Background
One of the most exciting developments in the world of refractive surgery has been the advent of laser in situ keratomileusis (LASIK). The surgical technique involves the creation of a hinged lamellar corneal flap, after which an excimer laser is used to make a refractive cut on the underlying stromal bed. LASIK is a fusion of old and new technologies, with its roots in keratomileusis and automated lamellar keratectomy (ALK). However, as currently practiced, it perhaps is best thought of as photorefractive keratectomy (PRK) performed under a flap instead of on the corneal surface.
LASIK has been available in the United States as an off-label procedure since the mid 1990s. FDA approval of excimer lasers for LASIK dates to about 1999. [1] Many millions of procedures have been performed worldwide. According to the American Society of Cataract and Refractive Surgery, about 850,000 procedures a year are currently performed in the United States.
History of the Procedure
Jose Barraquer generally is credited with much of the early work leading to corneal lamellar refractive procedures as they are currently practiced. He noted that refractive change could be accomplished in the cornea by tissue addition or subtraction. He subsequently developed the idea of resecting a corneal disc and freezing it, followed by shaping the disc with a cryolathe. [2, 3, 4] However, the technique was limited by complexity of the equipment and tissue damage to the resected corneal disc caused by freezing.
Ruiz and Barraquer performed keratomileusis in situ in the late 1980s. Using principles developed by Krumeich, this technique involved first removing a corneal disc with a microkeratome. Refractive change was accomplished by performing a second plano cut with the microkeratome. The thickness and diameter of this second disc of tissue determined the end refractive result; then, the first disc was sutured back onto the cornea. Problems included complexity, poor predictability, and irregular astigmatism.
Burratto and Pallikaris were the first to combine the use of the excimer laser and microkeratome technology. Burratto's original work involved performing a corrective excimer laser ablation on the back of a resected disc of corneal tissue. This disc was replaced and sutured onto the cornea. Pallikaris developed the technique of performing the excimer laser corrective ablation in the corneal stromal bed under a hinged flap. He first studied the procedure in rabbits, followed by blind human eyes in 1989, and then sighted eyes in 1991.
In 1993, Steve Slade added the refinement of using an automated microkeratome to create the flap and was one of the first US surgeons to perform LASIK.
Indications
As of 2023, LASIK was approved by the US Food and Drug Administration (FDA) for several different laser platforms, including the Allegretto Wavelight, VISX Star S4, Technolas, Mel 80, and NIDEK lasers. The approved range for myopic, hyperopic, and custom treatments varies slightly between platforms.
Table 1 summarizes these devices and their FDA status.
Table 1. Device Summary and FDA Status (Open Table in a new window)
|
Myopia (MRSE) -Conventional LASIK |
Custom Parameters |
Wavelight ® EX500, Allegretto (Alcon) |
< -12.0 D sph with < -6.0 D cyl |
Wavefront-Optimized: < -7.0 D sph with < -3.0 D cyl |
iDesign Refractive Studio (VISX Star S4) |
< -14.0 D sph;-0.50 D to -5.0 D cyl |
Wavefront-Guided: ≤ -6.0 D sph with ≤ -3.0 D cyl |
Technolas 217 (B&L) |
< -11.0 D sph with ≤ -3.0 D cy |
(217z): < -7.0 D sph with ≤ -3.0 D cyl |
Carl Zeiss Meditec Mel 80 Excimer Laser System |
< -12.0 D sph with < -6.0 D cyl |
N/A |
| NIDEK EC-5000 | -1.0 D to -14.0 D sph; ≤ 4.0 D cyl | Topography-assisted: -1.0 D to -4.0 D sph; -0.5 D to -2.0 D cyl |
Source: http://www.fda.gov/cdrh/LASIK/lasers.htm 4/20/23. |
||
Relevant Anatomy
The cornea is a thin layer of transparent tissue that protects the intraocular contents and refracts light. Average central corneal thickness is about 550 µm, increasing to about 700 µm in the periphery. The cornea has a diameter (from the front surface) of about 11 mm vertically and 12 mm horizontally. The air-tear interface is the first refractive surface that light encounters and accounts for about 80% of the eye's total refractive power; the average corneal curvature (K readings) in the adult cornea is approximately 44.00 diopters (D).
Anatomically, the cornea consists of 5 layers: epithelium, Bowman layer, stroma, Descemet membrane, and endothelium.
Three types of cells are present in the epithelium: (1) basal columnar cells attached to the epithelial basement membrane via hemidesmosomes, (2) wing cells noted for thin winglike projections, and (3) surface cells joined by connecting bridges and covered by microvilli. Mucin is attached strongly to the surface. Usually, 5-7 layers of cells are present. Unlike stratified squamous epithelium in other areas of the body, the epithelium in the eye has an exceptionally smooth and regular surface, contributing to the transparency and light transmission characteristics of the cornea.
