Overview
Uveal melanoma is the most common primary intraocular tumor in adults. Iris melanoma is the least common site for uveal melanoma (2-5%). The incidence varies between 0.2 and 0.9 per million people. [1] Melanomas arise from the malignant proliferation of the neuroectodermally derived iris stromal melanocytes, which replace the normal iris stromal architecture. While melanoma is the most common primary malignancy of the iris, it accounts for only 3%-10% of all uveal melanomas. Considerable controversy exists regarding the histopathologic classification and the malignant potential of iris melanomas.
See The Case of the Middle-Aged Woman with Sudden Unilateral Vision Loss, a Critical Images slideshow, to help identify and treat malignant intraocular tumors.
See Ciliary Body Melanoma, Choroidal Melanoma, and Conjunctival Melanoma for complete information on these topics.
Pathophysiology and Etiology
A pre-existing Nevus is the most common origin of iris melanoma; however, they can develop de novo. Epidemiologic studies suggest that high levels of solar ultraviolet B (UV-B) radiation play a role in the pathogenesis of iris melanomas. [2] Loss of chromosome 3, and loss of chromosomal region 9p21 (entails tumor suppressor gene CDKN2A), play a role in iris melanoma. There is evidence that individuals with light skin and iris color are more prone to iris melanoma. [3]
Epidemiology
The mean age of patients varies between 45 and 65 in different studies (range 10-89). It is more common in males than in females. Prevalence does not appear to vary by geographic location. [4]
Rates of transformation of a suspicious iris nevus to melanoma are reported at 4% in 10 years and 11% by 20 years. [5] In a study surveying 3680 iris tumors based on patient age at presentation, Shields et al found that nevus (42%), iris pigment epithelium (IPE) cyst (19%), and melanoma (17%) were the most common specific diagnoses at all ages. [6]
Clinical Presentation
Patient history
Many patients with iris melanoma provide a history of a nevus existing since childhood that has suddenly undergone rapid growth. This may be noticed by the patient or during a routine eye examination. Patients may present because of cosmetic concerns. Some patients may experience pain caused by increased intraocular pressure or a change in vision caused by cataract development.
Physical examination
Iris melanomas may be circumscribed or diffuse. Circumscribed melanomas have a nodular shape, and almost 80% arise in the inferior half of the iris. After inferior quadrant, temporal, nasal, and superior are affected in a sequential order. [7] They vary in size, shape, and clinical behavior. They are typically yellow, tan, or brown in color with a flat or rounded anterior contour. The variability of pigmentation (a sign of cellular heterogeneity) is more often associated with malignant histology, fast progression, and poor prognosis. [8] Iris melanomas can grow anteriorly into the anterior chamber or posteriorly into the posterior chamber, usually being limited by the lens, and giving a “lion’s paw” appearance on ultrasonographic biomicroscopy (UBM).
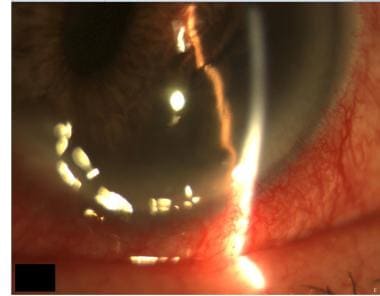 Inferior iris melanoma of the left eye that is pushing the pupil superiorly and nasally and distorting the normal appearance of the iris. Image courtesy of MERSI, Waltham, MA.
Inferior iris melanoma of the left eye that is pushing the pupil superiorly and nasally and distorting the normal appearance of the iris. Image courtesy of MERSI, Waltham, MA.
Diffuse melanomas present differently from circumscribed melanomas, usually as a unilateral dark iris (acquired heterochromia) without focal thickening. [9] Diffuse melanoma may also be associated with glaucoma, which tends to respond poorly to medical management and causes severe disc cupping and functional loss. Diffuse melanomas also tend to be of the epithelioid cell type and carry a higher risk for metastasis than do circumscribed melanomas. Distant metastasis occurs in 13% of patients with diffuse iris melanomas. [9]
General signs indicative of malignant transformation include rapid growth of the lesion and the development of prominent blood vessels.
Ring melanomas involve more than two thirds of the angle and have associated glaucoma. Tapioca melanomas are multifocal nodules projecting into the anterior chamber that may be associated with glaucoma.
