Practice Essentials
Meningioma is a primary central nervous system neoplasm that is commonly encountered by neurosurgeons. The management of the meningioma depends on its location, size, shape, and involvement of the surrounding neurovascular structures. Observation, surgery, and radiation all are considered among the potential treatments when evaluating patients with meningioma. When considering surgical treatment, it is important to recognize patients’ lifespan, comorbidities, and physical status to assess whether the surgery may be tolerated. Various surgical approaches have been developed to maximize tumor resection while preserving important neurovascular structures. The extent of resection often is determined by the involvement of the neurovascular structures. Histopathology of the tumor may determine the postoperative treatment course for patients.
Background
In 1614, Felix Plater first described meningiomas at an autopsy. In 1938, Cushing and Eisenhardt first used the term "meningioma" to describe these lesions and introduced it as a separate category of extraparenchymal tumors. [1, 2] Meningiomas are believed to arise from the meningothelial cell (arachnoid cap cell) and usually are attached to the inner surface of the dura mater. [3] The parasagittal region, cerebral convexities, skull base, and falx are the most common locations for meningiomas, although they may arise at any location where meninges exist. [4] See the image below.
Meningiomas account for approximately 13-20% of all brain tumors [5] and 34-36.4% of all primary brain tumors, making meningiomas the most common primary brain tumors. [6, 7] Meningiomas represent only 4% of all orbital tumors. [8] Of lesions in the supratentorial compartment, sphenoid wing meningiomas represent about 15-20% of all meningiomas. Sphenoid wing meningiomas also are known as “orbitosphenoid meningiomas,” “meningiomas en plaque of the sphenoid wing,” and “sphenoid wing meningiomas with osseous involvement.” [9] Sphenoid wing meningiomas may be associated with hyperostosis of the sphenoid ridge and may be very invasive, spreading to the dura of the frontal, temporal, and orbital regions. [9, 10, 11, 12]
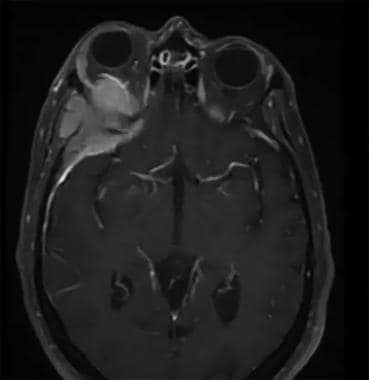 This is a typical appearance of a sphenoorbital meningioma on the MRI. The image depicts a contrasted MRI of the brain which shows an enhancing mass along the sphenoid ridge, orbital apex, and even the temporalis muscle. The orbital involvement of the tumor causes significant proptosis of the affected eye.
This is a typical appearance of a sphenoorbital meningioma on the MRI. The image depicts a contrasted MRI of the brain which shows an enhancing mass along the sphenoid ridge, orbital apex, and even the temporalis muscle. The orbital involvement of the tumor causes significant proptosis of the affected eye.
Two different growing patterns of sphenoid wing meningioma have been described: meningioma en masse, forming a nodular space-occupying lesion, and meningioma en plaque, which is flat and demonstrates a carpet-like growth pattern.
Meningiomas of the anterior skull base are defined as arising anterior to the chiasmatic sulcus that separates the middle cranial fossa from the anterior cranial fossa. [13] Sphenoid wing meningiomas are the most common of the basal meningiomas. Medially, they may expand into the wall of the cavernous sinus, anteriorly into the orbit, and laterally into the temporal bone. Sphenoid wing meningiomas are categorized as lateral, middle, or medial (clinoidal), depending on the origin of the tumor along the sphenoid ridge. [14] Furthermore, medial (clinoidal) meningiomas are further differentiated into 3 subcategories based on their relation to the anterior clinoidal process.
Type I clinoidal meningiomas originate from the inferomedial surface of the clinoidal process proximal to the distal carotid ring. This type is very difficult to resect because of the absence of the arachnoid plane between the tumor and the internal carotid artery.
Type II clinoidal meningiomas originate from the superolateral surface, leading to widening of the sylvian fissure, and are relatively easy to remove.
