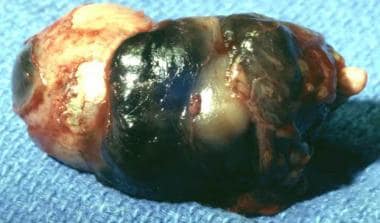
Practice Essentials
Choroidal melanoma is the most common primary malignant intraocular tumor and the second most common type of primary malignant melanoma in the body. It most often affects whites of northern European descent.
See The Case of the Middle-Aged Woman with Sudden Unilateral Vision Loss, a Critical Images slideshow, to help identify and treat malignant intraocular tumors.
Signs and symptoms
Choroidal melanomas remain asymptomatic for prolonged periods of time; they may be found incidentally during ophthalmoscopy. In general, the more anterior their origin, the longer the delay of any symptoms. Choroidal melanoma may present with the following symptoms:
-
Blurred visual acuity
-
Paracentral scotoma
-
Painless and progressive visual field loss
-
Floaters
-
Severe ocular pain
-
Weight loss, marked fatigue, cough, or change in bowel or bladder habits (suggests primary nonocular malignancy with choroidal metastasis)
Ophthalmologic examination may reveal the following:
-
Small choroidal melanomas typically take the form of a nodular, dome-shaped, and well-circumscribed mass under the retinal pigment epithelium
-
As choroidal melanomas grow, they may adopt more irregular configurations (eg, bilobular, multilobular, or mushroom shapes)
-
Diffuse choroidal melanoma, characterized by lateral growth throughout the choroid with minimal elevation, are more difficult to diagnose and often cause significant exudative retinal detachment [1]
-
Choroidal melanomas may have variable coloration, ranging from amelanotic to darkly pigmented; some are partially pigmented
-
If the tumor is light-colored, its abnormal vascularization usually can be seen ophthalmoscopically
-
Overlying the choroidal melanoma, there are usually retinal pigment epithelial changes (eg, drusen), patches of atrophy, and orange discoloration; orange changes can occur in both malignant and benign lesions
-
Choroidal melanoma may remain undetected underneath a large exudative retinal detachment, a subretinal hemorrhage, or a vitreous hemorrhage
-
Infrequent presentations of advanced choroidal melanoma are a painful blind eye with cataract and proptosis from tumor transscleral orbital extension
-
Anterior choroidal melanomas might show sentinel vessels (dilated episcleral blood vessels visible through the conjunctiva) that feed the metabolically active tumor
-
Transscleral growth of an anterior choroidal melanoma (mainly through emissary channels) may appear on examination as a small subconjunctival area of abnormal hyperpigmentation
Diagnosis
Liver enzyme levels are indicated in any patient with uveal melanoma, because the liver is the most common site of choroidal melanoma metastasis. The most sensitive tests of hepatic function are serum levels of the following:
-
Alkaline phosphatase
-
Glutamic-oxaloacetic transaminase
-
Lactate dehydrogenase
-
Gamma-glutamyl transpeptidase
Ultrasonography
-
A-scan ultrasonography of the eye is useful for tumors thicker than 2-3 mm
-
Choroidal melanoma characteristically shows an initial prominent spike, followed by low-to-medium internal reflectivity with diminishing amplitude and a significant echo
-
Vascular pulsations can be seen as fine oscillations of the internal spiking pattern within the tumor
-
Performing sequential A-scans, with accurate dimension measurements, in cases of diagnostic uncertainty is important
-
B-scan ultrasonography of the eye is a routine test used in the evaluation of any posterior segment mass; it is especially needed in patients with media opacity
-
For choroidal melanomas, B-scan ultrasonography is used to help establish the diagnosis, to evaluate possible extraocular extension, to estimate tumor size for periodic observation, and to plan therapeutic intervention
Intraocular melanomas have several distinctive features, as follows:
-
Low-to-medium reflectivity
-
Excavation of underlying uveal tissue
-
Shadowing of subjacent soft tissues
-
Internal vascularity
-
An acoustic quiet zone at the base of the tumor called acoustic hollowing
Ultrasound biomicroscopy (UBM) has the following advantages:
-
Provides excellent resolution for anterior ocular abnormalities
-
Can differentiate very anterior choroidal melanomas from those of ciliary body origin
-
Can help define the tumor’s anterior border
-
Is also helpful in assessing angle-closure glaucoma
Angiography
-
Fluorescein angiography and indocyanine green angiography do not show pathognomonic signs of choroidal melanoma but can help point to its diagnosis
-
Small choroidal melanomas may show fluorescein angiographic changes similar to some choroidal nevi, with such changes ranging from normal angiography to hypofluorescence secondary to blockage of background fluorescence
-
Larger melanomas may show a patchy pattern of early hypofluorescence and hyperfluorescence followed by late intense staining
-
Simultaneous fluorescence of retinal and choroidal circulation within the tumor is fairly distinctive of choroidal melanomas
Imaging studies
-
Obtain a chest x-ray to rule out possible lung metastases
-
CT scanning of the globe and orbit is useful for visualizing extraocular extension and may help differentiate between choroidal or retinal detachment and a solid tumor
-
MRI of the globe and orbit can be used to determine extrascleral extension of the melanoma and distinguish surrounding fluid from the tumor
Management
Several ocular modes of treatment are available for choroidal melanomas, as follows:
-
Tebentafusp, a first-in-class bispecific fusion protein, is approved for HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma
-
Observation may be acceptable for posterior uveal tumors where diagnosis is not well established; in particular, tumors of less than 2-2.5 mm in elevation and 10 mm in diameter can be observed until growth is documented
-
Enucleation is the classic approach to choroidal melanomas and has been the preferred treatment for large (basal diameter >15 mm and height >10 mm) and complicated tumors, which compromise visual function and for which other therapies tend to fail
-
Plaque brachytherapy is a widely accepted alternative to enucleation for medium-sized posterior uveal melanomas (< 10 mm in height and < 15 mm in diameter)
-
External beam irradiation with protons or helium ions is a frequently used alternative for the treatment of medium-sized choroidal melanomas, although it has been used for larger tumors
-
Pars plana vitrectomy endoresection
-
Block excision (sclerouvectomy) is an alternative treatment method reserved for small tumors covering less than one third of the globe’s circumference
-
Laser photocoagulation and transpupillary thermotherapy are used to treat selected small choroidal melanomas that are located away from the fovea and are less than 3 mm in thickness
-
Orbital exenteration is a radical treatment reserved for cases with widespread orbital extension; it should be considered only in rare cases where marked discomfort is associated with massive orbital spread of the melanoma
Adjuvant systemic chemotherapy is not advocated.
