Practice Essentials
Myopia is a condition of nearsightedness in which parallel rays from infinity are focused before they reach the retina with the accommodation at rest. Various refractive surgical procedures are used to treat myopia namely laser in situ keratomileuses (LASIK), photorefractive keratectomy (PRK), laser-assisted subepithelial keratectomy (LASEK) or Epi-LASEK, intracorneal ring segments (ICRS or INTACS), clear lens extraction (CLE) or phakic intraocular lens/IOL (PIOL) implantation.
Background
Myopia can be corrected by three different means, as follows [1] :
-
Optical devices (ie, glasses, contact lenses)
-
Corneal refractive procedures (ie, radial keratotomy [RK], automated lamellar keratoplasty [ALK], PRK, and LASIK [1]
-
Intraocular procedures (ie, clear lens extraction with or without lens implantation and the use of phakic intraocular lens [IOL] implants) [1]
The important facets of each procedure are the ease of application, the accuracy, the period of recovery, the quality of vision, the long-term stability of the results, and the minor or major complications. Also important are the possibilities of the reversal of the procedure and the successful management of complications.
Each intraocular procedure has its advantages, in terms of ease of implantation and tissue reactions causing change of position, inflammation, degeneration, and rise of intraocular pressure (IOP).
LASIK is currently the most popular type of refractive surgery. It is safe and effective, and technology advances such as femtosecond bladeless LASIK have further improved visual outcomes. However, not all patients are good candidates for LASIK surgery, including patients with severe myopia, patients who have hypermetropia or astigmatism, patients with an unusually thin or irregularly shaped cornea, and patients with eye conditions such as keratoconus, pellucid marginal dystrophies, or dry eye. [1]
Phakic IOL is preferable over LASIK surgery in most patients with severe myopia. In such patients, outcomes of phakic IOLs are superior to those of LASIK surgery in terms of both postoperative visual acuity and contrast sensitivity.
All the various phakic IOLs, whether angle supported, iris supported, or placed in the posterior chamber, provide good immediate postoperative results. However, frequent change in the designs of the angle-supported and the posterior chamber lenses makes conclusions about long-term stability difficult. The design of the iris claw lens has practically remained unchanged in the past 15 years.
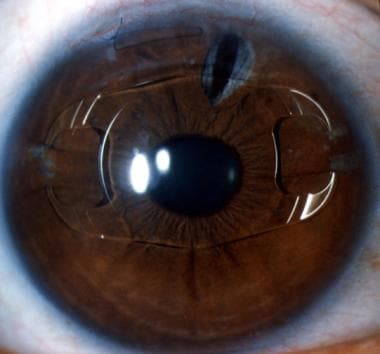 Phakic myopia lens of -20.0 diopters, 7 years postoperatively, in a 30-year-old man. There is slight upward decentration. The lens appears well tolerated.
Phakic myopia lens of -20.0 diopters, 7 years postoperatively, in a 30-year-old man. There is slight upward decentration. The lens appears well tolerated.
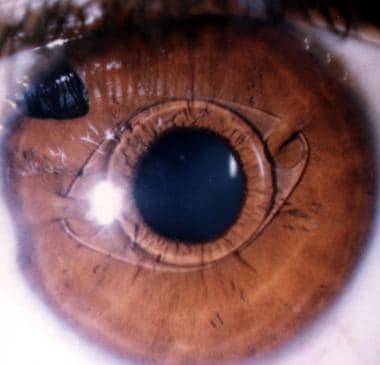 Phakic myopia iris claw lens, 11 years postoperatively. The claws of the lens are shifted upwards. This configuration makes iris fixation in the claws easier at the time of surgery. It reduces the chances of pressure by the lens haptics and the optic on the natural lens and the iris, in case a bigger knuckle of iris is drawn inside the claw.
Phakic myopia iris claw lens, 11 years postoperatively. The claws of the lens are shifted upwards. This configuration makes iris fixation in the claws easier at the time of surgery. It reduces the chances of pressure by the lens haptics and the optic on the natural lens and the iris, in case a bigger knuckle of iris is drawn inside the claw.
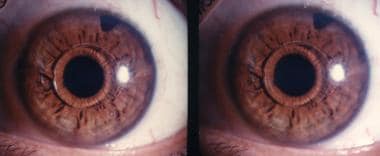
 Anterior segment fluorescein angiography in a case of iris claw lens (Artisan lens) in a phakic myope. There is no dye leakage. Courtesy of Professor Jan J. F. Worst, MD.
