Background
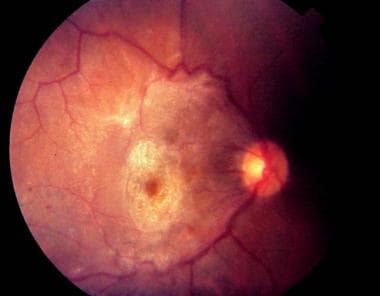
An epiretinal membrane (ERM) is a collection of collagenous cells that occurs on the inner surface of the central retina. These membranes have contractile properties and can lead to visual changes and metamorphopsia because of their effect on the underlying retina. See the image below.
This ocular pathology was first described by Iwanoff in 1865, and it has been shown to be a relatively common entity, occurring in as much as 34% of the population. [3, 44] Epiretinal membranes have been called various names, including epimacular membranes, cellophane maculopathy, preretinal macular gliosis, preretinal macular fibrosis, macular pucker, preretinal vitreous membranes, epiretinal astrocytic membranes, surface wrinkling maculopathy, internoretinal fibrosis, and silk-screen retinopathy; all of which pertain to clinico-anatomic descriptions of pathologic findings produced by epiretinal membranes of varying severity and differing morphologic characteristics.
Epiretinal membranes can be associated with various ocular conditions, such as posterior vitreous detachments (PVD), retinal tears, retinal detachments, retinal vascular occlusive diseases, ocular inflammatory diseases, and vitreous hemorrhage. However, a large proportion of cases do not occur in the context of any associated disease or known history and therefore are classified as idiopathic epimacular membranes (IEMM). Idiopathic and postdetachment membranes are the most common epiretinal membranes and, as such, are the focus of this article.
Pathophysiology
Epiretinal membranes (ERM) are avascular, fibrocellular membranes that proliferate on the surface of the retina and can lead to varying degrees of visual impairment. These cells, once in contact and attached to the retina, may proliferate and form sheets of membranes over the surface of the retina. Through their contractile properties, the underlying retina is, in turn, distorted. The effect on vision is variable and determined by the severity of the distortion, the location, and other secondary effects on the retina.
The source of the cells producing these membranes has been the source of great debate. Earlier reports proposed that glial cells (primarily fibrous astrocytes) from the inner layers of the neurosensory retina proliferated through breaks in the internal limiting membrane (ILM) produced after a retinal tear or a posterior vitreous detachment. Modern vitrectomy specimens have shown that epiretinal membranes comprise glial cells, retinal pigment epithelial cells, macrophages, fibrocytes, and collagen cells. These cells are found in varying proportions in accordance with the etiology of the membrane. Membranes associated with retinal breaks, previous retinal detachments, or cryopexy are composed mainly of dispersed RPE cells, while cells of glial origin predominate in the ERM. Furthermore, these cells also possess the ability to change into cells with similar appearance and function.
The incidence of associated PVD in cases of ERM range from 75-93%, and PVD is present in virtually all eyes with retinal breaks or retinal detachments and subsequent epiretinal membrane formation. It has been suggested that PVD may contribute to epiretinal membrane formation in many ways. PVD can lead to retinal breaks that may liberate RPE cells that initiate membrane formation. Small breaks in the ILM after PVD also may provide retinal astrocytes access to the vitreous cavity, where they may subsequently proliferate. Finally, vitreous hemorrhage, inflammation, or both associated with a PVD also may stimulate epiretinal membrane formation.