When distant metastases are found during the initial systemic workup or later in the disease course, systemic chemotherapy is the primary treatment. In addition to standard chemotherapeutic agents, several FDA-approved immunotherapy options are currently available, including immunomodulators and oncolytic virus therapy for advanced malignant melanoma.
Overview
Choroidal melanoma is the most common primary malignant intraocular tumor and the second most common type of primary malignant melanoma in the body. It is nevertheless an infrequently found tumor.
Choroidal melanoma is a subtype of uveal melanoma. Uveal melanomas can be divided into two categories: (1) anterior uveal melanomas, in which the tumor arises in the iris, and (2) posterior uveal melanomas, in which the tumor arises in either the choroid or the ciliary body. Intraocular melanomas simultaneously can involve more than one uveal structure.
The ocular tissue where these tumors arise, the uvea, is a densely pigmented layer that forms part of the wall of the eye. The uvea is subdivided into the iris, the ciliary body, and the choroid. The choroid underlies the retina and its pigment epithelium throughout the ocular fundus. The main function of the uvea is to provide oxygen and other nourishment to the highly metabolically demanding retinal photoreceptors. It is primarily a vascular tissue, with fenestrated capillaries and stroma containing melanocytes.
Go to Ciliary Body Melanoma, Conjunctival Melanoma, and Iris Melanoma for complete information on these topics.
Pathophysiology
Primary choroidal melanoma arises from melanocytes within the choroid. Most choroidal melanomas are believed to develop from preexisting melanocytic nevi, though de novo growth of choroidal melanomas also occurs. Three distinct cell types are recognized in choroidal and other uveal melanomas: (1) spindle A, (2) spindle B, and (3) epithelioid. The last cell type usually has the most aggressive behavior and carries a poorer prognosis for the patient’s long-term survival.
Choroidal melanomas may have variable coloration, ranging from darkly pigmented to purely amelanotic. They typically are dome-shaped. As they enlarge, if they break through the Bruch membrane, they can assume a mushroom configuration. Other shapes found for these tumors are bilobular, multilobular, and diffuse. The last of these is characterized by lateral growth throughout the choroid with minimal elevation; it occurs in about 5% of cases. Rarely, choroidal melanomas may arise in a multicentric distribution in 1 or both eyes.
Choroidal melanomas affect the retinal pigment epithelium as they push against it and deprive it of normal choroidal circulation. Overlying retinal pigment epithelium usually develops areas of atrophy, drusen, and localized pigment epithelial detachments.
Areas of phagocytic activity, where cellular debris from melanocytes is digested, give the pigment epithelium patches of coloration change. Macrophages within these typically orange areas contain melanin and lipofuscin. These changes can lead to choroidal neovascularization over the tumor, with consequent subretinal exudation, hemorrhage, and fibrous plaque formation.
Growth of choroidal melanomas can occur silently until it produces enough visual loss through various mechanisms. [2] The tumor’s disruption of choroidal circulation and consequent ischemia typically cause degeneration of retinal photoreceptors and other retinal neurons. The retina overlying the tumor can separate into cystoid spaces and larger schisis cavities. There may be associated cystoid macular edema.
In general, the farther the tumor’s origin is from the optic nerve and fovea, the larger the tumor can become before the patient notices a visual field defect. Exudation of fluid into the subretinal space with consequent retinal detachment may enlarge the field loss. This exudation can lead to total retinal detachment. Rarely, choroidal melanomas can impinge into underlying posterior ciliary nerves, causing severe ocular pain.
Other signs and symptoms can result if the tumor grows anteriorly, pathologically involving the ciliary body, trabecular meshwork, and lens, with consequent ocular hypotension or hypertension and cataract. Large choroidal melanomas can induce iris rubeosis. Erosion of the melanoma into blood vessels in adjacent tissues, or areas of necrosis within the tumor, can lead to vitreous hemorrhage or hyphema.
Choroidal melanoma ultimately causes death, practically always secondary to distant metastases rather than local spread. Its metastatic potential depends on the histopathologic aggressiveness of the tumor cells. Unfortunately, it not infrequently metastasizes before diagnosis. If the melanoma does not show extraocular extension, it can only spread hematogenously, because there are no lymphatic vessels in the eye. It most often metastasizes to the liver; other organs of dissemination include the lung, bone, skin, and central nervous system (CNS).
Less frequently, choroidal melanoma can grow transsclerally, through emissary channels, and metastasize locally into the orbit or rarely the conjunctiva. Choroidal melanoma almost never extends through the optic nerve; when it does, it is usually in juxtapapillary tumors or in diffuse choroidal melanomas.
Etiology
A particular predisposition exists for choroidal melanomas to occur in people with light-colored irides. Evidence points to sunlight exposure as a likely contributor to the development of choroidal melanoma.
Predisposing diseases for uveal melanomas include a family history of uveal melanoma, uveal nevus, congenital ocular melanocytosis, dysplastic nevus syndrome, and xeroderma pigmentosum.
Epidemiology
United States statistics
Incidence of primary choroidal melanoma is about 6 cases per 1 million population. Perhaps because of increased sunlight exposure, there appears to be a higher incidence of uveal melanoma in the southern latitudes of the United States. Alternatively, this might be the effect of a tendency of older Americans to retire in the South.
International statistics
Incidence of choroidal melanoma is much higher in countries with large numbers of people of northern European descent than elsewhere in the world. In Denmark and other Scandinavian countries, incidence is about 7.5 cases per million per year.
Age-related differences in incidence
Incidence of choroidal melanoma is highest around age 55 years. In Asians, although it is a very infrequent tumor, reports indicate a peak incidence at a somewhat younger age. Choroidal melanoma is exceptional in children.
Sex-related differences in incidence
Choroidal melanoma is found slightly more frequently in men for all age groups, except from 20-39 years, when a small predilection exists for women.
Race-related differences in incidence
Choroidal melanoma and other uveal melanomas most often affect whites of northern European descent. Incidence of choroidal melanoma among blacks is extremely rare. Hispanics and Asians are thought to have a small but intermediate risk compared to whites and blacks.