Anterior segment fluorescein angiography in a case of iris claw lens (Artisan lens) in a phakic myope. There is no dye leakage. Courtesy of Professor Jan J. F. Worst, MD.
Compared with the corneal procedures, the intraocular procedures suffer from one glaring handicap, that is, patients with the lens implants have to be under careful supervision of the ophthalmic surgeon throughout their lives to prevent or treat any adverse development. The role of microtrauma or macrotrauma as a result of blinking, squeezing, and minor rubbing during the waking hours and involuntary hard rubbing during some phases of sleep, in producing tissue changes, cannot be overemphasized.
The future of phakic IOLs shall be determined by the newer techniques of corneal refractive surgery, especially the wave-guided ablations. Phakic IOLs cannot take care of preexisting astigmatism. Surgery might introduce astigmatism of its own. Also, with the passage of months and years, patients with phakic IOLs tend to miss detailed follow-up examinations. Situations do exist where phakic IOLs and corneal procedures can be combined to provide the best refractive results. Whether a phakic myopia IOL in a young patient will be a problem in future decades is still unknown.
Phakic Intraocular Lens Types
Various phakic IOLs are available.
The Visian implantable collamer lens (ICL), marketed by Staar Surgical, is a posterior-chamber phakic IOL, meaning it is positioned behind the iris and in front of the crystalline lens. It received FDA approval in 2005 for correcting myopia ranging from -3.00 to -20.00 D. The Visian ICL is made of a soft biocompatible collagen copolymer. Owing to its flexibility, the lens can be folded during implantation, requiring only a small surgical incision.
The Verisyse (Abbott Medical Optics) is an anterior-chamber phakic IOL, meaning it is positioned in front of the iris. In 2004, the Verisyse phakic IOL received FDA approval for correcting moderate to severe myopia ranging from -5.00 to -20.00 D. The Verisyse lens is made of medical-grade plastic (polymethylmethacrylate [PMMA]) and is rigid in form. In Europe, it is approved and marketed under the trade name Artisan. Verisyse IOLs are not typically noticeable in the eye, although they may be visible to patients upon close inspection with a mirror. This iris claw lens has been in use in India by Professor Dr. Daljit Singh by the name of Singh's modification of Jan Worst's lens since the late 1970s. Dr. Singh has implanted this lens in more than 100,000 eyes over the past 30 years.
The Visian ICL and Verisyse phakic IOL are FDA approved to correct myopia only.
History of the Procedure
The phakic myopia lens was introduced by Strampelli and later popularized by Barraquer in the late 1950s. The design was a biconcave angle-supported lens. These lenses were abandoned following serious angle- and endothelium-related complications. In the mid 1980s, Dveli restarted phakic myopia lenses with 4 soft angle-supported loops. Baikoff introduced a myopia lens with Kelman-type haptics. This design had many problems, leading to its design modification a number of times. Fyodorov introduced the concept of a soft phakic lens in the space between the iris and the anterior surface of the crystalline lens. Earlier, the material used was silicone; now, the material used is collamer.
Worst, who introduced the iris claw lens in 1977, implanted an opaque iris claw lens in the phakic eye of a patient who had unbearable diplopia in 1979. Fechner and Worst introduced a phakic myopia lens of iris claw design in 1986. According to Ophthec, the maker of this lens (now called Artisan), more than 16,000 such lenses have been implanted worldwide in phakic myopes. During the same period, more than 250,000 iris claw lenses have been implanted in aphakes, which suggested that the design is well tolerated by the iris, where the lens is fixated.
During the past 20 years, the coauthor has implanted more than 160,000 iris claw lenses (ie, Artisan lenses) in his hospital (ie, Daljit Singh Eye Hospital, Amritsar, India); during this same period, the author has implanted approximately 20,000 lenses. In India, also during this same period, approximately 100,000 lenses have been implanted. Ophthec reports that more than 15,000 lenses are implanted in Europe every year. [2]
Problem
Myopia is a common refractive error, which exists from a young age. A unilateral myopia, with or without amblyopia, might remain undiscovered for a long time. The treatment of unilateral myopia is not easy. Since the vision is very good in the other emmetropic eye, the child is not impressed by the glass or the contact lens for the affected eye because the child prefers to use the nonaffected eye. Most parents give up all efforts out of sheer frustration, and the magnitude of wasted sight is immense.