According to Shields, criteria for a clinical diagnosis of melanoma are as follows [10] :
-
Larger than 3 mm in diameter and 1 mm in thickness
-
Replaces the stroma of the iris
-
Three of the following 5 features are present: photographic documentation of growth, secondary glaucoma, secondary cataract, prominent vascularity, or ectropion irides
Ciliary body involvement is associated with a higher incidence of malignancy. Medial location and pigment dispersion onto the iris or the angle structures are associated with tumor growth.
Shields et al described clinical features predictive of growth from iris nevus to iris melanoma based on evaluation of 1611 eyes at an ocular oncology center. They state that the risk factors for growth may be identified by the following [11] :
-
Age: Younger than 40 years at presentation
-
Blood: Past episode of hyphema
-
Clock hour: Inferior, 4 o’clock to 9 o’clock hour location of tumor
-
Diffuse configuration: Involving entire iris surface
-
Ectropion uveae
-
Feathery tumor margin
A thorough ophthalmologic examination, including transillumination and indirect examination with scleral depression, is essential for differentiating among iris cysts, primary iris tumors, and primary ciliary body melanomas.
Gonioscopy and UBM of the entire ciliary body must also be performed to rule out involvement before any therapeutic decisions are made.
Complications
Potential complications of iris melanoma include the following:
-
Glaucoma: Secondary glaucoma in iris melanomas may result from several different mechanisms, including (1) invasion of malignant cells into the trabecular meshwork (seeding), (2) decreased aqueous outflow due to pigment-ingesting macrophages blocking the angle, (3) angle closure, or (4) neovascularization
-
Cataract (usually sectoral)
-
Hyphema
-
Pupillary distortion
-
Ectropion uveae
-
Metastases
-
Death
Untreated iris melanoma can lead to complications such as recurrent hyphema and glaucoma with irreversible optic nerve damage, which is why treatment of slow-growing iris melanoma must be considered to prevent complications. [12]
Differential Diagnoses
The differential diagnoses of iris melanomas include the following:
-
Primary iris cysts
-
Iris nevi
-
Iris metastases
-
Iris foreign body
-
Iridocorneal endothelial syndrome
-
Koeppe or Busacca nodules
-
Lisch nodules
-
Peripheral anterior synechiae
-
Ciliary body melanoma with anterior extension
-
Other iris tumors (eg, leiomyoma, rhabdomyosarcoma)
Imaging Studies
Sequential photography
Sequential photographs of iris lesions are extremely helpful for monitoring the lesions’ size and growth and are done usually prior to dilation.
Ultrasonographic biomicroscopy
Ultrasonographic biomicroscopy (UBM) can be extremely helpful in differentiating solid iris masses from iris cysts. Melanomas involving the anterior chamber angle can also invade the ciliary body. Thus, in these tumors, gonioscopy and UBM of the ciliary body are critical. The anterior chamber angle can also be viewed, and ciliary body involvement can be evaluated. Nodular melanomas with posterior extension delimited by the lens may show a “lion’s paw” appearance on UBM. UBM has recently been used to help monitor the characteristics of these lesions after brachytherapy. [13]
B-scan ultrasonography
B-scan ultrasonography can be helpful in visualizing the extension of the lesion and determining the level of variation in appearance.
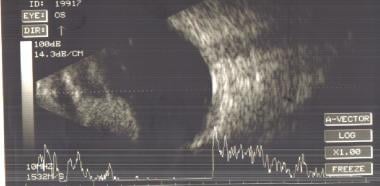 B-scan ultrasonogram of a patient with an iris melanoma showing a solid echogenic nodule. Image courtesy of MERSI, Waltham, MA.
B-scan ultrasonogram of a patient with an iris melanoma showing a solid echogenic nodule. Image courtesy of MERSI, Waltham, MA.
Anterior segment optical coherence tomography
Anterior segment optical coherence tomography (AS-OCT) is superior to B-scan for imaging small lesions pertaining to the anterior iris, but B-scan is better for imaging larger iris lesions with posterior or ciliary body extension. [14] Hau et al suggested that optical coherence tomography can be used in minor iris tumors with a low degree of pigmentation, whereas UBM is suggested for tumors of the ciliary body and those with heavy pigmentation. [14]
Other studies
Fluorescein angiography may show irregular vascular channels with late filling. However, this modality rarely is helpful and usually is not performed in clinical practice.