Clinical Presentation
Patient history
Choroidal melanomas remain asymptomatic for prolonged periods of time; they may be found incidentally during ophthalmoscopy. In general, the more anterior their origin, the longer the delay of any symptoms. Choroidal melanoma may present with the following symptoms:
-
Blurred visual acuity consequent to various mechanisms, including growth of the melanoma into the subfoveal retina, cystoid macular edema, retinal detachment, vitreous hemorrhage, cataract, and blockage of the visual axis directly by the tumor
-
Paracentral scotoma, occurring as the tumor affects the perifoveal retina
-
Painless and progressive visual field loss, occurring as peripheral melanoma grows or exudates subretinal fluid
-
Floaters, developing when areas of necrosis within the tumor or adjacent structures produce vitreous hemorrhage or hyphema
-
Severe ocular pain, occasionally occurring secondary to impingement of choroidal melanomas on posterior ciliary nerves or to high intraocular pressure from acute angle-closure glaucoma
-
History of weight loss, marked fatigue, cough, or change in bowel or bladder habits, which should prompt consideration of primary nonocular malignancy with choroidal metastasis
Physical examination
Patients with choroidal melanoma may present with painless visual loss or, occasionally, inflammation and pain from a complicated tumor. However, many patients have no symptoms, and melanomas are discovered on routine ophthalmologic examination.
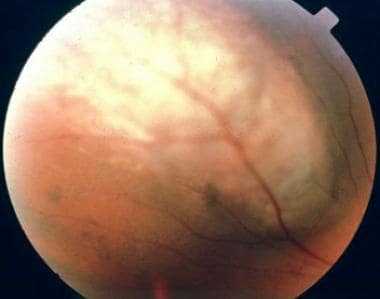
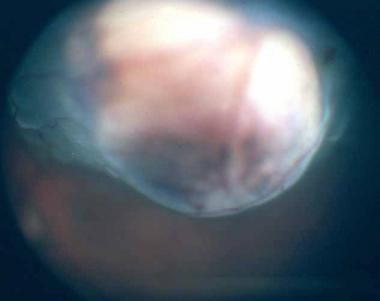
Small choroidal melanomas typically take the form of a nodular, dome-shaped, and well-circumscribed mass under the retinal pigment epithelium. As they grow, they may adopt more irregular configurations (eg, bilobular, multilobular, or mushroom shapes).
An unusual tumor type that may be found is diffuse choroidal melanoma, characterized by lateral growth throughout the choroid with minimal elevation. Diffuse melanomas are more difficult to diagnose and often cause significant exudative retinal detachment. [1]
Choroidal melanomas may have variable coloration, ranging from amelanotic to darkly pigmented. Some tumors are partially pigmented. If the tumor is light-colored, its abnormal vascularization usually can be seen ophthalmoscopically. Overlying the choroidal melanoma there usually are retinal pigment epithelial changes (eg, drusen), patches of atrophy, and orange discoloration. Orange changes in the pigment epithelium traditionally have been regarded as strongly indicative of malignancy; however, it is now well known that they can be seen over benign lesions as well.
Sometimes, a choroidal melanoma may remain undetected underneath a large exudative retinal detachment, a subretinal hemorrhage, or a vitreous hemorrhage. Infrequent presentations of advanced choroidal melanoma are a painful blind eye with cataract and proptosis from tumor transscleral orbital extension.
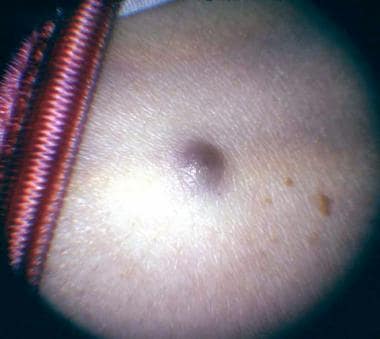
Anterior choroidal melanomas might show sentinel vessels (dilated episcleral blood vessels visible through the conjunctiva) that feed the metabolically active tumor. Transscleral growth of an anterior choroidal melanoma (mainly through emissary channels) may appear on examination as a small subconjunctival area of abnormal hyperpigmentation.
Transillumination can be used to find the borders of the tumor, especially if it is surrounded by exudative retinal detachment. Its precision is dependent on even tumor pigmentation and if associated hemorrhage is present.
Overall accuracy of the clinical diagnosis of choroidal melanoma, using modern diagnostic tools, was shown to be 99.7% in a preliminary report from the Collaborative Ocular Melanoma Study. [3] Thorough evaluation of choroidal melanoma should include a complete physical examination, with particular attention to the hepatic-abdominal region and the skin and subcutaneous tissues, which are frequent sites of metastatic spread.
Differential Diagnosis
The differential diagnosis includes the following:
-
Choroidal Detachment
-
Intraocular Foreign Body
-
Chronic Angle Closure Glaucoma
-
Glaucoma, Hyphema
-
Neovascular Glaucoma
-
Cavernous Hemangioma
-
Vitreous Hemorrhage
-
Hyphema
-
Ciliary Body Melanoma
-
Conjunctival Melanoma
-
Iris Melanoma
Benign and malignant tumors, cysts, and other abnormal masses in the choroid, retina, and pigment epithelium must be distinguished from choroidal melanomas. Other problems to be considered include the following:
-
Melanocytic nevus
-
Melanocytoma
-
Metastatic tumors
-
Medulloepithelioma (diktyoma)
-
Choroidal osteoma
-
Adenoma
-
Adenocarcinoma
-
Combined hamartoma of the retina and pigment epithelium
-
Congenital hypertrophy and reactive hyperplasia of the retinal pigment epithelium
-
Retinal cavernous hemangioma
-
Presumed acquired retinal hemangioma
-
Lymphoid tumor
-
Hemangiopericytoma
-
Leiomyoma
-
Neurofibroma
-
Glioneuroma
-
Astrocytoma
-
Rhabdomyosarcoma
-
Posterior uveitis
-
Sarcoid nodules
-
Tubercular granuloma
Other choroidal masses can present with characteristics very similar to those of choroidal melanoma, making it a challenging diagnosis.
Laboratory Studies
The most common site of choroidal melanoma metastasis is the liver. Liver enzyme levels are indicated in any patient with uveal melanoma. The most sensitive tests of hepatic function are serum levels of alkaline phosphatase, glutamic-oxaloacetic transaminase, lactate dehydrogenase, and gamma-glutamyl transpeptidase.