While there is some experience with such modalities as the excimer laser for use as PRK or LASIK and the newly designed phakic IOLs, they have been used in adult patients, and there is little or no experience in the group of young patients who need the treatment. [3, 4] Slight or moderate myopia is hardly a problem as far as the vision is concerned. These patients do extremely well with glasses or contact lenses. However, high myopes do experience serious handicaps, both cosmetic and visual, for which surgery becomes a matter of importance.
Myopia is not merely a refractive problem. The importance of regular retinal examination should not be overlooked.
Etiology
Much remains unknown about the developing eye. It is known that myopia results when the refracting optics of the eye focus parallel rays from infinity to a point in front of the retina (relative to the length of the eyeball).
Newborns are moderately hyperopic, but they have a broad distribution of refractive errors. As the eye grows, there is a shift toward emmetropia. Perhaps, myopia represents an extension of the emmetropization process.
Pathophysiology
The pathophysiology differs in the three types of IOLs, the angle-supported, the iris-fixated, and the precrystalline varieties.
Angle-supported lenses
Two types of angle-supported lenses for use in phakic eyes are available. One has a Kelman-type haptic design, the other has a somewhat different design. The optic is biconcave. The lens is vaulted at 20°. The effective optic diameter is 4 mm in one design and 5 mm in the other design. Both are polymethyl methacrylate (PMMA) lenses.
 Phakic minus iris claw lens, 12 years postoperatively, in a 36-year-old patient shown in 3D. The lens is seen to sit on top of the iris cone. This picture helps to understand why the much larger angle-supported lenses have to be vaulted by 20° to keep clear of the iris.
Phakic minus iris claw lens, 12 years postoperatively, in a 36-year-old patient shown in 3D. The lens is seen to sit on top of the iris cone. This picture helps to understand why the much larger angle-supported lenses have to be vaulted by 20° to keep clear of the iris.
The angle-supported lens becomes fixated somewhere in the angle structures. There cannot be a perfect size of the IOL. A well-fixed lens is supposed to rest its haptics on the scleral spur. In actuality, the haptics press against the corneoscleral trabeculae and the Schlemm canal and, sometimes, the blood vessels in the vicinity that overlie the scleral spur. The haptics impinge on the nerve endings in the angle of the anterior chamber. However, nothing is known about this interaction. The haptics press on the segmental blood supply of the iris, leading to ischemia and iris atrophy, which results in progressive ovalization of the pupil. The haptics can erode through the angle tissues and become lodged in the ciliary body.
A loose angle-supported lens can move around and damage the corneal endothelium. Tissue reactions and damage to the angle of the anterior chamber can cause breakdown of the blood-aqueous barrier, uveitis-glaucoma-hyphema (UGH) syndrome. [5] Anterior segment inflammatory pathology may find an echo in the development of cystoid macular edema. [6] Microtrauma and macrotrauma of any nature can accelerate the pathological changes in the angle.
Iris-supported lenses
Iris claw lenses also are made of PMMA; the overall length of the implant varies from 7.2-8.5 mm, the size of the optic varies from 5-6 mm. The optic is convexo-concave, with a maximum height of 0.96 mm. The lens also is vaulted at the haptic to clear the natural vault of the iris.
The haptics become fixed to the midperiphery of the iris through the medium of the claws, located on either side of the lens. The amount of iris engaged into the claw depends on the judgment of the surgeon at the time of surgery.
The size of the IOL precludes all angle-related complications. No pupil- or posterior pigment epithelium–related problems occur because at no time does the lens come into contact with either of them. The author and the coauthor have used these lenses extensively, and they are very satisfied with the results. A concern does exist regarding chronic inflammation as a result of continued compression of the iris tissue in the claws. The inclusion of excessive iris tissue in the claws during surgery can push the lens against the iris and the crystalline lens. This can interfere with the free circulation of the aqueous through the pupil, possibly resulting in the formation of posterior synechia and inflammation.
Micromovements, such as blinking and squeezing, do not bring together the endothelium of the cornea and the optic of the IOL. However, forcible rubbing for any reason has the potential to damage the endothelium. [7]
Posterior chamber lenses
The phakic posterior chamber lens or the precrystalline lens is made of a soft material, such as silicone or collamer. The overall length varies from 11-13 mm. The central biconcave optical zone varies from 4.5-5.5 mm. The average thickness of the haptic is 60 µm. In an ideal lens implant situation, it is supposed to remain clear of the crystalline lens by 100-200 µm. The posterior chamber lens is sized empirically by adding 0.5 mm from the white-to-white corneal diameter.
