Practice Essentials
Nonexudative (dry) age-related macular degeneration (AMD or ARMD) accounts for more than 90% of patients diagnosed with AMD. [1] AMD is the leading cause of irreversible visual loss in the United States, [2] with variable degrees of age-related macular changes occurring in 19.8 million (12.6%) of the population aged 40 years and older in 2019. Just under 1% (1.49 million) of these had vision-threatening illness. The prevalence of AMD rose with age from 2% among individuals aged 40 to 44 years to 46.6% among persons aged 85 years and older. [3]
Drusen can be detected early in this disease without visual loss. As dry AMD progresses to retinal atrophy and central retinal degeneration, loss of central vision often occurs. Generally, nonexudative AMD has a much slower (over decades), progressive visual loss relative to exudative (wet) AMD (over months). [1, 4]
See Exudative (Wet) Age-Related Macular Degeneration (AMD).
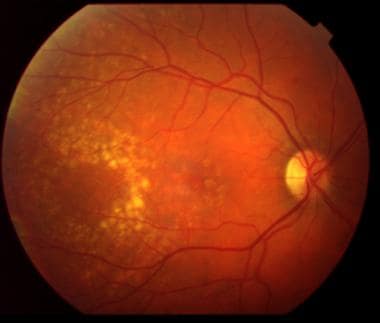 Moderate nonexudative age-related macular degeneration is shown with the presence of drusen (yellow deposits) in the macular region.
Moderate nonexudative age-related macular degeneration is shown with the presence of drusen (yellow deposits) in the macular region.
Signs and symptoms
Signs and symptoms of dry AMD include the following [1, 3] :
-
Difficulty with night vision and with changing light conditions (specifically, changes in Amsler grid self-evaluation and trouble with reading)
-
Slow adjustment to indoor light after having been in the bright outdoors
-
Straight lines appearing broken or distorted (eg, power lines appear crooked, window shades and slats appear crooked, letters appear missing while reading)
-
Visual fluctuation (ie, some days, vision is poor; other days, vision appears improved)
-
Difficulty with reading and making out faces
-
Metamorphopsia (distortion of visual images): Not a major patient complaint for dry AMD, but it may be present as the atrophy slowly progresses. However, rapid onset of metamorphopsia is concerning for the development of wet AMD.
See Clinical Presentation for more detail.
Diagnosis
Funduscopic examination in patients with suspected AMD includes the following findings [3] :
-
Early to intermediate nonexudative AMD: Significant for the presence of multiple drusen for early AMD. For intermediate AMD, drusen may appear confluent with significant pigment changes and pigment accumulation in the posterior pole. In addition, the retinal pigment epithelium (RPE) often appears atrophic, with easier visualization of the underlying choroid vascular plexus
-
Advanced stages of nonexudative AMD: Coalescence of focal islands of atrophy and formation of large zones of atrophy with severely affected vision
-
Nonexudative wet AMD: This is a newly defined state of AMD in which new blood vessels are visible on ocular coherence tomography angiography (OCTA) but no edema or fluid leakage from these new vessels
-
Advanced AMD (wet): Choroidal neovascularization: RPE elevation, exudate, subretinal fluid or hemorrhage
-
Fluorescein angiography: Has value in patients with AMD who note a recent onset or worsening of vision associated with metamorphopsia
-
Amsler grid evaluation: Cornerstone of evaluation of nonexudative AMD
-
Ocular coherence tomography (OCT) of the retina
-
OCTA of the retina
-
Slit-lamp biomicroscopy
-
Biopsy and histologic examination
-
Fundus photography and fundus autofluorescence: Best modality to follow nonexudative AMD
-
Optical coherence tomography (optional; may be used to follow disease progression): To examine retinal thickness
-
Multifocal electroretinography (optional; may be used to follow disease progression): To evaluate functional response of retinal rods and cones
See Workup for more detail.
Management
Prevention is the best treatment for nonexudative AMD, because no satisfactory method exists to treat this condition. Accumulated evidence suggests that AMD is a genetic disease.
Management of exudative AMD may include the following [1, 3, 5] :
-
Intravitreal injection with ranibizumab, bevacizumab, aflibercept, or pegaptanib sodium
-
Photodynamic therapy with verteporfin
-
Thermal laser photocoagulation surgery
Nonpharmacotherapy for both wet and dry AMD
-
Screening for impaired visual acuity
-
Wraparound shades (eg, orange-tinted, blue blocker lenses): Effective solution for delayed dark adaptation and to protect eyes from direct sunlight
-
Avoidance/cessation of tobacco use
-
Frequent follow-up for risk assessment of conversion to exudative AMD
See Treatment and Medication for more detail.
