-
Clot irrigation with trabeculectomy
-
Anterior chamber maintainer
Currently, the preferred technique is evacuation of the hyphema with vitrectomy instrumentation. The initial clear corneal incision is made with a diamond blade. To avoid both the iris and the lens, the blade is oriented and pushed into the anterior chamber in such a manner that it is parallel to the plane of the iris. A 20-gauge Ocutome or similar guillotine instrument, attached to an infusion line of balanced salt solution plus (BSS-Plus), is gently placed into the anterior chamber. The bottle of BSS-Plus should be 30-40 cm above the eye to maintain normal intraocular pressure. With the Ocutome cutting port half open and the infusion line in place, irrigating and aspirating free blood from the formed clot are possible. The suction mode is initially set at 4, and the cutting speed is set at 150 for the procedure. An anterior chamber maintainer can help stabilize fluctuations in intraocular pressure during clot evacuation. [65]
Extreme care is required to avoid any contact with the iris, the lens, or the corneal endothelium. Directing the guillotine port anteriorly and always keeping the port in view generally avoids intraoperative uveal tissue injury. This operative procedure is used to remove the central portion of the clot. Removing the entire clot in the periphery of the anterior chamber is not necessary.
In traumatic hyphemas, Yu et al suggest the use of an anterior chamber maintainer (ACM) when there is increased intraocular pressure unresponsive to medication with persistent corneal staining. They describe the use of two paracenteses to facilitate anterior chamber washout while maintaining intraoperative intraocular pressure and minimizing corneal endothelium irritation. The first limbal paracentesis accommodates a 20-gauge ACM connected to a bottle of Balanced Salt Solution, which is then raised to 50 cm above the patient’s head to maintain a continuous positive 30 mm Hg intraocular pressure. The second limbal paracentesis then facilitates release of the hyphema.
The hydrostatic pressure created from the ACM encourages liquified blood and blood clot evacuation without additional surgical instruments deep into the anterior chamber and iatrogenic corneal damage. The authors advise surgeons to leave blood clots firmly adherent to the iris or angle to dissolve naturally to avoid the risk of rebleeding. [66]
If a secondary hemorrhage occurs during the operative procedure, the authors recommend tamponade of the bleeding by elevation of the infusion bottle to approximately 70 cm above the eye for several minutes. If the bleeding continues, filling the anterior chamber with an air bubble after evacuating the clot is helpful. If bleeding persists, bimanual bipolar diathermy is extremely helpful when the bleeding site is visible. [67]
At the end of the surgical procedure, filling the anterior chamber with an air bubble is helpful. This also helps to control any secondary bleeding. The corneal incision is closed with two 10-0 nylon sutures. The response in lowering intraocular pressure with the Ocutome instrumentation has been quite successful. Each eye operated on with this technique has shown an initial decrease in intraocular pressure associated with the surgery.
Paracentesis causes little surgical trauma and relieves the elevated intraocular pressure. Paracentesis is especially beneficial in patients with sickle cell trait or sickle cell disease. However, the decrease in intraocular pressure may be transient, and appreciable reduction may not occur in the amount of the formed clot.
Irrigation by a single or double needle technique has the advantage of a small incision. The authors prefer using a diamond blade and entering at the 1-o'clock position in the right eye and at the 11-o'clock position in the left eye. The entry should be through a clear cornea. The irrigating needle should extend just through the corneal endothelium, and a slow push-pull maneuver with the single needle technique washes out the erythrocytes from the anterior chamber clot, often leaving the fibrin matrix. To reduce the likelihood of rebleeding during the operative procedure, care should be undertaken not to produce violent alterations in the anterior chamber pressure. If rebleeding does occur, an air bubble can be effectively introduced for tamponade. After a 5-minute wait, irrigation maneuvers can be resumed. Using the single or double needle technique, the surgeon must be particularly careful to have direct visualization of the anterior chamber.
This technique has some disadvantages. Sometimes, maintaining the position of the needle tip in the anterior chamber during the procedure is difficult. A hazardous situation is created when the collar-button type of formed clot occupies both the anterior and posterior chambers. This produces a pupillary block with anterior displacement of the iris-lens diaphragm.
Generally, trabeculectomy is not used in smaller hyphemas. However, in patients with total hyphema, trabeculectomy with peripheral iridectomy should be considered. Trabeculectomy is performed with gentle irrigation of the anterior chamber hyphema. This surgery is relatively safe and should be performed early for patients with total hyphema unless the elevated intraocular pressure is medically controlled and resolution of the hyphema is clearly imminent.