The vaulting of the lens is produced by the elastic single piece lens getting lifted from the ciliary body. This puts some constant pressure, however small, on it. The vaulting may let it stay clear of the crystalline lens, but it physically pushes the iris anteriorly, a tissue normally in touch with the crystalline lens in the central part. This unnatural constant contact pressure and friction might lay the foundation for ciliary body reactions and shedding of posterior pigment epithelium. It also may prevent free passage of aqueous across the pupil. A mistakenly larger sized lens will put more pressure on the iris and push it forward by what may be called the spinnaker effect (blowing sail of a sailboat).
The optimal size remains a matter of conjecture. A mistaken small size allows the lens to rub against the natural crystalline lens (causing cataract) and knock against the ciliary body (setting up uveal reaction) and the posterior surface of the iris (releasing the pigment). The free volume of the restricted posterior chamber space is encroached upon by the implanted IOL and an increase in the size of the natural crystalline lens with age. If the peripheral iridectomy becomes blocked, iris bombé and consequent angle-closure glaucoma will occur. Some of the developments occurring behind the iris cannot be visualized clinically. They can only be speculated.
During blinking, squeezing, and rubbing, the results of pressure and friction between the IOL and the surrounding tissues are unknown. Even if the frictional force is very small, it is bound to continue for life. When shed from the iris and ciliary processes, the pigment may lead to glaucoma. Patients with diabetes can have problems. This disease causes changes in the posterior pigment epithelium of the iris, which may cause excessive shedding of pigment. An inflammatory process can bind the lens to the iris, the ciliary body, or even the crystalline lens. An intermittent or a continuous touch with the anterior lens capsule can produce lens opacification. Sometimes, the presence of the artificial lens in the posterior chamber can crowd the angle of the anterior chamber, leading to glaucoma.
In all phakic IOLs, increased crystalline lens size with increasing age and cataract formation leading to a decrease in the depth of the anterior chamber and crowding in the posterior chamber tend to exaggerate the pathologic processes. While the results of phakic IOLs are highly predictable, long-term data are limited.
Frequency
The prevalence of myopia is approximately 20% in the United States. This incidence rate frequently varies with age, sex, race, ethnicity, occupation, environment, and other factors in various sampled populations. The condition is more common in central and eastern Europe than in northern Europe, Britain, and the United States.
Incidence
The incidence of simple myopia is quite high since it occurs as a normal chance variant in the biological series, which include emmetropia and hypermetropia. In comparison, high and degenerative myopia are relatively rare. It has been estimated that myopia of more than -6.00 diopters (D) represents 27-32% of the myopic population and more than -8.00 D represents 6-18% of the myopic population.
Mortality/morbidity
Unilateral high myopia (more than about -6.00 D) of early onset can cause severe amblyopia. Severe symmetric refractive error (isometropia) may cause bilateral amblyopia of mild-to-moderate degree. However, myopia, even when extreme, rarely causes bilateral amblyopia because the sharply focused images of objects held close to the eyes support normal visual development.
Race
Race exercises a considerable influence over myopia. High degrees with degenerative changes are very common in certain races, such as Chinese, Japanese, Arab, and Jewish persons. Myopia is uncommon in black, Nubian, and Sudanese persons. The variation probably is due more to heredity than habit.
Sex
Sex appears to have an influence on the incidence. Although in the lower degrees of myopia, the sexes seem to be affected equally, with a probable excess in males; females are more prone to the higher degrees and to degenerative changes. Surgical correction of myopia in girls assumes significance in certain societies in which wearing of glasses and/or contact lenses is not looked upon kindly.
Age
Age at which surgery is performed is of great importance. The ideal age should be at around age 18 years when the refraction stabilizes. However, in specific circumstances, in the interest of the minor patient, the parents and the surgeon can opt to perform phakic lens implantation at an earlier age.
Presentation
Clinical manifestations are as follows:
-
Defective uncorrected vision
-
Defective corrected vision - Myopic retinal degeneration and deprivation amblyopia
-
Strabismus
-
Nystagmus
Clinical findings are as follows:
-
The eyeball may be normally placed, prominent, or deeply set.