Type III clinoidal meningiomas originate at the optic foramen and extend into the optic canal and thus present some difficulty in gross total removal. [15] Other frontal skull base meningiomas can arise from the olfactory groove or planum sphenoidale. Planum sphenoidale meningiomas arise 2 cm posterior to olfactory groove meningiomas, and they may be symmetrical around the midline or may extend to the side. Of these 2 subtypes, about 15-20% grow into the ethmoid sinuses. [16, 17]
Pathophysiology
The most widely known risk factor for meningiomas is ionized radiation exposure. [18, 19, 20, 21, 22] For example, children with tinea capitis who are treated with an average single dose or multiple doses of 1.5 Gy have a relative 9.5% risk of developing meningioma. [23] Dental radiography is a common source of radiation in the United States. The risk of meningioma has been found to double after full-mouth series. [24] In addition, radiation-induced tumors may develop after previous radiation treatment of another lesion. In a recent pooled analysis, survivors of childhood cancer who received radiation treatment were 30 times more likely to develop meningioma later in life than those survivors who did not receive radiation treatment. [25] Cahan et al [26] were the first to propose criteria for identifying radiation-induced tumors, and these criteria have been applied to diagnose radiation-induced meningiomas. Radiation-induced tumors were defined as lesions arising after a latency period (4 years in the original article) within a previously irradiated field that have different histology than the radiated tumor. [26] These criteria have been augmented by others over the years with additional features, and the full set of criteria now includes the following [27, 28, 29] :
-
The tumor must arise in the irradiated field.
-
The histologic features must differ from those of any previous neoplasm in the region.
-
The tumor must occur after an interval sufficient to demonstrate that the neoplasm did not exist prior to irradiation (usually years).
-
This type of tumor must occur frequently enough after irradiation to suggest a causal relationship.
-
This type of tumor must have a significantly higher incidence in irradiated patients than in an adequate control group.
-
There must be no family history of a phakomatosis
-
The tumor must not be recurrent or metastatic.
The most commonly reported radiation-induced tumor is meningioma. [27, 29] The Childhood Cancer Survivor Study in the United States reported a cumulative incidence of 3.1% and a relative risk of 2.7% for meningioma development at 30 years after the primary tumor diagnosis. [21] Radiation-induced meningiomas develop more frequently over the convexities than the skull base, at a ratio of 1.9:1 for both high-dose and low-dose radiation. [29]
Radiation-induced meningiomas more commonly occur as multiples and in younger age groups than do spontaneous meningiomas. [29] Some authors reported that these tumors exhibit more malignant behavior, as indicated histologically by high cellularity, pleomorphism, multinucleation, and giant cell pseudoinclusions. [30] These findings have not yet been confirmed by others. [29]
There is a wide variation in the literature regarding the latency period for the development of radiation-induced meningioma. [27, 30, 31] It has been suggested that this variation is related to variation in the radiation dose, with a short latency for high doses and long latency for low-dose radiation. [27] The recognized latency period can be as short as 14 months and as long as 63 years, although the average latency period is 30-40 years in most cases. [29, 30, 31]
Aside from radiation, other factors that have been studied as potential causes of meningioma include genetic abnormalities, hormonal factors, and viral infections.