Background
Age-related macular degeneration (AMD or ARMD) is the most common cause of irreversible vision loss in the developed world. [1, 3] AMD is associated with the presence of drusen, without visual loss early in the disease. However, the disease often slowly progresses over years to retinal atrophy and central retinal degeneration with associated loss of central vision. The early form of dry AMD is characterized by small to intermediate sized drusen, without significant vision loss. The intermediate form of dry AMD is associated with loss of retinal pigment epithelium (RPE) and the overlying retinal layers (atrophy), with loss of contrast sensitivity, loss of reading speed, and difficulty with adaptation to changing light conditions. The advanced, nonexudative form of AMD is characterized by the presence of atrophy that can be associated with severe central visual-field loss. In all forms of dry AMD, peripheral visual acuity is preserved. Exudative AMD is associated with the development of choroidal neovascular membranes that results in the development of exudate, subretinal fluid, and hemorrhage, as well as relatively rapid central vision loss and visual distortion.
Greater than 90% of patients diagnosed with AMD have nonexudative (dry) AMD; nonexudative AMD is generally associated with much slower (over decades), progressive visual loss compared with exudative (wet) AMD, which is generally associated with more rapid (over months) visual loss. [3] However, patients with the more advanced cases of dry AMD can have as profound a visual loss as those with exudative AMD.
AMD describes a collection of inherited diseases (multifactorial) that share common features, including age predilection, positive family history, presence of yellow-gray material in the Bruch membrane (ie, drusen), RPE changes (eg, atrophy, clumping, RPE detachments) in the posterior pole or periphery, and visual disturbances (eg, abnormal reading, stereo and/or color vision disturbances, dark/light adaptation disturbances).
RPE degeneration is accompanied by variable loss of both the overlying photoreceptors and the underlying choroidal perfusion. When the appropriate age and clinical findings are accompanied by the loss of visual acuity, visual field, or other visual functions, the condition is often classified as AMD. Early AMD may be diagnosed prior to the onset of visual loss if the patient has characteristic drusen and relevant family history.
AMD usually manifests after age 50 years. The disease is often bilateral and may be asymmetrical, and affected patients report a significant history of disease in family members who have lived to later years of their life. Approximately 10% of patients develop a more rapid form of visual loss secondary to the development of neovascularization from the choroid (CNV), wet AMD. The abnormal vessels may be located either below (type 1) or above (type 2) the RPE. Dry, or nonexudative, AMD is the most prevalent form of this disease. When the dry form of AMD progresses with larger areas of RPE atrophy, the condition is referred to as geographic atrophy (GA). GA usually is bilateral but not necessarily symmetrical. GA can also develop neovascularization and can result in a more rapid loss of vision.
Antioxidant multivitamin therapy (consisting of vitamin A at 25,000 IU, vitamin C at 500 mg, zinc at 80 mg, copper at 2 mg, and vitamin E at 400 mg) has been shown in a large clinical trial, the Age-Related Eye Diseases Study (AREDS), to be helpful in decreasing the risk of visual loss with nonexudative AMD. Most of the decrease in visual loss appeared to result from a reduced risk of conversion to wet AMD. The AREDS notably did not show any benefit with the use of these vitamins in very early AMD or in subjects without AMD at baseline. AREDS also showed a relative increased risk of lung cancer among smokers who used high-dose vitamin A. The AREDS2 study showed that a formulation that replaced vitamin A/beta-carotene with a combination of lutein and zeaxanthin was safer and likely more effective at preventing AMD progression than the initial AREDS formula.
The Women's Antioxidant and Folic Acid Cardiovascular Study looked at a cohort of women without any evidence of AMD. The randomized, double-blind, placebo-controlled trial included 5442 female healthcare professionals and noted that a combination of folic acid (2.5 mg/d), pyridoxine hydrochloride (50 mg/d), and cyanocobalamin (1 mg/d) reduced the relative risk of developing visually significant AMD by approximately 40%. The study also demonstrated a risk of developing non–visually significant AMD that was reduced by a similar amount. [9]
A phase 1 study demonstrated visual benefit and decreased progression of AMD in subjects with advanced dry AMD who were provided with an intravitreal implant that secreted ciliary neurotrophic factor (CNTF). Current molecules under investigation aim to inhibit GA progression via various mechanisms, including reducing toxic byproduct generation and accumulation, slowing the visual cycle, and inhibiting the complement pathway, which have all been shown to be involved in the pathogenesis of AMD. Targeting the alternate complement pathway has shown the most promise for dry AMD treatment. More specifically, complement factor H mutations have been linked to dry AMD, and smoking, a known risk factor for AMD progression, alters activity of complement factor H. There are both phase 2 and 3 trials underway to address the potential role of complement inhibitors to treat nonexudative AMD. Multiple other studies have shown early promise but failure in later stages, including a promising phase 2 study using a complement factor D inhibitor that failed in phase 3 (Genentech).
