Background
Photorefractive keratectomy (PRK) consists of the application of energy of the ultraviolet range generated by an argon fluoride (ArF) excimer laser to the anterior corneal stroma to change its curvature and, thus, to correct a refractive error. The physical process of remodeling the corneal stroma by ultraviolet (193 nm wavelength) high-energy photons is known as photoablation.
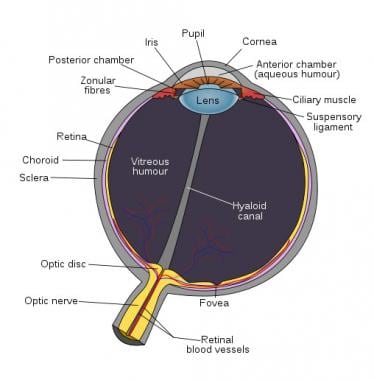
An image depicting human eye anatomy can be seen below.
History of the Procedure
During the 1980s, several applications of the 193-nm ArF excimer laser were investigated, including its use on human corneas for the correction of refractive errors. In 1988, Munnerlyn, Kroons, and Marshall reported an algorithm relating diameter and depth of the ablation to the required dioptric change.
McDonald performed the first excimer PRK for the correction of myopia on a normally sighted human eye in the United States. That same year, the Food and Drug Administration (FDA) organized a phase 3 trial, the PRK study (which ended in 1996), to demonstrate the safety, predictability, and stability of PRK for the treatment of myopia. At the end of this trial, 2 ophthalmic companies, VISX and Summit, were allowed to manufacture excimer lasers for widespread use in the United States. Since then, Nidek also has obtained approval for the manufacture of excimer lasers in the United States, and several hundred thousand patients have undergone this procedure throughout the world. The first excimer lasers used to perform PRK in the late 1980s have been improved significantly in terms of size, efficiency, and accuracy.
Problem
Several epidemiological studies, including the Beaver Dam population-based survey taken in the United States, show a prevalence of myopia greater than 0.5 diopters (D), ranging from 43.0% in people aged 43-54 years to 14.4% in individuals older than 75 years.
Pathophysiology
The mechanism of ablation of the excimer laser appears to be photochemical in nature and is known as photochemical ablation or ablative photodecomposition. This highly localized tissue interaction is based on the fact that each photon produced by the ArF excimer laser has 6.4 eV of energy, enough to break covalent bonds. [1]
The intramolecular bonds of exposed organic macromolecules are broken when a large number of high-energy 193-nm photons are absorbed in a short time. The resulting fragments rapidly expand and are ejected from the exposed surface at supersonic velocities. This mechanism explains why only the irradiated organic materials are affected, whereas the adjacent areas are not affected.
The return of corneal innervation up to 5 years after PRK was measured. Corneal subbasal nerve density does not recover to near preoperative densities until 2 years after PRK, as compared to 5 years after laser in situ keratomileusis (LASIK). [2, 3]
A study comparing transepithelial PRK and laser surgery found that both offer effective correction of myopia at 1 year, but LASIK seemed to result in less discomfort and less intense wound healing in the early postoperative period. [4]
Indications
Clinical indications for PRK include the following:
-
Myopia (-1.0 D to -6.0 D) - Higher corrections are associated with a greater risk of corneal haze formation; therefore, LASIK is generally the preferred procedure.
-
Astigmatism (0.75 D to 3.0 D) - Higher corrections are associated with regression of the effect; therefore, LASIK is the preferred procedure.
-
Hyperopia (+1.0 D to +4.0 D) - Haze and regression of the PRK effect have made LASIK the preferred procedure for most of these patients.
-
Patients with documented evidence of a change in manifest refraction of less than or equal to 0.5 D (both cylinder and sphere components) per year for at least 1 year prior to the date of preoperative examination
-
Patients aged 18-20 years for the reduction or elimination of myopia of less than or equal to -6.0 D spherical equivalent at the corneal plane
-
Patients aged 21 years for the reduction or elimination of myopia from 0 D to -6.0 D spherical myopia at the spectacle plane with up to -3.0 D of astigmatism
-
Patients aged 21 years or older with naturally occurring hyperopia from +1.0 D to +4.0 D spherical equivalent, with no more than 1.0 D of refractive astigmatism
-
Correction of refractive errors following other ocular surgery, including cataract surgery - PRK has been performed in patients who had previous radial keratotomy (RK) surgery or penetrating keratoplasties, but, in those cases, LASIK appears to be preferable. A significant risk of corneal haze formation exists if PRK is performed on an eye with any previous corneal surgery, so LASIK is generally the procedure of choice because of its minimal haze risk
-
PRK in corneas previously treated with LASIK
-
Treatment of anisometropic amblyopia in children
Relevant Anatomy
PRK ablation of the anterior stroma takes place after removing the epithelium, which is approximately 40-50 µm in thickness. The Bowman layer is destroyed in the process of PRK with no known deleterious consequences. A residual stromal thickness of at least 250 µm after PRK is necessary to prevent future corneal ectasia. A residual stromal thickness of 400 µm or more is preferred. The epithelium can be removed via mechanical, laser, or chemical means.
Contraindications
Contraindications include collagen vascular, autoimmune, or immunodeficiency diseases; pregnancy or breastfeeding; keratoconus; medications, such as Accutane (isotretinoin) or Cordarone (amiodarone hydrochloride); and a history of keloid formation. A recent report on the outcome of PRK in African Americans, including those with a known history of dermatologic keloid formation, revealed that a history of keloid formation does not appear to have an adverse effect on the outcome. These results question whether known dermatologic keloid formation should be a contraindication to photorefractive keratectomy.
-
Human eye anatomy.