In cytogenetic studies, the most commonly reported genetic abnormality is the loss of NF2 tumor suppressor gene on long arm of chromosome 22 (monosomy 22). This genetic alteration leads to loss of expression of NF2 protein product (neurofibromin) and has been reported in 40-70% of meningiomas. [32, 33, 34] Seventy-five percent of NF2 patients develop meningioma during their lifetime. Ten percent of these are multiple lesions. [35, 36] Other commonly reported genetic alterations in meningioma include deletion of short arm of chromosome 1; loss of chromosomes 6, 10, 14, 18, and 19; and gain of 1q, 9q, 12q, 15q, 17q, and 20q. [32]
Hormonal factors (eg, estrogen, progesterone, androgen, steroid) have been studied extensively as risk factors for meningiomas because of the striking predominance of meningiomas in women; the female-to-male ratio is 2:1 for intracranial tumors and 10:1 for spinal meningiomas. [32] Other evidence to substantiate the implication of sex-specific hormones comes from data showing increased growth of meningiomas during pregnancy and hormonal replacement therapy. [37] Estrogen receptor (ER) has been found in 30% of meningiomas in 1 series, predominantly the ER-beta receptor isoform. [38]
The progesterone receptor is the best candidate among the sex-specific factors as a cause for meningiomas. Progesterone receptors have been shown to be expressed in 81% of women and 40% of men with meningiomas. [39, 40] Other studies indicate that progesterone binds to meningiomas in 50-100% of tested specimens. [39] Although progesterone receptor expression has been observed more frequently in benign meningioma (96%) than the malignant type (40%), no relation has been found between progesterone receptor status and age, sex, location of tumor, or menopausal state. [5, 40] These findings have prompted researchers to develop antiprogesterone medications, such as mifepristone (RU-486), which appears to inhibit tumor growth in vitro and in vivo. [41]
Androgen receptors also have been found in approximately 50% of meningiomas, but their receptor expression is variable, making them less likely candidates in the pathophysiology of meningiomas. [42] Similarly, meningiomas vary in expression of receptors for other hormones (eg, vascular endothelial growth factor receptor [VEGFR], epidermal growth factor [EGF], platelet-derived growth factor [PDGF], fibroblast growth factor, and insulin-like growth factor-1 [IGF-1]), making them less likely candidates for oncogenesis of meningiomas. It has been suggested that the direct stimulatory effect of EGF on PDGF or PDGF itself may be partially responsible for angiogenesis and even oncogenesis in meningiomas. PDGF is a particularly attractive candidate because it has structural homology with the product of c-sis oncogene on chromosome 22. [36]
Some viruses have been found within meningiomas, including polyoma virus, simian vacuolating virus 40 (SV-40), and adenovirus. A suggested role for these viruses or parts of viruses is related to the proteins involved in the induction or maintenance of tumor growth and transformation. [18] However, this association has not been proven.
Among the other potential factors for inducing meningiomas that have not been proven are head trauma and electromagnetic field exposure. Head trauma and skull fractures have been suggested as a risk factor for meningioma development by some authors. [43] However, a large population-based 2014 study from Taiwan found no association between head injury and meningioma development in 2 cohorts of patient with and without head injury. [44]
Similarly, electromagnetic field exposure, especially with the widespread use of cell phones, has generated interest in relation to the pathophysiology of brain tumors. Many studies suggest that little, if any, evidence supports the implications of cell phone use on meningioma development, although there generally is a lack of well-conducted studies to date and a significant amount of debate in this regard. [5]
Epidemiology
According to the most recently published Central Brain Tumor Registry of the United States (CBTRUS) report, meningioma is the most common nonmalignant brain and central nervous system (CNS) tumor, making up 37.6% of reported tumors (7.86 per 100,000 population). [45] The CBTRUS report also indicates that meningioma is the second most frequently reported brain and CNS tumor overall in adolescents and young adults (age 15-39 years), accounting for 15.9% of tumors. It is the most common tumor in patients aged 35-39 years, accounting for 25.1% of tumors, and is least common in patients aged 15-19 years, accounting for 4.9% of tumors. These numbers increase steadily with age. [46]
The annual incidence of meningiomas can be as low as 0.74 per 100,000 individuals younger than 34 years and as high as 18.86 per 100,000 individuals older than 85 years. It is 2.5 times more common in females than in males and 1.1% more common in Blacks than in Whites. [6, 47] In children, meningioma accounts for 4.6% of all primary brain tumors. [32, 48] Incidental meningioma is found in 0.52-0.9% of brain images. [49, 50]
Frequency
United States
Meningiomas account for approximately 13-20% of all brain tumors.
International
Worldwide, meningiomas account for approximately 13-20% of all brain tumors.
