Overview
Autoimmune retinopathies (AIR) belong to a spectrum of uncommon ophthalmic disorders in which autoantibodies directed at various retinal proteins cause progressive vision loss. [1, 2] AIR may be classified as either paraneoplastic autoimmune retinopathies (pAIR) or non-paraneoplastic autoimmune retinopathies (npAIR). pAIR are characterized by retinal antibodies in the setting of an underlying malignancy, whereas npAIR are characterized by auto-antibodies directed against retinal proteins without a known malignancy. [3] The onset of visual symptoms and detection of antibodies may precede the diagnosis of malignancy by months to years, the longest reported interval being 11 years. [4] In some cases, patients with an underlying malignancy have been found to have high titers of antiretinal antibodies but no evidence of visual loss.
pAIR predominantly includes cancer-associated retinopathy (CAR), [5, 6] melanoma-associated retinopathy (MAR), [7] and cancer-associated cone dysfunction. [8] Examples of npAIR include antienolase retinopathy [9] and anticarbonic anhydrase II retinopathy. [8] CAR and MAR usually involve the retinal pigment epithelium membrane, whereas npAIR do not. [10] In most cases of CAR, vision loss occurs before a malignancy has been diagnosed. In contrast, MAR often occurs after a previously diagnosed cutaneous melanoma, and the vision loss often is accompanied by a recurrence or metastasis. [11] Paraneoplastic syndromes involving the optic nerves are less common than those involving the retina. The best-defined of these syndromes is associated with collapsin response-mediator protein-5 (CRMP-5)–immunoglobulin G (IgG) and manifests as bilateral optic neuropathy with retinitis and vitritis. [12]
The clinical features of AIR mostly are similar between the differing types. Patients typically present with rapid, painless vision loss associated with photopsias, photosensitivity, dyschromatopsia, and nyctalopia. [13] Symptoms usually are bilateral, occasionally sequential, and progressive over weeks to months. In patients with anti-enolase retinal antibodies, symptoms often are less acute and progression is slower. [9]
Findings on retinal examination usually are normal early in the course of the disease, posing a diagnostic challenge in some cases. Markedly abnormal electroretinographic (ERG) findings indicate the correct diagnosis, which usually can be confirmed with immunofluorescence techniques to identify circulating retinal antibodies.
Epidemiology
In general, pAIR and npAIR are uncommon disorders; however, their exact prevalence is unknown due to a lack of population-based epidemiologic studies. Presumed npAIR is more prevalent than pAIR, whereas CAR is thought to be the most common form of pAIR. [14] The malignancy most commonly associated with this disorder is small-cell lung cancer, followed by breast and gynecologic (uterine, ovarian, and cervical) cancers. [15, 16] Occasional cases have been associated with non-small-cell lung cancer, Hodgkin lymphoma, and pancreatic, [17] prostate, [18] bladder, laryngeal, and colon cancer. [1, 19]
MAR appears to be increasing in frequency relative to CAR, perhaps secondary to a decrease in cases of lung cancer. AIR usually affects older adults, with a mean age of onset ranging from 55 to 65 years, although patients as young as 3 years have been described. [11] Patients with npAIR have been found to be typically younger relative to those with pAIR. [21] MAR has demonstrated a slight male preponderance, [11] whereas females are affected twice as often as men in CAR and npAIR.
Clinical Findings
Symptoms and signs depend on which retinal elements are affected. CAR affects both rods and cones, whereas MAR typically is characterized by antibodies directed toward bipolar cells that interfere with rod function. Patients with cone-associated retinopathy have dysfunction limited to cones.
Patients with AIR generally experience bilateral subacute vision loss, scotomas, photopsias, and nyctalopia. Patients may present with asymmetric symptoms between eyes and predominant symptoms depending on which retinal cells primarily are affected. Individuals with cone dysfunction experience photosensitivity, prolonged glare after light exposure (hemeralopia), reduced visual acuity and central vision, and loss of color vision. Individuals with rod dysfunction have nyctalopia, prolonged dark adaptation, and peripheral field loss. In either case, positive visual phenomena are prominent, including photopsias, flickering, smoky or swirling vision, and other entoptic symptoms. Some patients report transient dimming of vision, which may be mistaken for retinovascular disease. Occasional cases with overlapping features occur.