Additional therapies that have been tried include rheopheresis (apheresis) and laser to drusen. While these therapies demonstrated a small benefit over the short term (1-3 y), they did not prove to have any significant benefit after that time. In fact, the Complications of Age-related Macular Degeneration Prevention Trial (CAPT) has demonstrated that laser to drusen is ultimately not beneficial and may potentially be harmful.
Pathophysiology
Clinical pathophysiology
The clinical definition of early age-related macular degeneration (AMD or ARMD) varies with the source consulted. A clinically useful guideline is when drusen in the posterior pole are greater than 5 in number and at least 63 µm in size. With time, drusen enlarge and result in shallow elevation of the RPE that overlies the Bruch membrane. These deposits may merge over time, and they can be associated with pigmentation change visible on ophthalmoscopy.
Evaluation of the AREDS results provided a clinically useful method of determining risk of advanced AMD using simple criteria: (1) presence of large drusen (greater than 125 µm in size) and (2) the presence of pigment abnormalities. Thus, one eye having both large drusen and pigment abnormalities has a score of 2 (1 for each criterion), and if both eyes have each risk factor, the score is 4. Using this simplified criteria, the AREDS found that over 5 years, eyes with a risk factor score of 0 had only a 0.4% move to advanced AMD, while those with 1 risk factor had a 3.1% move to advanced AMD. However, when eyes had 2, 3, or 4 risk factors, the rate of advanced AMD conversion (either large geographic atrophy or neovascularization) increased to 12%, 26%, and 47% respectively. [10]
Because the above matrix is a simple and powerful tool to determine the persons who will develop advanced AMD, it is useful to recollect the criteria when performing a clinical examination. For example, the presence of a large druse (125 µm in size) would be most easily remembered by looking for drusen whose shortest diameter is approximately 125 µm (or as large as the diameter of a retinal vein at the optic disc margin). Only drusen within 2 disc diameters of the center were used during the analysis. Pigment abnormalities included any areas of hyperpigmentation or hypopigmentation, as well as noncentral areas of geographic atrophy.
Visual acuity loss or visual-field loss occurs when the RPE atrophies and results in secondary loss of the overlying photoreceptor cells that it supplies. The variety of fundus changes described above defines dry AMD. When the damaged RPE results in the development of choroidal neovascularization with late leakage on fluoresce in angiography and a decrease in vision and metamorphopsia, exudative (wet) AMD is said to occur.
Molecular pathophysiology
Dry AMD is an inherited autosomal dominant disease that appears to be affected by nutrition and environmental factors. Nonexudative AMD is characterized by the degeneration of the retina and the choroid in the posterior pole due to either atrophy or RPE detachment. The atrophy is generally preceded (or coincident in some cases) by the presence of yellow extracellular deposits adjacent to the basal surface of the RPE called drusen. Multiple studies now point to the role of inflammation in AMD. AMD can be thought of as degeneration of the RPE cells due to chronic inflammation. Abnormalities in complement function (complement factor I [CFI], complement factor H [CFH]) are slightly more likely to trigger the complement system. Similarly, long-term cigarette smoking, Alu RNA, and other inflammatory mediators activate NLRP3 inflammasome complex, leading to cell death.
Drusen are composed of vitronectin (a multifunctional plasma and extracellular matrix protein), lipids, immune and inflammatory related proteins, and amyloid associated proteins, as well as other poorly characterized substances. While drusen were thought to be the result of accumulated waste material from subretinal tissues, data now suggest that the accumulation is due to the presence of inflammation in the subretinal space. This extracellular material in the Bruch membrane is composed of various substances, including vitronectin and proteinaceous material. The products of oxidative stress also trigger a chronic low-grade inflammation (pathophysiological parainflammation) process in patients with AMD. In early AMD, soft drusen contain many mediators of chronic low-grade inflammation such as C-reactive protein, adducts of the carboxyethylpyrrole protein, immunoglobulins, and acute phase molecules, as well as the complement-related proteins C3a, C5a, C5, C5b-9, CFH, CD35, and CD46. Inappropriate activation of the complement system, a mainly alternative pathway, mediates chronic autologous pathophysiological parainflammation in dry and exudative AMD.