Epiretinal membrane formation without PVD may predispose patients to vitreomacular traction syndrome (VMT). Chang et al evaluated patients with VMT using a spectral-domain ocular coherence tomography (SD-OCT) and ultrastructural correlation using samples obtained during surgery. [1] They were able to document fibrocellular proliferation between the inner surface of the retinal and posterior surface of the vitreous, resulting in increased vitreoretinal adhesion. [1]
Bovey and Uffer observed a phenomenon of ILM tearing associated with epiretinal membrane. [2] They hypothesize that the presence of ILM tears and folds are more likely when the epiretinal membrane forms prior to a posterior vitreous detachment, resulting in the subsequent cleavage plane being between the ILM and the inner retina rather than at the ILM surface. [2]
Recent attempts to classify ERM are based on OCT findings for the purposes of aiding surgical decision making and prognostic value of post-operative visual acuity and symptoms. Govetto et al described changes within the central boquet of the fovea, the central 100 microns of densely packed cone photoreceptors. They identified three stages on SD-OCT: (1) cotton ball sign (fuzzy hyperreflective areas between the ellipsoid zone and interdigitation zone) (2) foveolar detachment, (3) acquired vitelliform lesion. The authors suggested that these findings represented a continuum of change related to foveolar traction and that visual prognosis worsened as SD-OCT findings transitioned between these stages. Ex vivo pathologic analysis of peeled ERM also implicated foveal Mueller cells as major factor for transmission of traction to central cones. [48]
Visual acuity changes and symptoms in ERM are related to tractional effects on the retina. Visual acuity loss is hypothesized to be due to multiple fators, including the light filtering and scattering effect of the ERM, distortion of retinal elements, intracellular edema, RPE detachment from traction and disruption of axoplasmic flow. Metamorphopsia is suspected to be due to tractional effects on the inner retina while aniseikonia (most commonly macropsia) may be related to distributional changes of the photoreceptors. [44, 49] Metamorphopsia tended to show improvement post-operatively while aniseikonia did not show improvement and in some cases continued to progress after surgery. The OCT thickness of the inner nuclear layer (INL) seemed predictive of degree of aniseikonia. [49]
Epidemiology
Frequency
United States
The frequency at which epiretinal membranes occur varies according to the underlying disease. The idiopathic variety of epiretinal membranes has been shown to be present in up to 7% of the population. Bilateral cases have been seen in as much as 30% of the population. A 2016 examination of the Beaver Dam Eye Study cohort using OCT suggested a higher prevalence of epiretinal membrane in the population (34.1%). [3]
Clinically significant epiretinal membranes occur in 3-8.5% of eyes after successful primary retinal detachment surgery. Patients noted to be at the greatest risk for epiretinal membranes are those with preoperative signs of proliferative vitreoretinopathy, including rolled retinal edges, star folds, and equatorial ridges.
One study noted no significant difference in the frequency of epiretinal membranes in eyes that underwent retinal detachment repair with subretinal fluid drainage compared to those that had nondrainage procedures. While there is the belief that excessive cryotherapy or laser photocoagulation may lead to increased risk of ERM formation, the possible risk of epimacular development in eyes that have undergone cryotherapy or laser photocoagulation for retinal tears is difficult to quantify because it is almost impossible to determine whether the cellular dispersion was caused by the retinal tear itself or the subsequent therapy for it.
The incidence of epiretinal membrane formation associated with other ocular pathologies, such as retinal vascular occlusive disease, ocular inflammation, or vitreous hemorrhage, is unknown.
Mortality/Morbidity
The visual loss depends on the severity of the distortion of the retina, the location of the wrinkling of the retina, and any other secondary effects of the membrane on the retina (eg, edema, hemorrhage). While vision loss is the most frequent symptom, patients may present with disabling metamorphopsia and aniseikonia despite having relatively good Snellen visual acuity. [44]
Sex
Both sexes appear to be affected in relatively equal percentages.
Age
Epiretinal membranes occur more frequently in the older population, with postmortem studies showing 2% prevalence in individuals aged 50 years and as much as 20% prevalence in individuals aged 75 years. The Beaver Dam Eye Study found a 34% prevalence of ERM in individuals over 60 years of age. [3, 44]
Prognosis
Multiple studies have examined the natural history of ERM with good vision and progression of these patients to surgery. Over the course of 1 to 7 years, 13-30% of patients would progress to surgery. [12, 44] Persistence of vitreomacular adhesion appeared, in one study, to be a risk factor for progression of ERM to surgery, as compared to patients who had ERM in the presence of a posterior vitreous detachment and no residual vitreomacular traction. [44]
Numerous studies have addressed the potential benefit of surgery to remove the epiretinal membranes. These studies have looked at the quantification of the postoperative visual acuity improvement as well as the subjective improvements through postoperative quality of life questionnaires. They have also looked at other prognostic factors that may influence visual outcomes.
