Practice Essentials
Patients with diabetes often develop ophthalmic complications, such as corneal abnormalities, glaucoma, iris neovascularization, cataracts, and neuropathies. The most common and potentially most blinding of these complications, however, is diabetic retinopathy, [1, 2, 3] which is, in fact, the leading cause of new blindness in persons aged 25-74 years in the United States. In 2021, an estimated 9.60 million people in the United States were living with diabetic retinopathy, or 26.43% of individuals living with diabetes. [4]
Diabetes mellitus (DM) is a major medical problem throughout the world. Diabetes causes an array of long-term systemic complications that have considerable impact on the patient as well as society, as the disease typically affects individuals in their most productive years. [5] An increasing prevalence of diabetes is occurring throughout the world. [6] In addition, this increase appears to be greater in developing countries. The etiology of this increase involves changes in diet, with higher fat intake, sedentary lifestyle changes, and decreased physical activity. [7, 8]
Important aspects of workup regarding diabetic retinopathy include fasting glucose and hemoglobin A1c (HbA1c), fluorescein angiography, optical coherence tomography, and B-scan ultrasonography. Controlling diabetes and maintaining the HbA1c level in the 6-7% range are the goals in the optimal management of diabetes and diabetic retinopathy.
The exact mechanism by which diabetes causes retinopathy remains unclear, but several theories have been postulated to explain the typical course and history of the disease. [9, 10] See the image below.
In the initial stages of diabetic retinopathy, patients are generally asymptomatic, but in more advanced stages of the disease patients may experience symptoms that include floaters, distortion, and/or blurred vision. Microaneurysms are the earliest clinical sign of diabetic retinopathy. (See Clinical Presentation.)
Renal disease, as evidenced by proteinuria and elevated blood urea nitrogen (BUN)/creatinine levels, is an excellent predictor of retinopathy; both conditions are caused by DM-related microangiopathies, and the presence and severity of one reflects that of the other. Aggressive treatment of the nephropathy may slow progression of diabetic retinopathy and neovascular glaucoma. (See Treatment and Management.)
A study by Ito et al indicated that in patients with type 2 diabetes, the presence of reduced peripheral nerve conduction velocity is associated with the existence of early diabetic retinopathy. The report included 42 patients with type 2 diabetes (42 eyes), who had either no diabetic retinopathy or mild nonproliferative diabetic retinopathy. The investigators found that the latter group had significantly lower sural sensory conduction velocity and tibial motor conduction velocity than did patients with no diabetic retinopathy, with logistic regression analysis showing these velocities to be independent risk factors for the mild nonproliferative eye disease. [11]
According to The Diabetes Control and Complications Trial controlling diabetes and maintaining the HbA1c level in the 6-7% range can substantially reduce the progression of diabetic retinopathy. (See Treatment and Management.)
One of the most important aspects in the management of diabetic retinopathy is patient education. Inform patients that they play an integral role in their own eye care. (See Patient Education.)
For more information, see Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus.
Signs and symptoms of diabetic retinopathy
In the initial stages of diabetic retinopathy, patients are generally asymptomatic; in the more advanced stages of the disease, however, patients may experience symptoms that include floaters, blurred vision, distortion, and progressive visual acuity loss. Signs of diabetic retinopathy include the following:
-
Microaneurysms: The earliest clinical sign of diabetic retinopathy; these occur secondary to capillary wall outpouching due to pericyte loss; they appear as small, red dots in the superficial retinal layers
-
Dot and blot hemorrhages: Appear similar to microaneurysms if they are small; they occur as microaneurysms rupture in the deeper layers of the retina, such as the inner nuclear and outer plexiform layers
-
Flame-shaped hemorrhages: Splinter hemorrhages that occur in the more superficial nerve fiber layer
-
Retinal edema and hard exudates: Caused by the breakdown of the blood-retina barrier, allowing leakage of serum proteins, lipids, and protein from the vessels
-
Cotton-wool spots: Nerve fiber layer infarctions from occlusion of precapillary arterioles; they are frequently bordered by microaneurysms and vascular hyperpermeability
-
Venous loops and venous beading: Frequently occur adjacent to areas of nonperfusion; they reflect increasing retinal ischemia, and their occurrence is the most significant predictor of progression to proliferative diabetic retinopathy (PDR).