-
The cornea may have a normal diameter, or some degree of microcornea or megalocornea may be present.
-
Axial length - 20 mm to greater than 30 mm
-
Keratometry reading - 38-50 D
-
Depth of the anterior chamber - 2.5-4 mm
-
Myopia may be moderate or severe, even going beyond -20.0 D.
-
IOP should be normal, and the patient should not be under treatment for any kind of glaucoma.
-
Strabismus
-
Nystagmus
-
Fundus examination may show various degrees of lattice degeneration, with or without one or more holes. The macular area may show disturbed macular reflex, chorioretinal atrophy, or posterior staphyloma.
-
Visual field defects of myopia may be present.
History findings are as follows:
-
Deteriorating vision
-
Difficulty in handling glasses or contact lenses; intolerance to contact lenses
-
Optical problems in daily life, especially during driving
-
Cosmetic concerns of the patient
-
Problems in such professions as sports, stage, and service regulations
Physical findings are as follows:
-
All patients should undergo a complete ophthalmic examination.
-
Manifest and cycloplegic refraction
-
Uncorrected visual acuity
-
Spectacle and/or contact lens corrected visual acuity
-
Slit lamp examination of the anterior segment and ocular adnexa
-
IOP
-
Pupil size measurement under scotopic conditions
-
Corneal endothelial cell count with specular endothelial microscopy
-
Biometry to calculate axial length of the eyeball and the anterior chamber
-
White-to-white corneal diameter measurement, if contemplating angle-supported or posterior chamber implants
-
Videokeratography and keratometry
-
Fundus examination by indirect ophthalmoscopy
-
Field charting
Indications
Indications include unilateral or bilateral, moderate or severe myopia; cosmetic needs; and professional and service requirements.
The current practical options for the cornea are PRK and LASIK; for intraocular implantation procedures, an angle-supported lens, an iris-supported lens, or a posterior chamber lens are practical options. Surgeons in different parts of the world may have differing opinions about these three lenses.
When planning for an IOL implant in phakic myopes, consider the following:
-
What is the minimum age at which the lens is to be implanted?
-
What is the minimum or the maximum refractive error to be treated?
-
What should be the lowest limit for anterior chamber depth?
-
What is the lowest corneal diameter at which lens implantation will be refused?
-
How accurate is the white-to-white diameter on the basis of which the length of an implant lens is to be derived?
-
What is the smallest size of the lens available?
-
How can the risk of complications be minimized? What are those complications? What are the chances of occurrence?
The final decision to do lens implantation comes after careful contemplation and detailed consultations and discussions.
The author and the coauthor are very interested in amblyopia and, to date, have helped more than 10,000 children and young adults with various forms of amblyopia, including those with amblyopia due to high myopia (unilateral and bilateral).
A new study by Alio et al shows promise in treating anisometropic amblyopia with phakic IOLs in adults. [8] The study reported a mean gain in vision of three lines, with a range of 0 to 7 lines. No patient lost any vision. PRK and LASIK previously have been studied to correct anisometropic amblyopia. In comparison to these studies, the visual results were similar after phakic IOL implantation for amblyopia. However, phakic IOL implantation may be a better option than LASIK or PRK because this study’s population had a much higher initial spherical equivalent. While effective in adults, phakic IOLs—in the right size—might work even better in children.
These IOLs may now have a new niche in the correction of amblyopia in adults. Alio et al evaluated the use of phakic IOLs to correct amblyopia in an adult population, with surprisingly good results. [8]
According to Alio et al, "This improvement is accounted for by the magnification on the retina caused by the intraocular lens, as opposed to the minimization induced by optical correction at the spectacle plane and the decrease in the spot size resulting from optical aberrations. This improvement was related to optical factors involved in the correction of the high myopia with a phakic IOL."
PRK and LASIK have previously been studied to correct anisometropic amblyopia and have had some success. According to Alio et al, "If excimer laser procedures can improve amblyopia in adults, phakic IOLs should be capable of doing so as well. This, along with the advantages of phakic IOLs regarding better quality of vision and lack of induction of aberrations resulting from corneal laser ablation, makes them an excellent alternative for the treatment of anisometropic amblyopia."
Fifty-nine eyes of 48 patients with anisometropic amblyopia underwent analysis by Alio and colleagues. They received angle-supported phakic IOLs (Domilens-Chiron, Lyon, France). Thirty-seven patients were unilaterally implanted, and 11 patients were bilaterally implanted.