Patheja reviewed 49 studies in the literature including both prospective and retrospective studies to identify preoperative ocular coherence tomography (OCT) signs predictive of good post-operative visual acuity. He reported that the weight of evidence pointed to the degree of photoreceptor integrity on pre-operative OCT as the most important factor for post-operative visual acuity. [47]
Surgical removal of clinically significant epiretinal membranes usually results in improvement in both visual acuity and biomicroscopic appearance of the retina. Studies have shown that, postoperatively, 78-87% of patients with idiopathic ERM and 63-100% of patients with postdetachment membranes improved at least 2 Snellen lines. Predictive factors for poor post-operative visual acuity include worse preoperative visual acuity at baseline, age >75 years and longer duration of symptoms (>6-12 mo). Patients with poorer preoperative vision tended to improve the most, but those patients with better preoperative vision obtained the best final results. [44, 45] Dawson et al reviewed 10 years of visual outcomes following epiretinal membrane surgery. They validated that the greatest improvement in visual acuity was seen in patients with the poorest initial visual acuity and that patients with the best preoperative visual acuity had the best visual results in this series. They also showed that surgical results improved over time. [4] Lee et al described microcystic changes noted on SD-OCT which would not necessarily be related to fluorescein leakage on fluorescein angiography. They used this distinction to differentiate between microcystic macular edema (MME) and cystoid macular edema (CME). They found that the presence of MME was a poor prognostic sign for postoperative visual acuity following epiretinal membrane surgery. [52] They also found that CME improved significantly following surgery and identified four factors that were significantly associated with favorable visual recovery: Absence of MME, poor initial visual acuity, increased central foveal thickness, an intact ellipsoid zone.
One study used SD-OCT characteristics at one month, three months and six months to define four patterns of response to epiretinal membrane surgery. Patients who did worst tended to have an increased retinal nerve fiber layer (RNFL) at one month and decrease outer nuclear layer (ONL) at 3 months and 6 months. The largest group in the study was patients who recovered rapidly over the course of one month but then showed minimal improvement thereafter. These patients showed a decreased ganglion cell layer/inner plexiform layer (GCL/IPL) ratio at one month. Patients with the greatest gain of visual acuity tended to have some visual recovery within one month but then continued visual improvement over the next 5 months. Finally, there was a group of patients who showed minimal visual recovery in the first month postoperatively but then significant visual improvement over the next 5 months. Both of these groups showed increased RNFL preoperatively with recovery of RNFL to more normal thickness after surgery. [54]
Visual acuity has also been evaluated through the use of postoperative questionnaires. A large-scale study showed that surgery improved the symptom of distortion the most, with moderate-to-severe symptoms most improved. Improvement was also seen in other daily tasks, such as reading small print.
Sometimes, however, the metamorphopsia may persist despite improvement in visual acuity. This is seen mostly in cases where there is incomplete peeling of the membrane. On the other hand, there are cases wherein the distortion is improved but the Snellen acuity remains unchanged. This mainly is encountered in cases where there is long-standing macular edema.
The presence of new or accelerated cataract formation has been shown to occur in the surgical treatment of epiretinal membranes.
Patient Education
Patient education is a key component of preoperative planning. While epiretinal membrane surgery can be performed in an outpatient setting under monitered local anesthesia similar to cataract surgery, the visual recovery from epiretinal membrane surgery is vastly different from cataract surgery. There is a wide range in visual recovery, including the potential for minimal recovery after surgery. [54] In some instances, visual recovery may be perceived by patients within a week of surgery but, more frequently, visual recovery may require weeks and even months to achieve its final levels. Furthermore, there may be the need for a gas tamponade and positioning after surgery. Most studies examining visual recovery following surgery assess vision at 1 month and 6 months, though anecdotal data suggests that visual recovery may continue even longer after that point. Thus, educating patients about the potential length time required for visual recovery as well as the potential use of gas tamponades postoperatively can mediate patient disappointment in the visual result of surgery.
-
Very dense epiretinal membrane with associated macular distortion.
-
Grade 2 epiretinal membrane causing striations in the retinal surface. Note the presence of a pseudohole.
-
Fluorescein angiogram demonstrating retinal vascular distortion. Note the leakage of the dye in the macular area, which represents secondary macular edema.