Diagnostic Procedures
Diagnostic procedures include the following:
-
Fine needle aspiration biopsy (FNAB) is recommended for a clinical suspicion of myeloma. [1]
-
Aqueous humor paracentesis (if metastasis is suspected)
-
Ultrasound-guided fine-needle aspiration biopsy for cytologic diagnosis before iridocyclectomy
-
Multifocal surgical iridectomy biopsy can be minimally invasive and effective and allows for partial and full-thickness iris biopsy. This can be performed using a 25-gauge aspiration cutting probe, allowing a small-incision surgery with no significant complications and a rapid rehabilitation. [15]
A multicenter study by Khan et al gathered information on biopsy-proven melanoma of the iris. The study found that the melanomas were most often brown and located in the inferior quadrants of patients with light irides. Commonly associated with angle blunting and spindle cell histopathology, melanomas are usually small and unifocal. [16]
In a 2012 study, liver function testing was not helpful for the early diagnosis of metastatic uveal melanoma, although the high negative predictive value of such tests suggests that they might allow clinicians to reassure patients when test results are negative. [17]
Histologic Findings
Malignant melanocytic stromal proliferation disrupts the normal iris stromal architecture. There are 3 types of iris melanomas: spindle cell, mixed cell, and epithelioid cells melanomas. Spindle cells or epithelioid cells may be morphologically found. The Jakobiec and Silbert classification categorizes the lesions as spindle cell melanoma, spindle and epithelioid melanoma, or epithelioid melanoma. According to a modified Callender classification system, most reported iris melanomas in the literature are diagnosed as spindle cell melanoma.
Small and unifocal melanomas are commonly associated with a spindle cell histopathology. [18]
There is evidence that the proliferation of melanocytes of the anterior iris surface and diffuse stromal invasion may be risk factors for local recurrence and metastasis. [19]
Treatment & Management
Medical care
Tebentafusp (Kimmtrak) is the first drug approved for treatment of HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma (mUM). It is a first-in-class bispecific protein comprised of a soluble T-cell receptor (TCR) fused to an anti-CD3 immune-effector function that specifically targets gp100, a lineage antigen expressed in melanocytes and melanoma. Immune-mobilizing monoclonal TCRs against cancer (ImmTAC) molecules bind cells that present a peptide derived from an antigen of interest, and recruit T-cells to lyse the target cells.
Approval of tebentafusp was based on the results of the phase 3 IMCgp100-202 clinical trial that evaluated overall survival (OS) of tebentafusp compared with investigator’s choice (either pembrolizumab, ipilimumab, or dacarbazine) in patients with previously untreated mUM. The trial randomly assigned 378 patients in a 2:1 ratio to either tebentafusp or investigator’s choice. Results demonstrated OS was 73% in the tebentafusp group compared with 59% in the investigator’s choice group (82% pembrolizumab; 13% ipilimumab; 6% dacarbazine) at 1 year (P< 0.001). Progression-free survival was also significantly higher in the tebentafusp group than in the control group (31% vs 19% at 6 months; P = 0.01).
Patients are observed closely with periodic slit-lamp examination, photographic documentation, and ultrasonographic biomicroscopy (UBM). Glaucoma can be controlled with medication if no tumor infiltration of the angle structures is present.
Surgical care
In general, sector iridectomy is performed for small tumors, and iridocyclectomy is performed to treat tumors that invade the angle. Other options include radiotherapy with a radioactive plaque (brachytherapy) or external proton-beam irradiation. Finally, enucleation may be required for diffusely growing tumors if radiotherapy is not possible.
The treatment of choice for growing lesions typically has been excision. Over the past 30 years, advances in microsurgical techniques and equipment have improved access to the iris and anterior chamber angle and have enabled more precise tumor resection with less trauma to the eye and fewer complications.
However, reports in the literature have described the successful treatment of iris melanomas with brachytherapy and proton-beam irradiation.