On examination, patients with CAR usually have prominent involvement of central vision, resulting in markedly decreased visual acuity, loss of color vision, and central scotomas. In some cases, visual-field testing shows paracentral scotomas that progress to classic ring scotomas.
Photostress recovery times typically are prolonged. In contrast, patients with MAR often have near-normal visual acuity, color vision, and central visual fields early in their course. [11] For example, in the series by Keltner et al, visual acuity was 20/60 or better in 82% at presentation but in only 30% at last follow-up. [11] However, most patients with MAR experience progressive visual loss, especially in the peripheral visual field.
Funduscopic findings at presentation often are normal in all forms of pAIR and npAIR, which may delay diagnosis particularly in the setting of preserved visual acuity in the early stages of the disease process. [14] A case series of 18 eyes from 13 patients with npAIR found 22% of eyes had normal fundus findings on color fundus photography. [22] However, characteristic changes occur over time, including thinning and mottling of the retinal pigment epithelium (RPE), attenuation of retinal arterioles, and occasional optic disc pallor. [23]
RPE-related changes usually are the most common finding and may present in the form of hyperplasia, bony spicules, or atrophy. [21] In rare cases of CAR or MAR, vitreous cells, arteriolar sheathing, and periphlebitis may be present, particularly late in the course of disease.
As reported by Keltner et al, [11] funduscopic findings in 43 patients with MAR were as follows: 19 (44%) patients had normal fundus findings at presentation, 13 (30%) had vascular attenuation, and 12 (28%) had RPE changes. Vitreous cells were present in 13 (30%) patients, and 10 (23%) had optic disc pallor.
Multimodal imaging may be beneficial as it often can reveal non-specific changes seen in AIR eyes, especially in the setting of a normal fundus exam and early in the disease process. Fundus autofluorescence (FAF) can be employed to detect subtle damage to the RPE and is recommended as an important tool in monitoring the progression of AIR.
The majority of AIR patients with abnormal FAF findings show a distinctive pattern of diffuse or granular, stippled hyper-autofluorescence throughout the posterior pole with primary concentration in the macula and peripapillary region. [21] A characteristic hyper-autofluorescent ring in the parafoveal region has been described in many cases. [24, 25] These findings are postulated to be secondary to the accumulation of lipofuscin derivatives in the cells of the metabolically hyperactive RPE, in addition to increased visibility of the RPE due to outer nuclear and photoreceptor layer thinning.
Ocular coherence tomography (OCT) studies in AIR have revealed outer retinal layer complex loss and ellipsoid zone (EZ) disruption in the parafoveal region with relative preservation subfoveally, creating a characteristic “flying saucer” sign. [22, 24, 26, 27] These findings often correlate with the FAF abnormalities previously described. These OCT findings also correlate with OCT angiography (OCTA) abnormalities of reduced deep vessel density in the parafoveal and perifoveal regions. [28] One case series found eyes that had intact external limiting membrane (ELM) and milder forms of npAIR demonstrated regeneration of the EZ on OCT. [22]
AIR eyes on average have been associated with a decrease in EZ extent over time; however, the integrity of the ELM appears to be predictive of regenerative ability of the EZ. EZ reflectivity recovery has been demonstrated following topical difluprednate treatment in a patient with npAIR. [29]
However, none of these changes are specific and can be present in other disease pathologies complicating the diagnosis. In addition to outer retinal changes and EZ disruption and loss, OCT is useful in the identification of cystoid macular edema (CME). CME is a relatively frequent finding in AIR, with an estimated prevalence ranging from 24 to 66%. [22, 30, 31] The presence of CME at initial presentation has been associated with a greater rate of EZ loss and larger decrease in ERG a- and b-wave amplitudes. [22, 32]
Fluorescein angiography often is performed to exclude other entities as potential causes of vision loss. Findings usually are normal, but in occasional cases, there is mild peripheral vascular leakage consistent with vasculitis. [33]
The findings from full-field (Ganzfeld) ERG almost always are abnormal. Specific findings depend on the predominance of cone versus rod dysfunction. Patients with CAR usually have absent cone responses with reduced a and b waves in both photopic and scotopic conditions. Findings in MAR include a markedly reduced or absent dark-adapted b wave (electronegative waveform), which indicates bipolar and Müller cell dysfunction with preserved photoreceptor function. [1] Multifocal ERG (mfERG) is useful for evaluating select cases in which visual-field loss is localized, for monitoring disease progression, and for correlating with visual-field loss. A case series of npAIR found all eyes displayed abnormal findings on full-field ERG and 73% of eyes had abnormal results on mfERG at initial presentation. [22]
Workup
It is important to maintain a high index of suspicion for AIR in patients who present with acute-onset, progressive vision loss in the setting of a normal-appearing fundus on examination. The initial workup should include a full assessment of the patient's visual function, including color vision and visual field testing. Goldmann perimetry is preferred because it readily tests the peripheral field and because kinetic perimetry may be more sensitive than static for detecting changes in this disorder. If automated perimetry is performed, the test should be adapted to include the peripheral field. Full-field ERG is crucial for localizing the disease process to the retina and for further defining the retinal elements involved. In select cases, mfERG may be helpful.