The authors currently perform trabeculectomy on patients with total hyphema persisting to day 4 and find it superior to clot evacuation. Several patients referred to the authors' institution have had attempts at clot evacuation. One patient sustained complete iridodialysis related to attempted clot evacuation. In addition, the authors have treated other patients who have been referred after optic atrophy developed with total hyphemas.
Topically applied mitomycin-C may be a useful adjunct in the prevention of long-term trabeculectomy failure, particularly in patients with trauma and, therefore, a predisposition to inflammation.
Because each of these surgical procedures has its own set of complications, the surgeon should approach each patient with caution and individualize the surgical strategy. Postoperative care should include meticulous control of nausea and emesis to avoid significant fluctuations in intraocular pressure.
Postoperative hyphemas may be seen at the time of surgery or within the first 2-3 days after surgery. If bleeding is identified intraoperatively, it must be identified and coagulated if it does not cease on its own. The surgeon can reduce postsurgical hyphemas by creating internal sclerostomy as anteriorly as possible to reduce bleeding during filtration surgery. In uveitis-glaucoma-hyphema (UGH) syndrome associated with archaic design anterior chamber IOLs and sulcus posterior chamber IOLs, the treatment may require removal of the lens that is causing the problem and replacing it with another lens.
A chaffing lens haptic can be diagnosed with ultrasound biomicroscopy (UBM) [68] or the video feature of endoscopic cyclophotocoagulation (ECP). ECP may also serve a role in treating areas of chaffing, potentially resolving UGH.
Questions & Answers
Overview
How is traumatic macrohyphema graded?
What causes anterior chamber hemorrhage in hyphema?
What is the pathophysiology of hyphema?
How does intraocular surgery cause hyphema?
What is the prevalence of hyphema in the US?
Which conditions should be considered in the differential diagnoses of hyphema?
What is the clinical presentation of elevated intraocular pressure in hyphema?
What is the significance of secondary hemorrhage in patients with hyphema?
What is the clinical presentation of postoperative hyphema?
What is peripheral anterior synechiae caused by hyphema?
What is optic atrophy caused by hyphema?
What are the possible complications of hyphema?
What is posterior synechiae caused by hyphema?
What are the risk factors for corneal bloodstaining in patients with hyphema?
What is the prognosis of hyphema?
What is the role of lab testing in the workup of hyphema?
What is the role of imaging studies in the workup of hyphema?
What is the role of fluorescein angiography in the workup of hyphema?
What is the role of gonioscopy in the workup of hyphema?
What is the role of topical medications in the treatment of traumatic hyphema?
What is the role of oral aminocaproic acid (ACA) in the treatment of hyphema?
What is the role of topical aminocaproic acid (ACA) in the treatment of hyphema?
What is the role of tranexamic acid in the treatment of hyphema?
What is the role of oral steroids in the treatment of hyphema?
What is the role of intracameral tissue plasminogen activator (t-PA) in the treatment of hyphema?
What is the role of topical antiglaucoma medications in the treatment of hyphema?
How is uncontrolled intraocular pressure treated in patients with hyphema?
When is outpatient treatment of hyphema indicated?
What is the role of surgery in the treatment of hyphema?
When is surgery indicated for the treatment of hyphema?
What are the major surgical approaches used in the treatment of hyphema?
What is the role of vitrectomy instrumentation in the treatment of hyphema?
What is the role of paracentesis in the treatment of hyphema?
What is the role of irrigation in the treatment of hyphema?
What is the role of trabeculectomy in the treatment of hyphema?
-
Air-fluid exchange two-needle technique: (a) Entry into the anterior chamber superiorly with gas-filled syringe. (b) After partial gas injection, entry into the deepened anterior chamber inferiorly with evacuation syringe, plunger removed. (c) Evacuation of hyphema with complete or near complete anterior chamber fluid-gas exchange. (d) Inferior needle is removed while superior gas-filled syringe is used to equilibrate intraocular pressure. Courtesy of Hindawi Publishing Corp under Creative Commons Attribution License [MomPremier M, Sadhwani D, Shaikh S. An Office-Based Procedure for Hyphema Treatment. Case Reports in Ophthalmological Medicine. Vol 2015; Article 321076; https://read.qxmd.com/doi/10.1155/2015/321076].