-
Intraretinal microvascular abnormalities: Remodeled capillary beds without proliferative changes; can usually be found on the borders of the nonperfused retina
-
Macular edema: Leading cause of visual impairment in patients with diabetes
Nonproliferative diabetic retinopathy
-
Mild: Indicated by the presence of at least 1 microaneurysm
-
Moderate: Includes the presence of hemorrhages, microaneurysms, and hard exudates
-
Severe (4-2-1): Characterized by hemorrhages and microaneurysms in 4 quadrants, with venous beading in at least 2 quadrants and intraretinal microvascular abnormalities in at least 1 quadrant
Proliferative diabetic retinopathy
-
Neovascularization: Hallmark of PDR
-
Preretinal hemorrhages: Appear as pockets of blood within the potential space between the retina and the posterior hyaloid face; as blood pools within this space, the hemorrhages may appear boat shaped
-
Hemorrhage into the vitreous: May appear as a diffuse haze or as clumps of blood clots within the gel
-
Fibrovascular tissue proliferation: Usually seen associated with the neovascular complex; may appear avascular when the vessels have already regressed
-
Traction retinal detachments: Usually appear tented up, immobile, and concave
-
Macular edema
See Clinical Presentation for more detail.
Diagnosis of diabetic retinopathy
Laboratory studies of HbA1c levels are important in the long-term follow-up care of patients with diabetes and diabetic retinopathy.
Imaging studies used in the diagnosis of diabetic retinopathy include the following:
-
Fluorescein angiography: Microaneurysms appear as pinpoint, hyperfluorescent lesions in early phases of the angiogram and typically leak in the later phases of the test
-
Optical coherence tomography scanning: Administered to determine the thickness of the retina and the presence of swelling within the retina, as well as vitreomacular traction
-
B-scan ultrasonography
See Workup for more detail.
Management of diabetic retinopathy
Pharmacologic therapy
This includes the following:
-
Triamcinolone: Administered intravitreally; corticosteroid used in the treatment of diabetic macular edema
-
Aflibercept, brolucizumab, faricimab, or ranibizumab: Administered intravitreally; monoclonal antibodies that can help to reduce diabetic macular edema and neovascularization of the disc or retina
Glucose control
The Diabetes Control and Complications Trial found that intensive glucose control in patients with type 1 diabetes (previously called insulin-dependent diabetes mellitus [IDDM]) decreased the incidence and progression of diabetic retinopathy. [12, 13, 14] It may be logical to assume that the same principles apply in type 2 diabetes (previously called non-insulin-dependent diabetes mellitus [NIDDM]).
Laser photocoagulation
This involves directing a high-focused beam of light energy to create a coagulative response in the target tissue. In nonproliferative diabetic retinopathy (NPDR), laser photocoagulation is indicated in the treatment of clinically significant macular edema.
Panretinal photocoagulation (PRP) is used in the treatment of PDR. [15, 16] It involves applying laser burns over the entire retina, sparing the central macular area.
Vitrectomy
This procedure can be used in PDR in cases of long-standing vitreous hemorrhage (where visualization of the status of the posterior pole is too difficult), tractional retinal detachment, and combined tractional and rhegmatogenous retinal detachment.
Cryotherapy
When laser photocoagulation in PDR is precluded in the presence of an opaque media, such as in cases of cataracts or vitreous hemorrhage, cryotherapy may be applied instead.
See Treatment and Medication for more detail.