Concern for a diagnosis of pAIR or npAIR based on clinical and ERG findings is supported by laboratory confirmation of antiretinal antibodies. Tests for these antibodies are available commercially (eg, from Athena Diagnostics and the Ocular Immunology Laboratory at Oregon Health Sciences University) and at several research laboratories, including the University of California at Davis, Ophthalmology Research Laboratories. However, results of such laboratory testing are not always definitive. On occasion, individuals without clinical evidence of retinopathy have these antibodies, and, in some cases of presumed AIR, the antibodies cannot be identified with current techniques. In one report, it is estimated that up to 35% of retinal antibodies are not detected in patients with presumed CAR. [21]
Patients who present with suspected AIR and without a known malignancy should undergo extensive investigation to rule out malignancy. A patient’s individual risk profile can help to stratify which tests may be indicated. A chest radiograph should be obtained. If the result is normal and the index of suspicion of CAR remains high, a chest CT likely is appropriate. Additional imaging studies to consider include CT of the abdomen and pelvis, brain MRI, mammography (in women), prostate evaluation (in men), and total-body positron-emission tomography (PET) or CT/PET. Complete physical examination, including pelvic and breast examinations for women, is recommended.
Differential Diagnoses
Acute or subacute unilateral or bilateral vision loss with a normal-appearing fundus suggests the possibility of retrobulbar optic neuropathy. Specific entities to consider include compressive orbital and intracranial lesions, demyelinating disease, ischemia, toxicity, and hereditary disorders. In the ideal case, the clinical findings are sufficiently distinct to distinguish optic nerve disease from retinal disease and therefore obviate extensive neurologic testing.
Symptoms of hemeralopia or nyctalopia (degradation of vision in bright or dim lighting, respectively), positive visual phenomena, prolonged photostress times (as determined from the history or examination), and ring scotomas indicate retinal disease, even in the absence of funduscopic abnormalities, and should prompt electrophysiologic studies. If the ERG findings clearly confirm a retinal disorder, additional neurodiagnostic testing is unnecessary.
Patients with cancer-associated cone dysfunction have bilateral central vision loss with poor color vision and central scotomas. These findings also are compatible with toxic-nutritional optic neuropathy or hereditary optic neuropathy. Patients with these findings should be questioned about tobacco and alcohol use, dietary habits, exposure to environmental toxins, use of potentially toxic medications, and a family history of similar problems. mfERG should be effective for distinguishing optic neuropathy from maculopathy in these patients.
In patients with unexplained vision loss and a history of malignancy, the differential diagnosis may be complex. Workup for metastatic disease as the cause of the vision loss should include contrast-enhanced MRI of the head and orbits and lumbar puncture for cytologic examination. Some chemotherapeutic agents, such as vincristine and carmustine (BCNU), can cause optic neuropathy. Patients who have received cranial radiation also are at risk for vision loss, which usually is identifiable on MRI. Vision loss in patients with metastatic disease may be due to infiltration of malignant cells around the optic nerve. Diffuse melanocytic proliferation is a possibility in cancers originating from the reproductive tract, retroperitoneal zone, or lungs. For reasons that are poorly understood, patients with this proliferation develop an orange pigment deposit at the level of the RPE; fluorescein angiography shows hyperfluorescence.