These test results are negative at the time of diagnosis in most patients with choroidal melanoma. If any of these laboratory test results are abnormal, order ultrasonography and computed tomography (CT) of the liver. Unfortunately, both imaging modalities have low sensitivity for metastasis smaller than 10-20 mm in diameter.
Ultrasonography
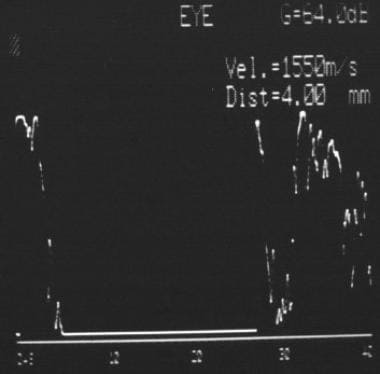
A-scan ultrasonography of the eye is useful for tumors thicker than 2-3 mm. Choroidal melanoma characteristically shows an initial prominent spike, followed by low-to-medium internal reflectivity with diminishing amplitude and a significant echo (see the image below). Vascular pulsations can be seen as fine oscillations of the internal spiking pattern within the tumor. Standardized ultrasonography has a diagnostic accuracy of more than 95%. Performing sequential A-scans, with accurate dimension measurements, in cases of diagnostic uncertainty is important.
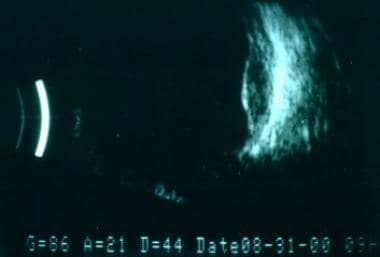
B-scan ultrasonography of the eye is a routine test used in the evaluation of any posterior segment mass. It is especially needed in patients with media opacity. For choroidal melanomas, B-scan ultrasonography is used to help establish the diagnosis, to evaluate possible extraocular extension, to estimate tumor size for periodic observation, and to plan therapeutic intervention.
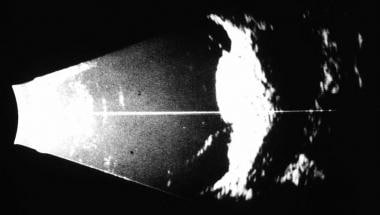 B-scan ultrasound showing acoustic hollowing in intraorbital extension of a posterior choroidal melanoma.
B-scan ultrasound showing acoustic hollowing in intraorbital extension of a posterior choroidal melanoma.
Intraocular melanomas have several distinctive features, as follows:
-
Low-to-medium reflectivity
-
Excavation of underlying uveal tissue
-
Shadowing of subjacent soft tissues
-
Internal vascularity
-
An acoustic quiet zone at the base of the tumor called acoustic hollowing
Ultrasound biomicroscopy (UBM) uses high-frequency waves, with excellent resolution for anterior ocular abnormalities. It can differentiate very anterior choroidal melanomas from those of ciliary body origin and can help define the tumor’s anterior border. It is also helpful in assessing angle-closure glaucoma.
Angiography and Radiography
Fluorescein angiography and indocyanine green angiography do not show pathognomonic signs of choroidal melanoma but can help point to its diagnosis.
Small choroidal melanomas may show fluorescein angiographic changes similar to some choroidal nevi, with such changes ranging from normal angiography to hypofluorescence secondary to blockage of background fluorescence. Larger melanomas may show a patchy pattern of early hypofluorescence and hyperfluorescence followed by late intense staining.
Some choroidal melanomas demonstrate intrinsic vascularization, visible throughout the angiogram. The angiographic sign, called the “double circulation pattern,” refers to simultaneous fluorescence of retinal and choroidal circulation within the tumor. When it occurs, it is fairly distinctive of choroidal melanomas.
Obtain a chest x-ray in patients with choroidal melanomas to rule out possible lung metastases.
Computed Tomography
Computed tomography (CT) scanning of the globe and orbit is more expensive than ultrasonography and is not as sensitive. It is useful for visualizing extraocular extension and may help differentiate between choroidal or retinal detachment and a solid tumor.
CT scan requires intravenous injection of contrast material. Choroidal melanoma shows enhancement with contrast, whereas exudation does not. CT scan also is sensitive in detecting calcium, a feature of some tumors that are different than uveal melanomas (characteristically choroidal osteoma).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) of the globe and orbit is more expensive than computed tomography (CT) scanning. Use of surface coil imaging and gadolinium as a contrast material greatly improves its resolution, but it still remains less sensitive than ultrasonography. Pigmented melanomas are seen as a high-density image in T1 and as a low-density image in T2. MRI also can be used to determine extrascleral extension of the melanoma and distinguish surrounding fluid from the tumor.
Biopsy
Fine-needle biopsy and incisional biopsy usually are not required but may be helpful in difficult diagnostic cases, particularly for distinguishing amelanotic melanomas from metastatic tumors, and in situations where the results of other ancillary tests are equivocal. Fine-needle biopsy is increasingly being performed for prognostic purposes.
In experienced hands, both biopsy techniques have an accuracy of more than 95% in tumors larger than 3 mm. Incisional biopsy is more invasive and may have more associated complications, but it has less false-negative and false-positive results. The most common complication for tumor biopsy is intralesional or perilesional hemorrhage.
Risk of spread of cancerous cells in the case of fine-needle biopsy is small for choroidal melanoma (unlike retinoblastoma). Follow biopsy with prompt treatment to prevent extrascleral extension.
Genetic analysis and karyotyping of biopsy specimens have gained increasing attention. Chromosome 3 monosomy and/or amplification of chromosome 8q in the choroidal tumor has been shown to be associated with a significantly greater risk of developing metastases. [4]
Loss of chromosome 1p and gain of chromosome 6p have been linked with a better prognosis.
Specific genetic errors are progressively better understood, such as inactivation of BAP1 (BRCA-associated protein 1), which is associated with metastatic spread, often through monosomy 3.
Gene expression profiling (GEP) outperforms monosomy 3 at predicting metastatic spread. This test is commercially available through DecisionDx-UM (Castle Biosciences).
Analysis of multiple gene expression has led to the subdivision of uveal melanomas into two types. Around half of ocular melanomas are class 1, which carry a low risk of metastasis. The other half are class 2, which have a different gene expression pattern, frequently showing chromosome 3 monosomy, and carry a high risk of metastasis. Class 2 tumors have been associated with previously known metastatic risk prognostic features, including ciliary body involvement, larger basal diameter, older age, presence of exudative retinal detachment, and increased tumor height.