Excision is recommended if the lesion is impinging on the pupillary margin and interfering with vision or if secondary glaucoma is not controlled with medication. Excision should be considered if the lesion grows rapidly or encroaches on the chamber angle or if the fine-needle aspiration biopsy specimen shows malignant histology. Excision must be complete—either a sector iridectomy or an iridocyclectomy if the lesion encroaches on the chamber angle.
Plaque radiation therapy with palladium 103 (103Pd) has been used for these patients. Preliminary results show a high rate of success, with the most common complication being cataract formation in more than 75% of phakic patients.
Proton-beam therapy is an effective treatment for nonresectable iris melanomas. The major complications include cataract and glaucoma. [20]
Glaucoma filtration procedures should not be attempted in the setting of a potential iris melanoma, as they may lead to seeding of the tumor cells and metastases.
As an alternative to enucleation, whole anterior segment fractionated proton-beam radiotherapy has offered excellent local tumor control in diffuse iris melanoma. Given the limited alternatives, the rate of complications appears acceptable and visual function could be preserved in most patients during follow-up. [21]
Consultations
Iris melanoma usually needs to be treated in coordination with an ophthalmologist/oncologist, especially if a metastatic lesion is suspected. After surgery, patients must be monitored at least every 6 months for metastatic development.
Further outpatient care
Patients should receive follow-up care as needed. They need to be monitored periodically for lesion recurrence and metastatic development as warranted. In addition, the intraocular pressure should be monitored closely if the patient had developed secondary glaucoma due to the melanoma itself or the seeding of tumor through the angle.
Deterrence/prevention
Exposure to ultraviolet light should be minimized, as high levels of solar UV-B radiation has been a suggested etiology.
Prognosis
Most primary tumors of the iris are benign. The prognosis of iris melanoma is usually better than that of melanoma of the ciliary body and choroid, although there is no clear reason for this. A possible explanation is that iris melanoma is smaller than its posterior segment counterparts at the time of diagnosis. [22]
Prognosis is generally good in terms of survival. Young adults tend to have a smaller melanoma basal dimension and a lower rate of tumor-related metastasis and death than do older populations. [23]
The mortality rate ranges from 0% to 11%, depending on the cell type, the presence or absence of metastases, and ciliary body involvement; in the absence of ciliary body involvement, the rate is 0% to 3%. Metastatic rate according to cell type are 2.6% for spindle cell, 10.5% for mixed cell, and 6.9% for epithelioid cell type.
Metastases occur in 2% to 10% of all iris melanomas; a higher rate of metastasis is observed in cases of ciliary body involvement. Clinical features that may predict metastasis from iris melanoma include the following [24] :
-
Older age at the time of diagnosis
-
Secondary elevated intraocular pressure and glaucoma
-
Posterior tumor margin at the angle or iris root
-
Tumor seeding in the angle
-
Extraocular extension
-
Recurrence following prior surgical treatment of the tumor
Questions & Answers
Overview
Which patient groups have the highest prevalence of iris melanoma?
Which clinical history findings are characteristic of iris melanoma?
Which ocular exam findings are characteristic of iris melanoma?
What are the criteria for a clinical diagnosis of iris melanoma?
Which clinical features are predictive of growth from iris nevus to iris melanoma?
How are cysts, primary tumors, and primary ciliary body melanomas differentiated?
What are the potential complications of iris melanoma?
Which conditions are included in the differential diagnoses of iris melanoma?
What is the role of sequential photography in the workup of iris melanoma?
What is the role of UBM in the workup of iris melanoma?
What is the role of B-scan ultrasonography in the workup of iris melanoma?
What is the role of AS-OCT in the workup of iris melanoma?
What is the role of fluorescein angiography in the workup of iris melanoma?
Which procedures are used to confirm the diagnosis of iris melanoma?
Which histologic findings are characteristic of iris melanoma?
What is the role of surgery in the treatment of iris melanoma?
Which specialist consultations are beneficial to patients with iris melanoma?
What is included in the long-term monitoring of iris melanoma?
How are iris melanomas prevented?
What is the prognosis of iris melanoma?
-
Inferior iris melanoma of the left eye that is pushing the pupil superiorly and nasally and distorting the normal appearance of the iris. Image courtesy of MERSI, Waltham, MA.
-
B-scan ultrasonogram of a patient with an iris melanoma showing a solid echogenic nodule. Image courtesy of MERSI, Waltham, MA.