The complement system is an alternative system (ie, independent of antibodies) of defense against infection. CFH is a robust anti-inflammatory agent, in that it protects host cells from complement-mediated damage by binding to the activated complement component C3b.
In 2005, four separate groups reported that a common variation in the CFH (complement factor H) gene increased susceptibility to dry AMD.
In 2006, two other genes were identified that increased the risk similarly. The CFH polymorphism that was most significantly associated with AMD is a T→C substitution that results in a tyrosine-to-histidine substitution of the CFH protein. Thus, it appears that in affected individuals, RPE cells may undergo damage via the complement system because of their inability to inhibit the complement cascade as effectively. Additional indirect evidence in support of this chain of events is noted by a publication that indicates that choroidal levels of C-reactive protein are elevated in homozygote CFH polymorphic individuals. [11]
Some studies have delineated a molecular pathway leading to geographic atrophy and visual loss. This pathway indicates that RPE death leads to secondary photoreceptor loss and consequent visual loss over time. Ambati et al found that RPE cells in patients with dry AMD have low levels of a RNA-cleaving enzyme, DICER1. Low levels of this enzyme lead to decreased breakdown of RNA-Alu molecules. Overabundance of cytoplasmic RNA-Alu molecules (non-coding sequences of RNA) activates inflammatory proteins (NLRP3 inflammasome), which activates a cascade of molecular responses that lead to RPE cell death. [12, 13]
Frequency
United States
Age-related macular degeneration (AMD or ARMD) is the leading cause of blindness in the United States for people older than 50 years. The actual frequency of the disease depends on specific racial group studies. AMD is more prevalent in Whites and likely has a more severe course in patients who have light-colored eyes. A liberal definition of AMD that includes all patients with significant drusen in the posterior pole, with or without visual loss, estimates the prevalence at greater than 20% of the population older than 60 years. A more rigorous, population-based survey with a definition that requires the presence of either late atrophy and/or choroidal neovascularization results in an incidence of 0% at age 50 years or younger, 2% at 70 years, and 6% at 80 years. In African Americans, dry AMD is noted to be approximately half the incidence rate stated above.
International
The incidence of AMD in Japanese and other Asian populations is lower than the white population in the United States, but reports suggest that the incidence is increasing. The Inuit people in Greenland have a significantly higher incidence, as well as a distinctive phenotype. Most black Africans and other people with darker-pigmented skin in general have a lower incidence of symptomatic macular degeneration. Similarly, it is evident that the lesions resulting from AMD in Asian populations are different from those in white populations. This is in agreement with the most accepted theory regarding AMD: that it is a multigenic inherited condition. The background and the specific gene affected would affect the phenotype.
Mortality/Morbidity
Age-related macular degeneration (AMD or ARMD) results in significant visual morbidity. The presence of neovascularization results in a blurry central visual field. Even in dry AMD, with relatively good vision, patients often report trouble adjusting to varying light conditions. Often, these patients note difficulty when initially placed in a dark environment from a relatively lighted one (eg, entering a restaurant from bright sunlight).
AMD patients, especially those with the exudative variant, have a higher incidence of cerebrovascular accidents and cardiac disease.
Geographic atrophy may also be associated with cognitive impairment, as assessed by mini-mental status exams and other similar tests. One case control study demonstrated a 3x increased odds for mild cognitive impairment in geographic atrophy subjects when compared to normal controls even when controlled for age, visual acuity and education level. [14] Additionally, 3000 subjects in the AREDS trial underwent a battery of cognitive tests. Among subjects with AMD, those with poorer vision had worse cognition. [15] Participants in the Cardiovascular Health Study underwent cognitive testing as well as mini-mental state testing and those with poorer cognition were at a slightly higher risk of having dry AMD. [16] Given the higher rate of impaired cognitive function in patients with dry AMD, it is important for the clinician to consider cognitive function when assessing low vision options and treatments.
Race
The incidence of AMD is higher in Whites compared with African Americans. [2] Some studies report a rate of approximately half in African West Indians in Barbados compared with whites in Baltimore, Maryland. The incidence in Asians is between the above 2 rates, although it appears that the incidence is increasing in this population.
Sex
No known difference exists between males and females in the incidence of AMD.
Age
As implied by its name, the incidence of AMD is related to the age of the patient. The incidence increases with each decade of life, with a significant rise in patients aged 70 years or older.
Prognosis
The prognosis for this disease is significantly better than the prognosis for wet age-related macular degeneration (AMD or ARMD). Patients likely will have steadily but slowly deteriorating visual acuity. It also is common to have other visual dysfunction (eg, loss of ability to quickly adapt to changing lighting conditions, loss of contrast sensitivity). Variability of vision from day to day is common.