Once it is clear that the patient's vision loss is due to photoreceptor dysfunction, the differential diagnosis is narrowed to paraneoplastic syndromes, hereditary photoreceptor degeneration (eg, cone-rod dystrophy, retinitis pigmentosa), and toxic retinopathy. The course in patients with hereditary retinopathies generally is longer than that of patients with acquired disease; progression occurs over years rather than weeks to months. A family history of retinitis pigmentosa should be excluded as patients may present with similar clinical features as AIR. It has been estimated that up to 37% of patients with retinitis pigmentosa may exhibit circulating antiretinal antibodies, further complicating the distinction of the two entities. [34, 35] Patients should be questioned regarding the use of potential retinal toxins, such as chloroquine, hydroxychloroquine, and thioridazine.
The clinical findings of white dot syndrome spectrum disorders, particularly acute zonal occult outer retinopathy (AZOOR), occasionally overlap with AIR, sometimes causing diagnostic confusion. In AZOOR, the non-seeing areas are more sharply demarcated from the surrounding areas, the involvement usually is unilateral, and the disease has a predilection for the peripapillary area. Although mfERG demonstrates the abnormality well, findings from full-field ERG generally are normal, in distinction from pAIR in which ERG findings are markedly attenuated or flat early in the course of disease. Furthermore, eyes affected by AZOOR may demonstrate characteristic, well-demarcated areas of hypo-autofluorescence that is distinctive from the typical hyper-autofluorescent ring seen in AIR. [36]
It is important to ask patients with a history of melanoma and other systemic cancers if they have ever received chemotherapeutic treatment. Several novel targeted agents, most notably mitogen-activated protein kinase (MEK) inhibitors (eg, binimetinib) and fibroblast growth factor receptor (FGFR) inhibitors (eg, erdafitinib) have been associated with dose-dependent, transient serous neurosensory detachment with eventual retinal atrophy and preserved retinal function referred to as MEK inhibitor-associated retinopathy (MEKAR) and FGFR inhibitor-associated retinopathy (FGFRAR), respectively. [37, 38, 39] Retinal findings related to these medication side effects should not be confused with those of a pAIR. [22]
Pathologic Findings
The first and most commonly identified antibody in patients with CAR is directed toward recoverin, a 23-kd retinal protein found in photoreceptors that also is expressed by some tumor cells. Increased expression of recoverin has been found in many cancers associated with CAR, including small cell lung cancer, breast cancer, cervical cancer, and ovarian cancer, among others. [41]
Anti-recoverin antibodies also have been identified in cases of npAIR with an absence of known cancer. [42] Since the original identification of recoverin and its role in the pathophysiology of CAR, antibodies that react with a number of other retinal antigens have been identified, including enolase, transducin, carbonic anhydrase II, arrestin, heat shock cognate protein, TULP1, and others.
Three different enolase isoforms (α, β, and γ) exist with anti-α-enolase antibodies most commonly reported in CAR. [43] Anti-enolase-associated CAR usually presents with characteristic clinical findings including impressive abnormalities in visual acuity, contrast sensitivity, and mfERG, but with almost normal full-field ERG. [41] Retinal antibodies directed toward retinal proteins approximating 40 kd have been identified in a patient with cancer-associated cone dysfunction. [23]
Newly discovered autoantibodies directed against four unique 23-kd proteins have been also identified: guanylyl cyclase-activating proteins (GCAP1 and GCPA2), heat shock protein 27 (HSP27), and Rab6A GTPase (Rab6A). It is suspected that these autoantigens may be involved in the pathogenicity of CAR and pAIR. [24] Efforts at standardization of laboratory testing and correlation between specific antiretinal antibodies and malignancies are ongoing. [25] Antibodies to the photoreceptor cell-specific nuclear receptor (PNR) gene product also have been identified in some patients with CAR. [26, 27] These antibodies initiate a cascade of events, leading to increased phosphorylation of rhodopsin, which, in turn, increases intracellular levels of calcium and activates apoptotic pathways, causing photoreceptor cell death. [28]
Patients with MAR have demonstrated IgG autoantibodies directed against rod bipolar cells, a 22-kd neuronal antigen [29] and transducing. [30] These same antibodies were identified in a patient with colon cancer; therefore, this finding is not specific. Anti-transient receptor potential cation channel, subfamily M, member 1 (anti- TRPM1) antibodies have been identified in some cases of MAR, in addition to some forms of congenital stationary night blindness, likely explaining some of the similarities in retinal findings and clinical presentation between the two pathologies. [52, 53]
Other antibodies that have been identified in association with MAR include anti-bestrophin-1, anti-aldolase A and C, anti-recoverin, anti-α-enolase, anti-transducin, anti-rhodopsin, and anti-interphotoreceptor retinoid-binding protein antibodies. [41]
Antibodies directed against the 35-kd retinal Müller-cell layer have been found in some patients with npAIR. Further antibodies identified in npAIR include anti-carbonic anhydrase II, anti-α-enolase, and anti-rod transducin-α antibodies. [41]
Postmortem examination of eyes with CAR demonstrates diffuse photoreceptor degeneration with or without inflammation. Ganglion cells and retinal vasculature are spared. In MAR, bipolar neurons in the inner nuclear layer are markedly decreased, with evidence of transsynaptic ganglion-cell atrophy.