Class 1 tumors are further subdivided into class 1a (lowest metastatic risk: low expression of genes CDH1 and RAB31) and class 1b (moderate metastatic risk: high expression of genes CDH1 and RAB31). Class 1b tumors tend to occur in younger individuals and have a posterior location compared with class 1a tumors.
Onken et al, as part of the Collaborative Ocular Oncology Group, evaluated the prognostic performance of a 15-gene expression profiling assay that assigns primary posterior uveal melanomas to prognostic subgroups: class 1 (low metastatic risk) and class 2 (high metastatic risk). A total of 459 patients with posterior uveal melanoma were enrolled from 12 independent centers. Tumor tissue was classified by GEP as class 1 or class 2. Patients were managed for their primary tumor and monitored for metastasis. The GEP was class 1 in 276 cases (61.9%) and class 2 in 170 cases (38.1%). Metastasis was detected in 3 (1.1%) class 1 cases and 44 (25.9%) class 2 cases. GEP class had a stronger independent association with metastasis than any other prognostic factor, including tumor size. [5]
This GEP assay can provide prognostic information in eyes with malignant melanoma that have been enucleated.
However, because no effective treatment is available for metastatic disease, the clinical impact of performing routine biopsies on choroidal melanoma is unclear at this point.
Histologic Findings
Histologic evaluation of the tumor after enucleation can confirm the diagnosis and determine the prognosis. [6]
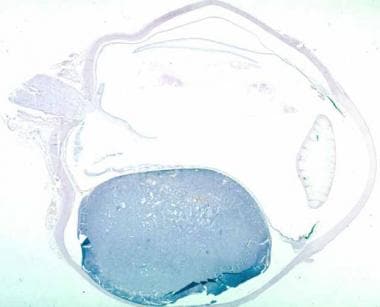
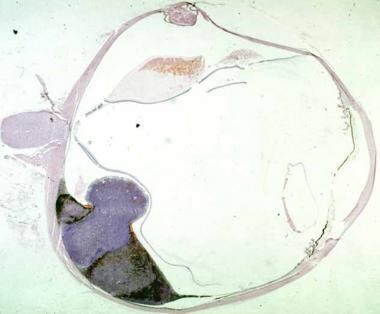 Histologic section of an enucleated eye showing a medium-sized mushroom-shaped choroidal melanoma with associated exudative retinal detachment.
Histologic section of an enucleated eye showing a medium-sized mushroom-shaped choroidal melanoma with associated exudative retinal detachment.
As noted (see Pathophysiology), 3 distinct cell types are recognized to occur in uveal melanomas: (1) spindle A, (2) spindle B, and (3) epithelioid. Spindle A cells have elongated nuclei and uncommonly have mitotic figures.
Spindle B cells have a prominent nucleolus. They are found more commonly and also have an elongated profile but are slightly larger than spindle A cells.
Epithelioid melanoma cells are highly anaplastic, poorly cohesive, and have considerable morphologic variation. They tend to resemble epithelial cells and contain frequent mitotic figures.
The most commonly used histologic classification of uveal melanomas is the modified Callender classification, which divides uveal melanocytic tumors into the following groups:
-
Spindle cell nevi
-
Spindle cell melanomas
-
Necrotic melanomas
-
Epithelioid cell melanomas
-
Mixed cell melanomas
The last 2 carry the poorest survival prognoses. Evaluation of vascular supply of the tumor, age at presentation, presence of extrascleral extension, tumor size, tumor cell types, mitotic rate, nucleolar area, and quantification of nucleolar organizer regions have been used for prognostic purposes.
Treatment & Management
Several modes of treatment are available for choroidal melanomas. Multiple factors are taken into account when deciding one approach over other approaches, such as visual acuity of the affected eye, visual acuity of the contralateral eye, size of the tumor, age and general health of the patient, ocular structures involved, and presence of metastases.
Observation may be acceptable for posterior uveal tumors where diagnosis is not well established. In particular, tumors of less than 2-2.5 mm in elevation and 10 mm in diameter can be observed until growth is documented. Photography and sequential ultrasonography for precise measuring of the tumor’s dimensions are usually necessary.
Choice of treatment of choroidal melanoma remains controversial in many respects. Although enucleation has been the treatment of choice in the past, it appears that vision-sparing approaches might offer similar degrees of ocular and metastatic tumor control—particularly because it is clear that in many patients at the time of diagnosis, posterior uveal melanomas already have spread through micrometastasis.
Early treatment versus watchful waiting
In a prospective cohort study of 3072 patients with choroidal melanoma, researchers found that, for some patients, early treatment, rather than watchful waiting, might better prevent metastatic death. [7, 8]
For the study, researchers correlated age at death, cause of death, age at treatment, and survival predictors. Tumors ranged in size from 2.4 to 23.8 mm in basal diameter and from 0.6 to 18.3 mm in thickness, and the largest basal diameter correlated with all survival predictors except chromosome 6p gain. Older age at treatment was correlated with largest basal tumor diameter, tumor thickness, TNM stage, ciliary body involvement, extraocular spread, chromosome 3 loss, and chromosome 8q gain. A total of 1005 patients died by the end of the study, and 561 of these patients died from metastatic disease from uveal melanoma. Median age at death was lower (68.6 years) for patients who died from metastatic melanoma than for patients who died from any cause (74.0 years). None of the survival predictors correlated with age at death among the patients who died of metastasis except for mitotic count, which had a weak correlation. [7, 8]
Systemic chemotherapy
Although undetected metastatic spread at the time of diagnosis and treatment of choroidal melanoma is a major concern in every patient, adjuvant systemic treatment is not advocated. This consensus comes from treatment trials with intraocular melanomas and extrapolation of the experience with cutaneous melanoma, where adjuvant treatment has shown no benefit.
When distant metastases are found during the initial systemic workup, treatment of the intraocular melanoma itself becomes palliative. Prior to 2011, systemic chemotherapy was the primary treatment for metastatic malignant melanoma discovered either at the initial workup or later in the disease course. Traditional chemotherapy types are no longer used as first-line therapy. Agents previously used individually or in combination included dacarbazine, temozolomide, cisplatin, fotemustine, lomustine, the taxanes, and vinblastine. Unfortunately, these approaches have had only minimal success in improving overall survival.