Pathophysiology
The exact mechanism by which diabetes causes retinopathy remains unclear, but several theories have been postulated to explain the typical course and history of the disease. [9, 10]
Growth hormone
Growth hormone appears to play a causative role in the development and progression of diabetic retinopathy. Diabetic retinopathy has been shown to be reversible in women who had postpartum hemorrhagic necrosis of the pituitary gland (Sheehan syndrome). This led to the controversial practice of pituitary ablation to treat or prevent diabetic retinopathy in the 1950s. This technique has since been abandoned because of numerous systemic complications and the discovery of the effectiveness of laser treatment. It should be noted that diabetic retinopathy has been reported in parients with hypopituitarism as well.
Platelets and blood viscosity
The variety of hematologic abnormalities seen in diabetes, such as increased erythrocyte aggregation, decreased red blood cell deformability, increased platelet aggregation, and adhesion, predispose the patient to sluggish circulation, endothelial damage, and focal capillary occlusion. This leads to retinal ischemia, which, in turn, contributes to the development of diabetic retinopathy.
Aldose reductase and vasoproliferative factors
Fundamentally, diabetes mellitus (DM) causes abnormal glucose metabolism as a result of decreased levels or activity of insulin. Increased levels of blood glucose are thought to have a structural and physiologic effect on retinal capillaries causing them to be both functionally and anatomically incompetent.
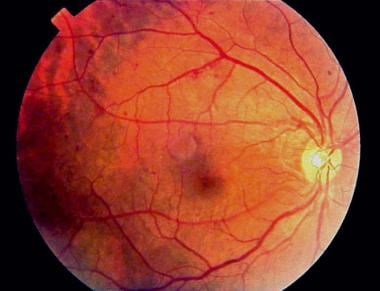
A persistent increase in blood glucose levels shunts excess glucose into the aldose reductase pathway in certain tissues, which converts sugars into alcohol (eg, glucose into sorbitol, galactose to dulcitol). Intramural pericytes of retinal capillaries seem to be affected by this increased level of sorbitol, eventually leading to the loss of their primary function (ie, autoregulation of retinal capillaries). This results in weakness and eventual saccular outpouching of capillary walls. These microaneurysms are the earliest detectable signs of DM retinopathy. (See the image below.)
Using nailfold video capillaroscopy, a high prevalence of capillary changes is detected in patients with diabetes, particularly those with retinal damage. This reflects a generalized microvessel involvement in both type 1 and type 2 diabetes. [17]
Ruptured microaneurysms result in retinal hemorrhages either superficially (flame-shaped hemorrhages) or in deeper layers of the retina (blot and dot hemorrhages). (See the image below.)
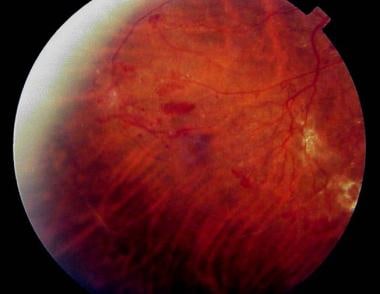
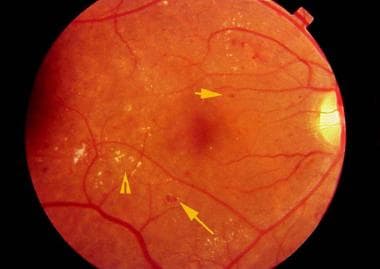 Retinal findings in background diabetic retinopathy, including blot hemorrhages (long arrow), microaneurysms (short arrow), and hard exudates (arrowhead).
Retinal findings in background diabetic retinopathy, including blot hemorrhages (long arrow), microaneurysms (short arrow), and hard exudates (arrowhead).
Increased permeability of these vessels results in leakage of fluid and proteinaceous material, which clinically appears as retinal thickening and exudates. If the swelling and exudation involve the macula, a diminution in central vision may be experienced.
Macular edema
Macular edema is the most common cause of vision loss in patients with nonproliferative diabetic retinopathy (NPDR). However, it is not exclusively seen in patients with NPDR; it may also complicate cases of proliferative diabetic retinopathy.