Mortality/Morbidity
In a 2016 study of 1549 operated meningiomas, the overall perioperative complication rate was 17.8-18.8%; of these, the morbidity rate was 1.2-2.2%. [51]
Among patients with skull base meningiomas, a 2016 study reported the overall mortality rate was 5%, with transient cranial nerve deficits occurring in 32% of cases, definite cranial nerve lesions in 18%, and cerebrospinal fluid (CSF) leak in 14%. [52] Another study reported an overall mortality rate of 5.8%, transient cranial nerve deficit rate of 11.7%, definitive morbidity of 5.8%, and second recurrence rate of 5.8%. [53]
Potential complications include bleeding, deep venous thrombosis and embolism, air embolism, venous infract, wound-healing deficits, paresis, sensory deficits, cranial nerve palsy, aphasia, seizures, brain edema, hygroma, CSF fistula hydrocephalus, ischemia, and pituitary insufficiency. These complications vary depending on preoperative morbid conditions, age, and tumor size and location.
Race
Previous studies argued that there is variability in the prevalence of meningiomas among Whites, Africans, African-Americans, and Asians and greater incidence among Blacks than Whites. [7, 54] A more recent report indicated that benign and malignant meningioma were more commonly found in African Americans compared with Caucasians in the United States. [45]
Sex
The incidence rate of meningioma is higher in females (10.87 per 100,000 population) than in males (4.98 per 100,000 population). [46]
No sexual predilection was found among Africans. [54]
Age
The average age at onset is 63 years. The incidence of meningiomas increases steadily thereafter. [51]
Prognosis
A 1983 study showed that recurrence-free survival rates after complete surgical removal in 114 patients (60% of which were sphenoid wing meningioma) at 5, 10, and 20 years were 80%, 70%, and 50%, respectively. [55] The extent of surgical resection and histological grade are very important prognostic factors.
In 1957, Simpson classified meningiomas based on the extent of resection as follows [56] :
-
Grade I - Macroscopically complete removal of the tumor with excision of its dural attachment and of any abnormal bone
-
Grade II - Macroscopically complete removal of the tumor and of its visible extensions with endothermy coagulation of its dural attachment
-
Grade III - Macroscopically complete removal of the intradural tumor without removal or coagulation of its dural attachment or extradural extensions
-
Grade IV - Partial removal of the tumor
-
Grade V - Simple decompression with or without biopsy
The 10-year risk for recurrence for grades I through IV were 9%, 19%, 29%, and 44%, respectively. [56]
The relevance of this system in the current era of microsurgical procedures advancement has been questioned by some authors53; however, a 2016 study of 458 patients with World Health Organization (WHO) grade I meningioma indicated that the extent of surgical resection based on Simpson grading is an important predictor of tumor recurrence. [57] Moreover, Borovich et al [58] introduced the idea of Simpson grade 0, which includes Simpson grade I resection plus an additional several cm of dural resection as a margin. This stemmed from the discovery that clusters of microscopic or even macroscopic tumor cells were observed in surrounding dura, as far as 4 cm from the margin of the tumor. [59] To corroborate this, Kinjo et al [60] observed no meningioma recurrence after 5 years for all of their Simpson grade 0 resections when they removed a 2-cm margin of dura. The overall tumor recurrence rates for Simpson resection grades I, II, III, and IV were 5%, 22%, 31%, and 35%, respectively. [57] When WHO histological grading of meningiomas is considered regarding survival, a 2016 study of 905 patients demonstrated that the 5-year overall survival rate was 85-90% for WHO grade I, 75-78% for WHO grade II, and 30-35% for WHO grade III tumors. [51] Recurrence rates of tumors graded according to the 2007 WHO classification of tumors of the central nervous system were 7-25% for WHO grade I, 29-52% for WHO grade II, and 50-94% for WHO grade III. [61]
Studies on the prognosis of sphenoid wing meningioma and spheno-orbital meningioma have generally showed good outcomes.