Liver-directed therapies have been used to treat hepatic metastasis with encouraging results in several trials. These include transarterial liver chemoembolization, intra-arterial chemotherapy with fotemustine, and isolated hepatic perfusion. [9]
Immunotherapy and targeted therapy for metastatic choroidal melanoma
Since 2011, multiple novel systemic therapies for metastatic skin melanoma (stage IV) have become available. These include immune checkpoint inhibitors and targeted therapy, which have led to durable responses and some improvement in overall survival and quality of life.
Several FDA-approved immunotherapy options are currently available for cutaneous melanoma. These include the immunomodulator checkpoint inhibitors ipilimumab, nivolumab, and pembrolizumab; the cytokines interferon alpha-2b and peginterferon alpha-2b; and oncolytic virus therapy with T-VEC.
Immunotherapy has dramatically improved outcomes for patients with advanced skin melanoma, but this clinical benefit has not been observed in metastatic uveal melanoma. Currently, FDA-approved targeted therapies for cutaneous melanoma includes the oral BRAF inhibitors dabrafenib (Tafinlar) and vemurafenib (Zelboraf) for melanomas with a mutated or activated BRAF gene and the oral MEK inhibitor trametinib for patients who have melanoma with a BRAF V600E or V600K mutation. BRAF and MEK inhibitors are usually given in combination. Other targeted therapies in clinical trials include KIT inhibitors. Tumor-infiltrating lymphocyte (TIL) immunotherapy is also under investigation for the treatment of metastatic melanoma. [10, 11] This is an area of intense medical research, with ever-increasing degrees of biological sophistication being applied to new clinical trials.
Tebentafusp
A new form of immunotherapy specific for uveal melanoma, tebentafusp (Kimmtrak), uses a bispecific fusion protein comprised of a soluble T cell receptor fused to an anti-CD3 immune-effector function, thus directing T-cells. It is the first drug approved for treatment of HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma. [12]
The mechanism of action of tebentafusp works by placing T cells in proximity to melanoma cells by targeting the gp100 protein, a lineage antigen expressed in melanocytes and melanoma. Approval was based on results from the phase 3 IMCgp100-202 trial assessing survival in naïve patients with metastatic uveal melanoma, was randomized 2:1 to receive tebentafusp or the investigator’s choice among dacarbazine, ipilimumab, or pembrolizumab. Overall survival was statistically significantly improved in patients randomized in the experimental group compared to the control group in the first pre-planned interim analysis (estimated 1-year survival rate of 73% for the study drug versus 59% with the investigator’s choice). Progression-free survival was also significantly higher in the tebentafusp group than in the control group (31% vs. 19% at 6 months; P = 0.01). [13]
Enucleation
Enucleation is the classic approach to choroidal melanomas and has been the preferred treatment for large (basal diameter >15 mm and height >10 mm) and complicated tumors, which compromise visual function and for which other therapies tend to fail.
Because of potential release of malignant cells into the bloodstream and orbital soft tissues during the surgical procedure, manipulation of the globe should be kept to a minimum. Particular care must be taken to avoid perforation of the globe during surgery. If transscleral extension is found (see the image below), the tumor should be removed in one piece, followed by cryotherapy of the involved orbital soft tissues.
The theoretical advantage of enucleation over globe-sparing treatments is a reduced risk of metastatic spread. However, the Collaborative Ocular Melanoma Study (COMS), in which medium-sized tumors were treated with either iodine-125 brachytherapy (see below) or enucleation, found that the mortality rates after brachytherapy did not differ from the mortality rates following enucleation for up to 12 years after treatment. [14, 15]
Some investigators have advocated pre-enucleation irradiation of the eye as a way to improve survival. However, the COMS demonstrated neither a positive nor a negative effect on the 10-year mortality rates among patients whose eyes containing large choroidal melanomas were randomly assigned to treatment with enucleation alone or to enucleation preceded by external radiation. [16]
Plaque brachytherapy
Plaque brachytherapy is a widely accepted alternative to enucleation for medium-sized posterior uveal melanomas (< 10 mm in height and < 15 mm in diameter).
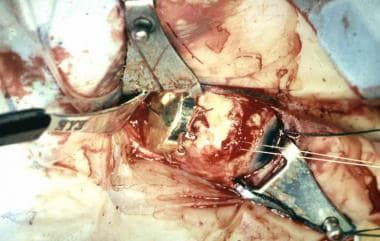 Intraoperative photograph showing placement of a radioactive plaque for posterior choroidal melanoma.
Intraoperative photograph showing placement of a radioactive plaque for posterior choroidal melanoma.
Plaques containing various radioactive isotopes (eg, iridium, cobalt, and ruthenium) have been used. The most common material used in modern plaques is iodine-125, because of its lower energy emission (lack of alpha and beta rays), its good tissue penetration, and its commercial availability.
Radiation from this source causes tumor destruction through damage of DNA in cancerous cells and tumor vessels, with consequent tumor necrosis and regression. However, it is not devoid of complications. Detorakis et al found that after iodine-125 brachytherapy for choroidal melanoma, iris and anterior chamber angle neovascularization developed in 23% of eyes. [17]
A computerized calculation is used to determine the dose and the duration of plaque application for a radiation delivery of approximately 400 Gy to the base and 80-100 Gy to the apex of the tumor, at 50-125 cGy/h.
The basal size of the melanoma is estimated preoperatively and confirmed during surgery. Appropriately sized plaques are sutured temporarily to the sclera and limbus underlying the melanoma. A margin of 2 mm over the largest tumor basal dimension is adequate. Intraoperative techniques, such as transillumination or ultrasonography, are used to ensure proper plaque placement under the tumor.
Postoperative imaging confirmation of correct plaque localization is required. Radioactive plaques are left in place for 3-7 days. The goal of successful treatment is to achieve arrest of tumor growth or regression in size.
Local recurrence, usually requiring enucleation, occurs at a rate of about 12-16%. Plaque brachytherapy can cause complications, including cataract, rubeosis, scleral necrosis, keratopathy, radiation retinopathy, and optic neuropathy, but at a reduced rate compared with external beam irradiation.