According to Alio et al, "After implantation of a phakic IOL, the visual acuity of myopic patients with anisometropic amblyopia showed a significant increase. This improvement is accounted for by the magnification on the retina caused by the intraocular lens, as opposed to the minimization induced by optical correction at the spectacle plane and the decrease in spot size resulting from optical aberrations."
Specifically, the mean gain in visual acuity was three lines, with a range of zero to seven lines. No eyes lost vision. Further, 54 eyes (91.5%) gained at least one line of visual acuity.
According to Alio and colleagues, "Our analysis … does not show evidence of neuroprocessing related to underlying mechanics in the rehabilitation of amblyopia in these anisometropic adult patients; instead, this improvement was related to optical factors involved in the correction of the high myopia with a phakic IOL."
Comparing these results with those from LASIK or PRK for amblyopia, the advantages of phakic IOLs are evident. As stated by Alio et al, "We obtained visual outcomes in our study group similar to those reported with other refractive procedures. Mean preoperative spherical equivalent was much higher in our series; thus, phakic IOL implantation was a better surgical option than LASIK or PRK."
Karl Stonecipher, MD, Medical Director, TLC Carolina, Greensboro, NC, agreed that phakic IOLs have a role to play in amblyopia correction but suggested better results could be obtained if they are implanted earlier in life prior to the development of the amblyopia. [9]
"I agree totally with the findings of Dr. Alio’s group and other reports of success with LASIK and refractive lensectomies that have been presented and published. Those studies show that improving the optics, as the study suggests, improves the overall visual function. I think it would be great if we could just interrupt the process earlier. Most older studies show you’re not going to significantly change the course of the problem after nine years of age. Yet, in these anisometropic amblyopic patients, any benefit can add to the functionality of the patient."
Unfortunately, current phakic IOLs are too large for young children. So other more conventional methods, such as glasses, contact lenses, atropine, and patching, may have to do in the meantime. Additionally, reports of LASIK for anisometropia in children have appeared and shown successful outcomes.
Stonecipher noted if phakic IOLs were made for children to treat anisometropic amblyopia, that would probably be a relief for parents who have to deal with contact lenses, patching, and the use of atropine drops. Parents often get tired of these methods to correct amblyopia because of their child’s complaints.
However, one potential problem with a smaller phakic IOL would be the fact that it would have to be explanted at some point and a larger IOL fitted or another refractive procedure performed as the eye grows. Further, putting kids to sleep to perform the phakic IOL procedure is also a risk, Stonecipher said.
Alio et al noted in their study that there indeed is room for improvement in amblyopia correction for children. Success rates of conventional methods, such as atropine and occlusion, range from 63-83%, respectively.
Relevant Anatomy
Important anatomical features for phakic posterior chamber lens implantation
The following anatomic features involved in phakic posterior chamber lens implantation are important: the space available for the implanted lens, the structures with which the lens will intimately contact, and the effect of age on the dimensions of various involved structures.
The pupillary margin normally rests on the crystalline lens. The contact is maximum in a mid-dilated condition. The posterior chamber, which has a volume of 65 µL, is a triangular space on cut section. The base is toward the periphery, and the apex is toward the pupillary margin (where the chamber depth is zero). Nothing is known about the variations of this space and its relationship with age, the depth of the anterior chamber, or with refraction.
Anteriorly, there is a floppy iris about 0.5 mm in thickness; the thickness does not change throughout life. Posteriorly, there is the firm crystalline lens, which has a volume of 140 µL at birth. The volume increases to 163 µL in the 30s and to 240 µL in the 80s, a change of 100 µL.
The anterior chamber depth decreases by 7% every decade. It is the increasing size of the crystalline with age that causes shallowing of the anterior chamber. At the same time, it is not unreasonable to assume that the restricted posterior chamber space is also progressively encroached upon by the increasing volume of the crystalline lens. By the time the patient is in the seventh decade, the space is nothing more than a mere slit. In the case of a posterior chamber lens, the thickest part of the implant, the optic, occupies the shallowest or the zero space.
If a plate haptic lens must stay clear of the crystalline lens, it should vault in the central area. This can happen only if the haptic rests strongly on the ciliary processes and bows forward. Staying clear of the crystalline lens can lead to rubbing against the posterior pigment epithelium. It also means impinging on the ciliary epithelium of the ciliary processes. If the ciliary epithelium sheds, then the implanted lens will further touch the ciliary vasculature. The ciliary capillary endothelium has fenestrations of 30-100 µm, which are permeable to plasma proteins and tracer elements.