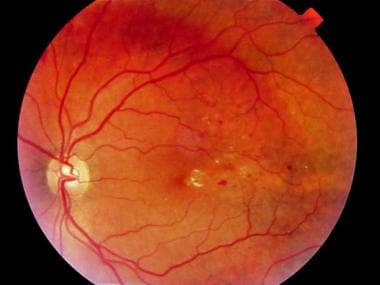 Fundus photograph of clinically significant macular edema demonstrating retinal exudates within the fovea.
Fundus photograph of clinically significant macular edema demonstrating retinal exudates within the fovea.
Another theory to explain the development of macular edema focuses on the increased levels of diacylglycerol from the shunting of excess glucose. This is thought to activate protein kinase C, which, in turn, affects retinal blood dynamics, especially permeability and flow, leading to fluid leakage and retinal thickening.
Hypoxia
As the disease progresses, eventual closure of the retinal capillaries occurs, leading to hypoxia. Infarction of the nerve fiber layer leads to the formation of cotton-wool spots, with associated stasis in axoplasmic flow.
More extensive retinal hypoxia triggers compensatory mechanisms in the eye to provide enough oxygen to tissues. Venous caliber abnormalities, such as venous beading, loops, and dilation, signify increasing hypoxia and almost always are seen bordering the areas of capillary nonperfusion. Intraretinal microvascular abnormalities represent either new vessel growth or remodeling of preexisting vessels through endothelial cell proliferation within the retinal tissues to act as shunts through areas of nonperfusion.
Neovascularization
Further increases in retinal ischemia trigger the production of vasoproliferative factors that stimulate new vessel formation. The extracellular matrix is broken down first by proteases, and new vessels arising mainly from the retinal venules penetrate the internal limiting membrane and form capillary networks between the inner surface of the retina and the posterior hyaloid face. (See the images below.)
In patients with proliferative diabetic retinopathy (PDR), nocturnal intermittent hypoxia/reoxygenation that results from sleep-disordered breathing may be a risk factor for iris and/or angle neovascularization. [18]
Neovascularization is most commonly observed at the borders of perfused and nonperfused retina and most commonly occurs along the vascular arcades and at the optic nerve head. The new vessels break through and grow along the surface of the retina and into the scaffold of the posterior hyaloid face. By themselves, these vessels rarely cause visual compromise, but they are fragile and highly permeable. These delicate vessels are disrupted easily by vitreous traction, which leads to hemorrhage into the vitreous cavity or the preretinal space.
These new blood vessels initially are associated with a small amount of fibroglial tissue formation. However, as the density of the neovascular frond increases, so does the degree of fibrous tissue formation.
In later stages, the vessels may regress, leaving only networks of avascular fibrous tissue adherent to both the retina and the posterior hyaloid face. As the vitreous contracts, it may exert tractional forces on the retina via these fibroglial connections. Traction may cause retinal edema, retinal heterotropia, and both tractional retinal detachments and retinal tear formation with subsequent detachment.
COVID-19
A study by Corcillo et al of 187 patients with type 1 (n=8) or type 2 (n=179) diabetes suggested that in individuals with diabetes who are hospitalized for coronavirus disease 2019 (COVID-19), the risk of intubation is more than five-fold greater in those with diabetic retinopathy. However, the investigators stated that because of the study’s cross-sectional design, they were unable to prove that diabetic retinopathy actually causes intubation, with further research required. [19, 20]
Etiology
Duration of diabetes
In patients with type I diabetes, no clinically significant retinopathy can be seen in the first 5 years after the initial diagnosis of diabetes is made. After 10-15 years, 25-50% of patients show some signs of retinopathy. This prevalence increases to 75-95% after 15 years and approaches 100% after 30 years of diabetes. Proliferative diabetic retinopathy (PDR) is rare within the first decade of type I diabetes diagnosis but increases to 14-17% by 15 years, rising steadily thereafter.