A multicenter randomized trial from the COMS Group that addressed conservative management revealed that patient survival after treatment of medium-sized melanoma is similar when plaque radiotherapy is compared with enucleation. [18]
External beam irradiation
External beam irradiation using charged particles, either protons or helium ions, is a frequently used alternative method for the treatment of medium-sized choroidal melanomas (< 10 mm in height and < 15 mm in diameter), although it has been used for larger tumors. Its indications and success rates are similar to those of plaque brachytherapy. [19]
After conjunctival incision and localization of the melanoma with transillumination, radiopaque tantalum rings usually are sutured to the sclera to serve as reference markers for alignment of the radiation beam. A collimated beam delivers about 70 Gy, usually divided into five sessions.
Vital ocular structures are avoided through careful positioning of the head and eye. Irradiation causes damage of DNA in cancerous cells and tumor vessels, much as in plaque brachytherapy, with consequent tumor necrosis and regression. Treatment may be complicated by exudative retinal detachment, radiation cataract, dry eye syndrome, epithelial keratopathy, rubeosis, radiation retinopathy, and optic neuropathy.
Patients treated with external beam irradiation seem to have a survival rate comparable to that of patients treated with enucleation. Treatment is successful when it achieves arrest of tumor growth or regression in size. About 10-15% of eyes ultimately require enucleation, often because of neovascular glaucoma or local recurrence.
Pars plana vitrectomy endoresection
In a nested case-control study, endoresection for posterior choroidal melanomas provided outcomes similar to or better than those obtained with iodine-125 plaque brachytherapy. [20] Metastatic spread was observed in 11 (20.4%) of the 54 patients in the iodine-125 brachytherapy group, compared with only 1 (3.7%) of the 27 patients in the endoresection group. No significant difference was found in overall or relapse-free survival between the 2 groups.
Block excision
Block excision (sclerouvectomy), is an alternative treatment method for choroidal melanomas. It is reserved for small tumors covering less than one third of the globe’s circumference.
The goal of block excision is to salvage the eye, with most of these patients retaining some useful vision. It consists of full-thickness excision with in-block removal of tumor, choroid, retina, and sclera. A 3-mm margin of healthy tissue around the melanoma is included, followed by closure with a graft of banked sclera. Surround treatment with cryotherapy or laser usually is added.
The most common complications are vitreous hemorrhage, retinal detachment, residual tumor, and cataract. Risks are improved by a modified approach, lamellar sclerouvectomy, which uses a partial-thickness scleral flap and minimizes altering the retina and vitreous. In about 15-20% of cases, local reappearance of the melanoma necessitates subsequent treatment, usually enucleation.
Laser photocoagulation and transpupillary thermotherapy
Laser photocoagulation and transpupillary thermotherapy are used to treat selected small choroidal melanomas, when the lesions are located away from the fovea and are less than 3 mm in thickness.
Orbital exenteration
Orbital exenteration is a radical treatment reserved for cases with widespread orbital extension. Patients with such advanced melanomas are likely to have extensive distant metastases and poor prognoses, with or without orbital exenteration surgery. The usefulness of such disfiguring surgery is not established; the procedure should be considered only in rare cases where marked discomfort is associated with massive orbital spread of the melanoma.
Consultations
It may be necessary to consult an oncologist and a radiation oncologist.
Further outpatient care
Irrespective of the treatment modality chosen, patients with choroidal melanomas need to be observed carefully and for many years. This is particularly true for small tumors, when the diagnosis is not established clearly. Close observation and measurement of the dimensions of the tumors with any of the diagnostic tools mentioned earlier is critical.
Repeat examinations usually are performed about every 3 months initially, and if no changes are seen, follow-up care is performed every 6 months. If growth of the lesion is detected, consider further treatment. Choroidal melanomas may show size regression starting several months after being treated with external beam irradiation or plaque brachytherapy. The goal of successful treatment is not necessarily reduction in size but long-term arrest of the tumor’s growth.
Repeat examinations and imaging tests are performed after all treatment modalities because of the possibility of intraocular or extraocular tumor recurrence. Follow-up care in patients with treated choroidal melanomas should include thorough physical examinations, liver function tests, and imaging of lungs, repeated about every 6-12 months. Early detection of distant metastases may affect management and survival.
Deterrence/prevention
Patients with choroidal nevi, a family history of uveal melanoma, congenital ocular melanocytosis, dysplastic nevus syndrome, and other predisposing conditions of uveal melanoma may benefit from annual careful ophthalmologic examinations.
Limiting excessive ocular sunlight exposure through sunglasses or other means may have a theoretical preventive effect in patients with a predisposition to intraocular melanoma.
Prognosis
Visual prognosis is guarded for choroidal melanomas. Choroidal melanoma normally leads to partial or total visual loss in the affected eye. This is the result of either tumor destruction of ocular structures or consequence to the treatment used. Patients with small- to medium-sized choroidal melanomas may be able to preserve very good central vision, even after treatment. [2]
Choroidal melanoma is a disease with a high mortality rate, usually irrespective of the chosen treatment modality. About 30-50% of patients with choroidal melanoma will die within 10 years from diagnosis and treatment. Death is usually secondary to distant metastases, and the risk is greatest in larger tumors.
For large melanomas, the Collaborative Ocular Melanoma Study found that the 10-year rates of death secondary to metastasis were 45% in the pre-enucleation radiation group and 40% in the enucleation alone group. There appears to be no survival benefit attributable to pre-enucleation radiation. The maximum diameter of the base of the tumor and older age were the primary predictors of time to death in patients with melanoma metastasis.
Previous publications have found several tumor features to correlate with increased mortality, including larger size, anterior location, transscleral extension, growth through the Bruch membrane, optic nerve extension, lack of pigmentation, and histologic characteristics (eg, mitotic activity and cell type). [21] Although metastases from the primary intraocular melanoma can first be detected years later, their highest incidence is in the first year after diagnosis. As yet, no effective treatment exists for metastatic uveal melanoma.
Questions & Answers
Overview
How is choroidal melanoma categorized?
What are the signs and symptoms of choroidal melanoma?
Which findings on ophthalmologic exam are characteristic of choroidal melanoma reveal?
What is the role of liver enzyme tests in the workup of choroidal melanoma?
When is A-scan ultrasonography indicated in the workup of choroidal melanoma?
When is B-scan ultrasonography indicated in the workup of choroidal melanoma?
Which ultrasound findings are characteristic of intraocular choroidal melanoma?
What are the advantages of ultrasound biomicroscopy (UBM) in the workup of choroidal melanoma?