Lying between the crystalline lens and the iris, the phakic posterior chamber lens produces resistance to the flow of aqueous. The increasing volume of the crystalline lens with age encroaches on the posterior chamber volume. The crystalline lens volume increases greatly, from 150 µL to 240 µL, in a matter of 60 years.
The average anterior chamber depth is 3.15 mm (2.6-4.4 mm). The volume of the anterior chamber is 250 µL, which decreases by 7.5% per decade.
Important anatomic features for iris claw and angle-supported lenses
The iris is 0.5 mm thick at the root and 0.6 mm at the collarette. An iris claw lens is attached to the peripheral anterior surface of the iris by pushing a fold of the iris into the two claws of the lens. Consider the following three points: the space available around the lens and the distance from the corneal endothelium and the crystalline lens; the anatomy of the iris, especially the vasculature, as it is fixed in the claws of the lens; and the pupil movements and the friction between the iris and the phakic iris claw lens.
The posterior surface of the IOL is concave and cannot touch the crystalline lens. There is a respectable distance between the various parts of the lens and the corneal endothelium. The maximum height of the optic, which is in the center, is less than 1 mm. The haptic of the lens in the periphery is 0.18 mm. With age, the growth of the crystalline lens tends to reduce the depth of the anterior chamber. The maximum width of the implant lens is 8.5 mm, which allows it to remain away from the structures of the angle of the anterior chamber.
The vasculature of the iris is peculiar. The vascular endothelium of the human iris is not fenestrated. The endothelial cells of the iris vessels are joined by two types of intercellular junctions, the zonular tight junctions and the gap junctions. The vessels are covered in layers in the following order:
-
Pericytes
-
0.5-3 µm wide basal lamina
-
7-µm zone of sparse, longitudinally directed collagen fibers
-
Granular ground substance
-
Another 10 µm-wide connective tissue layer
Vessels that are in the more cellular parts of the stroma (eg, anterior border layer) are invested in thicker and more cellular adventitia. These vascular peculiarities explain why the claw lens can grip the iris fold so well. Friction between the iris claw lens and the anterior surface of the iris is possible at the immediate vicinity of the two claws as well as along the edge of the lens. As observed during the past 25 years, an iris claw lens is well tolerated in aphakic eyes. Phakic eyes are different, and surgeons must be aware of any degenerative or inflammatory response. Furthermore, phakic lenses are being implanted in younger patients, and these patients will need to be observed beyond the lifetime of many of their surgeons.
Important anatomical features for angle-supported lenses
The angle of the anterior chamber is another place where phakic angle-supported lenses are made to rest their footplates. The tissues involved are the corneoscleral trabeculae, the ciliary body, and the periphery of the iris. In the depth of the trabecular meshwork, there is the Schlemm canal, stray blood vessels in its vicinity, and the scleral spur. The scleral spur is deeper to the Schlemm canal, and it ends at a few micrometers wide tip under the posterior part of the corneoscleral trabeculae. The recess of the angle does not end at the level of the scleral spur but further posteriorly.
The major arterial circle is situated in the anterior part of the ciliary body. From this circle arise branches that go to the root of the iris anteriorly and the ciliary processes posteriorly. The endothelium of the capillaries in the ciliary body and the processes has fenestrations of 30-100 µm, which are permeable to plasma proteins and tracer elements. The arteries to the iris arise along with the branches to the ciliary processes. The vessels travel toward the pupillary margin without anastomosing, that is, they are more or less end arteries
Contraindications
Contraindications include the following:
-
Myopia other than axial
-
Evidence of nuclear sclerosis or developing cataract
-
History of uveitis
-
Presence of anterior or posterior synechiae
-
Corneal dystrophy
-
Glaucoma or IOP higher than 20 mm Hg
-
Personal or family history of retinal detachment
-
Diabetes mellitus
-
Anterior chamber depth less than 2.75 mm
Some of the above contraindications are relative on the discretion of the surgeon and the needs of the patients.
Relative contraindications include the following:
-
The patient should not rub the eye. Young children who cannot follow these instructions should not be implanted with a phakic IOL.