The Bowman layer is not a membrane, but rather an acellular structure consisting of collagen and representing the most superficial layer of the stroma.
The stroma makes up about 90% of the corneal thickness and consists of regularly arrayed flattened bundles of collagen called lamellae. Approximately 200-250 lamellae are present in the human cornea. Each bundle extends the width of the cornea and is about 2 µm thick and up to 260 µm wide. The parallel arrangement of these bundles together with the uniform spacing between collagen fibrils helps explain corneal transparency. Although relatively acellular, stromal fibroblasts called keratocytes can be found scattered throughout the stroma between lamellae, and they are responsible for collagen production and wound healing.
The Descemet membrane is composed of a fine latticework of collagen fibers. It represents a true basement membrane, and it is produced by the corneal endothelium.
The endothelium is a single layer of hexagonal cells whose purpose is to act as a barrier to the influx of fluid into the cornea and to pump fluid out of the cornea keeping it deturgesced and clear. These cells are incapable of regeneration.
The cornea is richly innervated; myelin sheaths are present on the nerves as they traverse the superficial layers of the cornea. The nerve endings lose their sheath as they penetrate the epithelium. In terms of density, more nerve endings are present in the corneal epithelium than anywhere else in the human body. [5, 6]
Contraindications
Contraindications include unstable refractive error, active collagen vascular disease (especially in the presence of iritis or scleritis), pregnancy, any ongoing active inflammation of the external eye (eg, conjunctivitis, severe dry eye), and a refractive error outside the range of laser correction.
Other contraindications include leaving less than a calculated residual bed of 250 µm of untouched cornea, as well as signs, symptoms, or topographic findings consistent with keratoconus. Residual stromal bed (RSB) thickness is calculated by subtracting ablation depth plus flap thickness from the corneal thickness as measured by pachymetry. 250 µm generally is considered a higher risk threshold for RSB, and many surgeons use an RSB cutoff of 300 µm.
Patients who are on isotretinoin (Accutane), amiodarone hydrochloride (Cordarone), and sumatriptan (Imitrex) should be treated with caution, and patient counseling should be provided because these medications may adversely affect corneal wound healing.
A history of herpetic keratitis is a relative contraindication. Although patients have been treated safely with a history of herpes simplex keratitis and the appropriate use of prophylactic antivirals, reactivation of the virus following treatment remains a concern.
Patients who cannot cooperate with procedures under a topical anesthetic and cannot accurately fixate or lay flat without difficulty are poor candidates for refractive surgery.
-
Diffuse intralamellar keratitis (day 5).
-
Bacterial keratitis following LASIK.
-
Epithelial ingrowth.
-
Striae.
-
Thin, perforated flap.
-
Incomplete flap.
-
Buttonhole in flap.
-
Decentered flap and ablation.
-
Pupil alignment or visual axis alignment for laser ablation.
-
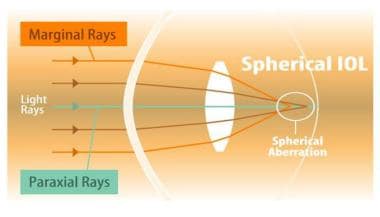
Spherical aberration: a schematic diagram for the human eye.
-
Zernike polynomials: pictorial representation.
-
Spherical aberration post-LASIK. The original refractive error was -10.00 diopters.
-
Coma in a patient with mild ectasia. This higher order optical aberration is also characteristic of decentered ablation zones and ectasia.
-
Postoperative ectasia: Orbscan. Note the elevation on anterior and posterior floats and the thinning of the central cornea on the pachymetry map.
-
Ectasia post-LASIK: Tracey WaveScan. Note the preponderance of higher order aberrations, including spherical aberration and coma. The Orbscan of this same patient appears in the image above.
-
Normal astigmatism pattern with corneal topography.
-
Normal corneal topography spherical pattern.
-
Keratoconus suspect; inferior and asymmetric corneal astigmatism pattern.
-
Keratoconus with elevation map; asymmetric and irregular astigmatism with inferior corneal elevation and steep area of inferior cornea.
-
Pre-operative Pentacam with slight inferior steepening and skew. Patients with borderline images such as this may require repeat or additional testing before deciding LASIK candidacy.
-
Pentacam image, Belin-Ambrosio Enhanced Ectasia Display. Note the absence of anterior or posterior elevation and normal "D" values.
-
Note the central elevation on both the anterior and posterior subtraction maps and the corresponding abnormal Df (front), Db (back), and overall D values. This patient will likely require additional testing or may not be a good candidate for LASIK due to the risk of ectasia.
-
Note the positive "donut sign," with a ring of epithelial thickening surrounding a central depression of epithelial thinning. The donut sign helps distinguish interior steepening on tomography due to early keratoconus from contact lens overwear; the latter would show inferior epithelial thickening.