In patients with type II diabetes, the incidence of diabetic retinopathy increases with the disease duration. Of patients with type II diabetes, 23% have nonproliferative diabetic retinopathy (NPDR) after 11-13 years, 41% have NPDR after 14-16 years, and 60% have NPDR after 16 years.
Hypertension and hyperlipidemia
Systemic hypertension, in the setting of diabetic nephropathy, correlates well with the presence of retinopathy. Independently, hypertension also may complicate diabetes in that it may result in hypertensive retinal vascular changes superimposed on the preexisting diabetic retinopathy, further compromising retinal blood flow.
Proper management of hyperlipidemia (elevated serum lipids) may result in less retinal vessel leakage and hard exudate formation, but the reason behind this is unclear.
Pregnancy
Pregnant women with proliferative diabetic retinopathy do poorly without treatment, but those who have had prior panretinal photocoagulation remain stable throughout pregnancy. Pregnant women without diabetic retinopathy run a 10% risk of developing NPDR during their pregnancy; of those with preexisting NPDR, 4% progress to the proliferative type. [21]
A study by Toda et al found that among pregnant women with diabetic retinopathy, those who showed progression of the eye disorder tended to have a longer duration of diabetes, to have had diabetic retinopathy prior to pregnancy, and to have higher blood pressure in the second trimester. [22]
For more information, see Diabetes Mellitus and Pregnancy.
Epidemiology
A study by Lundeen et al estimated, as stated above, that in 2021, 9.60 million people in the United States were living with diabetic retinopathy, or 26.43% of individuals living with diabetes. An estimated 1.84 million people (5.06% of people with diabetes), according to the report, were living with vision-threatening diabetic retinopathy, that is, severe nonproliferative retinopathy, proliferative retinopathy, panretinal photocoagulation scars, or macular edema. [4]
An increased risk of diabetic retinopathy appears to exist in patients of Native American, Hispanic, and African American heritage.
A literature review by Teo et al indicated that the global prevalence of diabetic retinopathy among persons with diabetes is 22.27%, while the prevalence of sight-threatening diabetic retinopathy is 6.17%. Africa was found to have the highest prevalence of diabetic retinopathy, at 35.90% of individuals with diabetes, with the prevalence also being among the highest in North America and the Caribbean, at 33.30%. The investigators projected that the number of adults worldwide with diabetic retinopathy would rise from an estimated 103.12 million in 2020 to 160.50 million by 2045, with sight-threatening diabetic retinopathy rising from an estimated 28.54 million adults in 2020 to 44.82 million by 2045. [23]
In a study using data from medical practices in East London, Nugawela et al found that among individuals with newly diagnosed type 2 diabetes, an increased risk of diabetic retinopathy existed in persons belonging to Indian, Pakistani, and African ethnic groups. Compared with the White population, the risk of sight-threatening diabetic retinopathy was highest in newly diagnosed people of African ethnicity, being 36% greater. [24]
With increasing duration of diabetes or with increasing age since its onset, there is a higher risk of developing diabetic retinopathy and its complications, including diabetic macular edema or proliferative diabetic retinopathy.
Nonetheless, a literature review by Sabanayagam et al indicated that although the prevalence of diabetes has increased worldwide, the incidence of diabetic retinopathy–related blindness has fallen, especially in developed nations. [25]
A retrospective study by Porter et al of patients under age 21 years with type 1 or type 2 diabetes mellitus seen at an urban tertiary eye-care center found the overall incidence of diabetic retinopathy in these individuals to be 3.8%. More specifically, the rates were 3.4% and 6% in patients with type 1 and type 2 diabetes, respectively. Moreover, diabetic retinopathy occurred after a shorter disease duration in patients with type 2 diabetes than in those with type 1. The investigators also found no diabetic retinopathy in patients with an HbA1c level below 8%. [26]
For more information, see Macular Edema.