-
The smallest available posterior chamber phakic lens is of 11 mm. The length of the lens is calculated by adding 0.5 mm to the white-to-white limbal diameter. The minimum white-to-white diameter should be 11 mm. Any degree of microcornea from this size is a contraindication. In the case of an iris claw lens, it is possible to have customized, smaller lenses. While the normal phakic lens is 8.5 mm wide, the iris claw lens may be made as small as 6 mm, thus greatly extending its application.
The presence of amblyopia should not be a contraindication for the following purposes:
-
To reduce the power of the glasses
-
To provide a greater freedom of movement
-
Later, to attempt to improve the eyesight with pleoptic exercises
Anterior chamber depth is an important consideration for all three types of lenses, whether it is angle-supported, iris claw, or posterior chamber lenses. A depth of less than 2.75 mm is a contraindication. The angle-supported or the posterior chamber lens may cause crowding of the angle, but the iris claw lens will encroach on the central depth of an already shallow anterior chamber.
-
Phakic myopia lens of -20.0 diopters, 7 years postoperatively, in a 30-year-old man. There is slight upward decentration. The lens appears well tolerated.
-
The drawing shows the relation of the phakic iris claw lens to the surrounding structures. Courtesy of Professor Jan J. F. Worst, MD.
-
Slit lamp photograph of optical section of the anterior segment showing a gap between the anterior surface of the crystalline lens and the posterior surface of the implanted myopia iris claw lens. Courtesy of Professor Jan J. F. Worst, MD.
-
Phakic minus iris claw lens, 12 years postoperatively, in a 36-year-old patient shown in 3D. The lens is seen to sit on top of the iris cone. This picture helps to understand why the much larger angle-supported lenses have to be vaulted by 20° to keep clear of the iris.
-
Phakic myopia iris claw lens, 11 years postoperatively. The claws of the lens are shifted upwards. This configuration makes iris fixation in the claws easier at the time of surgery. It reduces the chances of pressure by the lens haptics and the optic on the natural lens and the iris, in case a bigger knuckle of iris is drawn inside the claw.
-
Anterior segment fluorescein angiography in a case of iris claw lens (Artisan lens) in a phakic myope. There is no dye leakage. Courtesy of Professor Jan J. F. Worst, MD.
-
5-mm optic Artisan lens. Courtesy of Professor Jan J. F. Worst, MD.
-
6-mm diameter optic, Artisan lens. Courtesy of Professor Jan J. F. Worst, MD.
-
This 64-year-old patient had an iris claw lens implanted in his aphakic eye on the posterior surface of the iris, 10 years ago. The white line represents the pupil in its normal state. Note fine pigment on the surface of the intraocular lens in this area. Bigger pigment granules are seen in the periphery. It seems that the pigment dispersed as a result of friction between the anterior surface of the intraocular lens and the posterior surface of the iris, over a very long time. This happened in spite of the fact that the posterior chamber was very roomy due to aphakia. Courtesy of Kiranjit Singh, MD.
-
Gonioscopy in the same patient as in Image 9 with iris claw lens fixed to the back of the iris. The angle shows severe pigmentation of the angle of the anterior chamber. Only the very finely pulverized pigment is deposited in the angle. Courtesy of Kiranjit Singh, MD.
-
Gonioscopy in 3D of the other eye of the same patient as in Image 9 that received anteriorly fixed iris claw lens in the aphakic eye, 11 years ago. There is total absence of pigmentation in the angle of the anterior chamber. Courtesy of Kiranjit Singh, MD.
-
Postmortem appearances in an eye with an iris claw lens showing the ciliary body and the posterior surface of the iris. There is a total lack of inflammatory or degenerative changes. Courtesy of Professor Jan J. F. Worst, MD.
-
Besides producing optical problems, a decentered phakic intraocular lens, by taking the haptics closer to the limbus, makes the eye vulnerable to intermittent endothelial touch whenever the eye is forcibly rubbed. It can be prevented by a careful fixation during surgery. The decentered lens can be managed by opening the claws and refixation at the right place. Courtesy of Professor Jan J. F. Worst, MD.
-
Eleven years after phakic minus iris claw lens implantation in a 35-year-old patient, the pupil has been dilated for fundus examination. Iris claw lens does not affect the movements of the iris and the pupil, except at the point where the iris passes through the claw. The crystalline lens is not affected since the implanted lens remains far away from it.
-
This phakic intraocular lens is fixated to the midperiphery of the iris.