Which findings on angiography are characteristic of choroidal melanoma?
Which imaging studies are performed in the workup of choroidal melanoma?
How is choroidal melanoma treated?
What is the pathophysiology of choroidal melanoma?
What causes choroidal melanoma?
What is the prevalence of choroidal melanoma in the US?
What is the global prevalence of choroidal melanoma?
Which age groups have the highest prevalence of choroidal melanoma?
What are the sexual predilections of choroidal melanoma?
What are the racial predilections of choroidal melanoma?
Which clinical history findings are characteristic of choroidal melanoma?
Which physical findings are characteristic of choroidal melanoma?
Which conditions are included in the differential diagnoses of melanoma?
What is the role of lab tests in the workup of choroidal melanoma?
What is the role of ultrasonography in the workup of choroidal melanoma?
What are the distinctive features of intraocular choroidal melanoma on ultrasound?
What is the role of angiography in the workup of choroidal melanoma?
What is the role of radiography in the treatment of choroidal melanoma?
What is the role of CT scanning in the treatment of choroidal melanoma?
What is the role of MRI in the treatment of choroidal melanoma?
What is the role of biopsy in the workup of choroidal melanoma?
Which histologic findings are characteristic of choroidal melanoma?
What is the modified Callender classification for choroidal melanoma histology?
What is the role of enucleation in the treatment of choroidal melanoma?
What is the role of plaque brachytherapy in the treatment of choroidal melanoma?
What are the factors used to determine treatment selection for choroidal melanoma?
What is the role of watchful waiting in the treatment of choroidal melanoma?
What is the efficacy of early treatment of choroidal melanoma?
What is the role of systemic chemotherapy in the treatment of choroidal melanoma?
What is the role of immunotherapy in the treatment of choroidal melanoma?
What is the role of external beam irradiation in the treatment of choroidal melanoma?
What is the role of endoresection in the treatment of choroidal melanoma?
What is the role of block excision in the treatment of choroidal melanoma?
What is the role of orbital exenteration in the treatment of choroidal melanoma?
Which specialist consultations are beneficial to patients with choroidal melanoma?
What is included in long-term monitoring of choroidal melanoma?
How is choroidal melanoma prevented?
What is the prognosis of choroidal melanoma?
-
Color photograph of a dome-shaped choroidal melanoma.
-
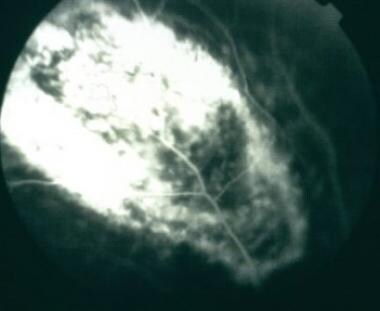
Early fluorescein angiogram of choroidal melanoma showing intrinsic vascularity.
-
Late fluorescein angiogram of choroidal melanoma showing early diffuse staining.
-
B-scan ultrasound showing acoustic hollowing and uveal excavation in posterior choroidal melanoma.
-
A-scan ultrasound of choroidal melanoma showing low-to-medium internal reflectivity.
-
B-scan ultrasound showing acoustic hollowing in intraorbital extension of a posterior choroidal melanoma.
-
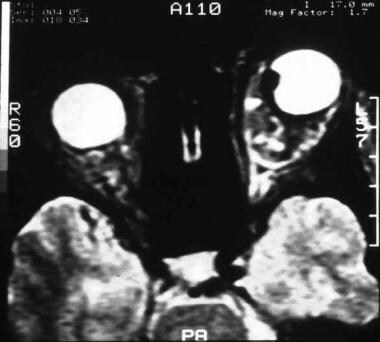
T2-weighted MRI showing a small anterior choroidal melanoma in the left eye.
-
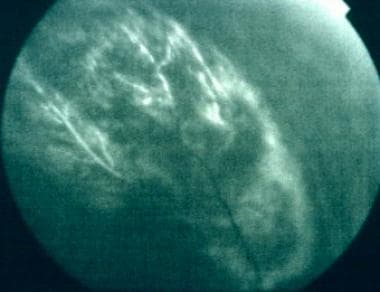
Transpupillary photograph showing a posterior choroidal melanoma.
-
Photograph showing an enucleated eye with advanced choroidal melanoma with transscleral extension.
-
Histologic section of an enucleated eye showing a large dome-shaped choroidal melanoma.
-
Histologic section of an enucleated eye showing a medium-sized mushroom-shaped choroidal melanoma with associated exudative retinal detachment.
-
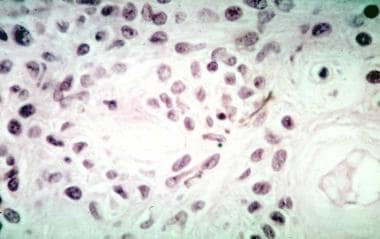
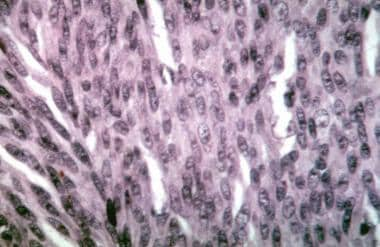
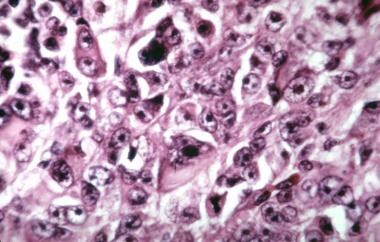
Choroidal melanoma. Histologic section showing spindle A cells in a uveal nevus.
-
Choroidal melanoma. Histologic section showing spindle B cells in a uveal melanoma.
-
Choroidal melanoma. Histologic section showing epithelioid cells in a uveal melanoma.
-
Photograph showing a skin metastasis of a posterior choroidal melanoma.
-
Intraoperative photograph showing placement of a radioactive plaque for posterior choroidal melanoma.
Tables
What would you like to print?
- Practice Essentials
- Overview
- Pathophysiology
- Etiology
- Epidemiology
- Presentation
- Differential Diagnosis
- Laboratory Studies
- Ultrasonography
- Angiography and Radiography
- Computed Tomography
- Magnetic Resonance Imaging
- Biopsy
- Histologic Findings
- Treatment
- Prognosis
- Questions & Answers
- Show All
- Media Gallery
- References