Prognosis
Prognostic factors that are favorable for visual loss include the following:
-
Circinate exudates of recent onset
-
Well-defined leakage
-
Good perifoveal perfusion
Prognostic factors that are unfavorable for visual loss include the following:
-
Diffuse edema/multiple leaks
-
Lipid deposition in the fovea
-
Macular ischemia
-
Cystoid macular edema
-
Preoperative vision of less than 20/200
-
Hypertension
The Early Treatment for Diabetic Retinopathy Study has found that laser surgery for macular edema reduces the incidence of moderate visual loss (doubling of visual angle or roughly a 2-line visual loss) from 30% to 15% over a 3-year period. The Diabetic Retinopathy Study has found that adequate scatter laser panretinal photocoagulation reduces the risk of severe visual loss (< 5/200) by more than 50%. [27, 28]
In a Danish nationwide matched case-cohort study, Mabala et al found evidence that in adults with type 1 diabetes mellitus, the risk for prevalent and incident cardiovascular disease (CVD) is greater in the presence of diabetic retinopathy. Moreover, the investigators reported that the risk for incident CVD grew in relation to the stage of macular degeneration, with the hazard ratio being 1.33 for the mildest stage, and 2.39 for the most severe stage. [29]
Patient Education
One of the most important aspects in the management of diabetic retinopathy is patient education. Inform patients that they play an integral role in their own eye care.
Excellent glucose control is beneficial in any stage of diabetic retinopathy. It delays the onset and slows down the progression of the diabetic complications in the eye.
The following symptoms and/or health concerns must be addressed in any patient education program for those with diabetic retinopathy:
-
Systemic problems (eg, hypertension, renal disease, and hyperlipidemia) may contribute to disease progression.
-
Smoking, although not directly proven to affect the course of the retinopathy, may further compromise oxygen delivery to the retina. Therefore, all efforts should be made in the reduction, if not outright cessation, of smoking.
-
Visual symptoms (eg, vision changes, floaters, distortion, redness, pain) could be manifestations of disease progression and should be reported immediately.
Diabetes mellitus, in general, and diabetic retinopathy, in particular, are progressive conditions, and regular follow-up care with a physician is crucial for detection of any changes that may benefit from treatment.
-
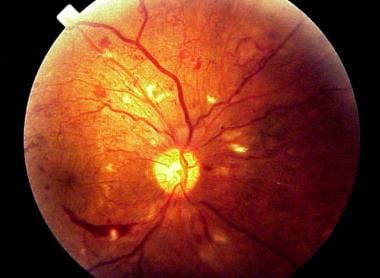
Fundus photograph of early background diabetic retinopathy showing multiple microaneurysms.
-
Retinal findings in background diabetic retinopathy, including blot hemorrhages (long arrow), microaneurysms (short arrow), and hard exudates (arrowhead).
-
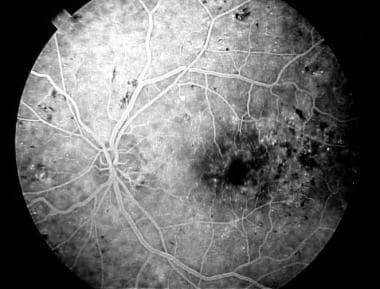
Fluorescein angiogram demonstrating an area of capillary nonperfusion (arrow).
-
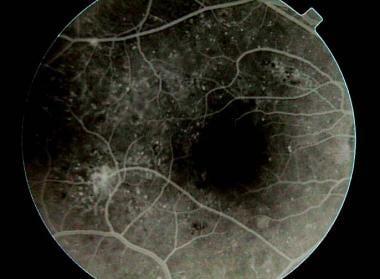
Fluorescein angiogram demonstrating foveal dye leakage caused by macular edema.
-
Fundus photograph of clinically significant macular edema demonstrating retinal exudates within the fovea.
-
New vessel formation on the surface of the retina (neovascularization elsewhere)
-
An area of neovascularization that leaks fluorescein on angiography.
-
Boat-shaped preretinal hemorrhage associated with neovascularization elsewhere.
-
Fibrovascular proliferations within the vitreous cavity
-
Extensive fibrovascular proliferations within and around the optic disc