Management
The overall prognosis of patients with pAIR is not good. Surgery, chemotherapy, and radiation therapy to treat the primary tumor do not appear to alter the visual prognosis. Corticosteroids (local or systemic) generally are recommended as first-line with or without systemic immunomodulation as second-line. Corticosteroids have been shown to decrease antibody titers in patients with CAR and may stabilize their vision, but they usually do not reverse vision loss. Case reports of improved visual and anatomical outcomes, however, have been reported with repeat intravitreal triamcinolone injections in CAR and intravitreal sustained-release fluocinolone acetonide implants in MAR. [54, 55]
Anecdotal reports describe improvement in both CAR and MAR with high-dose intravenous methylprednisolone, plasmapheresis combined with steroids, or intravenous immunoglobulin (IVIG); however, the treatment results are largely disappointing. [34] Case reports exist for both CAR and MAR of IVIG improving visual outcomes. [56, 57] Plasmapheresis has been postulated to help remove anti-renal antibodies, immune complexes, and cytokines in MAR. [11]
Meanwhile, conflicting data also exists for the use of plasmapheresis in npAIR. [59]
Various immunotherapies have been investigated in AIR and may result in modest visual recovery in some cases, but more often the most that can be accomplished is disease stabilization. [31, 32, 33] One retrospective study of 30 patients with CAR and npAIR found improvement in 21 (70%) using immunosuppressive agents. [30] In this report, the best response occurred in those with cancer-associated disease (improvement in 6 of 6 cases). Various agents were used, including corticosteroids (periocular and systemic), azathioprine, intravenous immunoglobulin, mycophenolate mofetil, cyclosporine, infliximab, and various combinations of these immunomodulatory medications.
Two IgG monoclonal antibodies specific for interleukin-6 (IL-6), tocilizumab and sarilumab, have demonstrated efficacy in the treatment of refractory CME in npAIR. [60, 61] Espandar et al described a beneficial response in a patient with CAR treated with alemtuzumab, a monoclonal antibody that is used for the treatment of various B-cell mediated disorders. [35] More recently, many case series and case reports have demonstrated the utility of rituximab in the improvement of both visual and anatomical outcomes in CAR and npAIR. [63, 64, 65, 66, 67, 68, 69, 70] However, a phase I/II clinical trial investigating the use of rituximab for npAIR revealed the drug was safely tolerated but had mixed efficacy including cases of non-sustained success, disease stabilization, and treatment failure. [71]
Calcium antagonists aimed at blocking antibody-mediated apoptosis were found to be protective against antirecoverin antibodies in an animal model, but the efficacy in humans has not yet been demonstrated. Other research efforts involve activation of recoverin-specific antitumor cytotoxic T lymphocytes. Last, antioxidants including lutein, vitamin C, vitamin E, and beta-carotene have been suggested to help stabilize the disease course and retinal degeneration. [14, 35]
Limited efficacy of current treatment options adds to the importance of early diagnosis and intervention in the hope of minimizing and stabilizing irreversible immunological damage to the retina. [21] Prospective, longitudinal studies and randomized controlled trials are warranted to better elucidate the potential efficacy of the therapies mentioned prior among others.