The results of a retrospective study of 25 patients who underwent surgical resection and orbital reconstruction with an average of 5 years of follow-up showed that a gross-total resection was achieved in 70% of patients, with surgery limited by the superior orbital fissure and the cavernous sinus. Proptosis improved in 96% of patients, with 87% improvement in visual function. Ocular paresis improved in 68%, although 20% of patients experienced temporary ocular paresis postoperatively. Overall, 95% of patients reported an improved functional orbit. There were no perioperative deaths or morbidity related to the surgical approach or reconstruction. Tumor recurrence occurred in 8% of patients. [62]
In another series of 67 patients with sphenoorbital meningioma who underwent surgical resection with orbital wall reconstruction, a total removal was achieved in 14 cases (82.3%), with only 1 recurrence (7.1%) over a mean follow-up period of 36 months. Proptosis was corrected in all cases, and visual acuity improved in 7 (70%) of 10 cases. Radical resection was followed by cranio-orbital reconstruction to prevent enophthalmos and to obtain good cosmetic results. No deaths or serious complications occurred in association with surgery. Revision of the orbital reconstruction was required because of postoperative enophthalmos (2 cases) or restricted postoperative ocular movement (1 case). [63]
A 2016 series of 33 patients with spheno-orbital meningioma who underwent resection without formal orbital wall reconstruction (mean follow-up, 4.5 years) showed improved proptosis in all patients, and only 2 patients had tumor recurrence at the orbit that required surgery. [9] An extension of this series evaluated visual outcomes of 33 subsequent patients with spheno-orbital meningioma. This study demonstrated that 86% of the patients had stable or improved vision, 100% had improved proptosis; and 0% developed pulsatile enophthalmos, even after no rigid orbital reconstruction. [64]
The authors of these studies emphasize aggressive removal of the hyperostotic bone to achieve satisfactory results and to decrease the risk for recurrence. For example, a study of 47 patients with spheno-orbital meningioma who underwent surgery via the frontotemporal approach without orbital wall reconstruction showed that complete resection was achieved in 51% of cases. At a mean follow-up of 52 months, proptosis normalized in 90.9% and improved in the remaining patients, visual acuity normalized in 20.8% and improved in 45.8% patients, and cranial nerve deficit subsided in all but 2 cases. The recurrence rate was 29.7%. [65] According to the authors, the high recurrence rate in this study was likely related to the incomplete removal of the invaded bone to minimize perioperative morbidity.
Patient Education
Some useful information is provided by the American Brain Tumor Association.
-
Coronal T1-weighted MRI with gadolinium enhancement of a sphenoid wing meningioma with some degree of encasement of bilateral cavernous sinuses.
-
T1-weighted MRI with gadolinium (coronal section) of same patient with sphenoid wing meningioma. A better visualization of en plaque growth of the meningioma along the convexity of the cerebral hemisphere on the left side is seen, in addition to better illustration of intracavernous carotid arteries bilaterally and en plaque growth of meningioma inferiorly and laterally around both temporal lobes.
-
T1-weighted gadolinium enhanced (sagittal section) of same patient with meningioma of the sphenoid wing.
-
CT scan brain bone window showing intraosseous meningioma involving left sphenoid wing, lateral orbital, superior orbital fissure, and the anterior part of the middle fossa floor.
-
MRI brain T2W (left) and T1W Fat-Sat (right) sequences showing involvement of the left sphenoid wing associated with dural thickening and the periorbita. Notice proptosis of the left globe secondary to left orbital wall thickening.
-
This is a typical appearance of a sphenoorbital meningioma on the MRI. The image depicts a contrasted MRI of the brain which shows an enhancing mass along the sphenoid ridge, orbital apex, and even the temporalis muscle. The orbital involvement of the tumor causes significant proptosis of the affected eye.
-
After elevation of the myocutaneous flap; note the underlying bone is infiltrated with tumor.
-
A frontotemporal craniotomy is performed to elevate the affected bone. Underlying it, dura is seen. The dura is also infiltrated with the tumor.
-
The tumor debulking is performed using the micro-instruments and high-speed diamond drill bit.
-
The dura is opened. Any intradural portion of the tumor is resected, if any. The involved dura is cut and discarded.
-
Important neurovascular structures are decompressed including the orbit, orbital apex, superior orbital fissure. The tumor has also infiltrated the periorbita. Some periorbital fat can be seen.
-
After gross total resection of the tumor (star = orbit, circle = frontal lobe, triangle = temporal lobe).
-
After closing the dura with a synthetic dural substitute, cranium is replaced and secured with titanium hardware. Medpore cranioplasty (white plates) are placed for further reconstruction and to restore cosmesis.