Illustrative Cases
Case 1
A 60-year-old plumber developed painless visual loss in the right eye accompanied by intermittent "swirling clouds of smoke", occasional dim flashes of light, and photophobia. Visual acuity was count fingers in the right eye and 20/25 in the left eye. The visual field in the right eye showed a ring scotoma and was normal in the left eye. Dilated funduscopic examination and fluorescein angiography were normal.
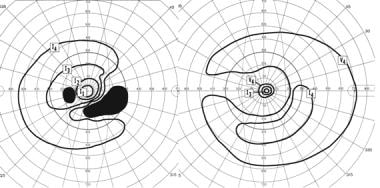 Goldmann perimetry in the above patient with bilateral sequential visual loss and photopsias. In the right eye, there is a dense ring scotoma breaking out to the periphery. In the left eye, there is an inferior arcuate scotoma that breaks out nasally.
Goldmann perimetry in the above patient with bilateral sequential visual loss and photopsias. In the right eye, there is a dense ring scotoma breaking out to the periphery. In the left eye, there is an inferior arcuate scotoma that breaks out nasally.
A retrobulbar optic neuropathy was suspected, but an MRI of the brain and orbits with gadolinium was normal. The patient received a tentative diagnosis of posterior ischemic optic neuropathy. Two months later, similar though milder visual loss developed in the left eye (see the image below). As before, the fundus appearance was normal.
A full-field ERG was nearly unrecordable under both scotopic and photopic conditions, indicating severe dysfunction of both rods and cones. Serologic testing showed antibodies to recoverin, diagnostic of cancer-associated retinopathy (CAR). A chest radiograph was normal, but CT of the chest revealed a small lesion, which, on biopsy, proved to be a small-cell lung carcinoma. His primary tumor was treated with radiation and chemotherapy, and he received a course of high-dose intravenous corticosteroids followed by rituximab. Vision stabilized but unfortunately showed minimal improvement.
Case 2
A 65-year-old dental hygienist presented with a 3-year history of flashing, squiggly lines around the periphery of her vision in both eyes, initially intermittent but more persistent over time. Visual acuity, color vision, and pupillary responses were normal. Goldmann perimetry (shown in the image below) showed several scotomas between 10 and 20 degrees in each eye, almost coalescing to form a ring scotoma. Fundus appearance and fluorescein angiography were normal.
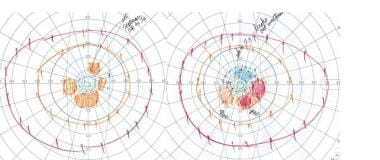 Goldmann perimetry in the above patient shows several scotomas between 10 and 20 degrees in each eye, almost coalescing to form a ring scotoma. While a normal foveal peak is noted, there is marked generalized decrease in the perifoveal responses in each eye.
Goldmann perimetry in the above patient shows several scotomas between 10 and 20 degrees in each eye, almost coalescing to form a ring scotoma. While a normal foveal peak is noted, there is marked generalized decrease in the perifoveal responses in each eye.
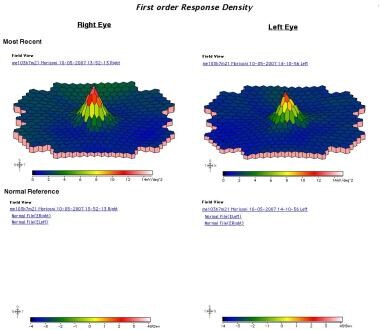
Multifocal ERG (images below) showed decreased amplitude surrounding fixation, corresponding to the location of the scotomas. Her serum was negative for anti-recoverin antibodies but positive for antibodies to 30 kd (carbonic anhydrase II). In addition, immunohistochemistry showed staining of some cells in the bipolar cell layer. She received a diagnosis of autoimmune retinopathy and has been followed conservatively.
-
Multifocal electroretinogram.
-
Multifocal electroretinogram.
-
Goldmann perimetry in the above patient shows several scotomas between 10 and 20 degrees in each eye, almost coalescing to form a ring scotoma. While a normal foveal peak is noted, there is marked generalized decrease in the perifoveal responses in each eye.
-
Goldmann perimetry in the above patient with bilateral sequential visual loss and photopsias. In the right eye, there is a dense ring scotoma breaking out to the periphery. In the left eye, there is an inferior arcuate scotoma that breaks out nasally.