Patient Education
Patients with geographic atrophy (GA) may have various types of visual dysfunction. The location of atrophy often suggests the type of visual dysfunction that will be experienced by the patient. Many patients with age-related macular degeneration (AMD or ARMD) report difficulty in adjusting to changing light conditions; specifically, they take a significantly longer time to adjust to indoor lighting after being outside in bright sunlight. Wrap-around outdoor sunglasses that have an orange tint work for some patients.
Patients who primarily have central atrophy often note trouble with reading and performing fine motor tasks. Magnification and increased contrast (via a monitor or increased illumination) are the best solutions for such visual dysfunction.
In contrast, other patients have GA that spares the foveal center but affects the entire perifoveal region. These patients often can see 20/20, but they are unable to navigate due to the small area of good visual acuity. Some of these patients must scan the screen to be able to see the 20/400 character. In these patients, excess magnification would be detrimental, because it would effectively decrease their limited visual field. Increased contrast and minification, by way of increased illumination and reverse telescopes respectively, may be beneficial for these patients.
Referral to comprehensive vision rehabilitation is indicated early in the disease process. The American Academy of Ophthalmology (AAO) recommends referral for vision rehabilitation when acuity is less than 20/40 or when a loss of contrast sensitivity, scotoma, or field loss is noted. The aim of early referral is to prevent the many negative consequences of vision loss. For example, when acuity is reduced to 20/50 or worse, patients have twice the risk of falling, 3 times the risk for depression, and 4 or greater times the risk for hip fracture. Rehabilitation aims to maximize patients’ use of their partial vision and to provide practical adaptation to reduce disability. Comprehensive rehabilitation addresses the “whole person,” as outlined in the AAO’s booklet of Vision Rehabilitation for Adults. Barriers to low-vision therapy access include poor insurance coverage and transportation. [17]
For patient education resources, see the Eye and Vision Center, as well as Macular Degeneration.
-
A normal-appearing macula of the left eye. Note the even pigmentation of the retinal pigment epithelium and the absence of any yellow excrescences (drusen) in the fovea. The optic nerve has unrelated changes.
-
In angiography, fluorescein dye is passed through a peripheral vein and transmits through the vascular system. The dye fluoresces in the vasculature, as seen here. No vascular prominences are seen in the macula or in any areas of dye pooling or staining. The abnormal vessels in the optic nerve, however, do show dye leakage.
-
Moderate nonexudative age-related macular degeneration is shown with the presence of drusen (yellow deposits) in the macular region.
-
Staining of drusen. Drusen absorb dye and, in the late frames of the angiogram, show hyperfluorescence. This staining is distinguished from the leakage that occurs when the dye spreads outside the boundary of the lesion.
-
A more advanced case of nonexudative age-related macular degeneration (ARMD). This image shows drusen that are larger, more confluent, and soft. Soft drusen are defined as drusen that have indistinct borders. Such drusen are more likely to convert to wet ARMD. A few areas of atrophy are noted, where the retinal pigment epithelium (RPE) has lost pigmentation. The retinal cells overlying atrophic RPE are generally nonfunctional and result in a scotoma.
-
The atrophic retinal pigment epithelium (RPE) demonstrates staining of the underlying choroidal vasculature. Normally, the intact RPE masks the presence of choroidal fluorescence. However, when the RPE atrophies, the underlying dye appears as an area of hyperfluorescence in the early stages of angiography. In the late stages, the drusen lose fluorescence in concert with (or with a small time lag) the rest of the retinal layers.
-
A more advanced case of dry age-related macular degeneration. Several areas of atrophy are present, as are areas of significant pigment mottling in the macula. The large drusen inferior to fixation are poorly distinguished from each other.
-
The atrophic areas are easily distinguished by the hyperfluorescence of the retinal pigment epithelium (RPE) in the mid phase of the angiogram. Hypofluorescence of dye, due to masking caused by the increased pigmentation, is seen. No areas of frank dye leakage or exudative age-related macular degeneration (ARMD) are apparent. A "hot cross bun" pattern of dry ARMD-related pigment changes is evident near the fovea.
-
High-definition optical coherence tomography scan of a 67-year-old woman showing retinal pigment epithelium mottling and pigment epithelial detachments temporal to fixation consistent with dry macular degeneration.
-
Fundus photo showing drusen in a 67-year-old woman with dry age-related macular degeneration.
-
Fluorescein angiogram 4 minutes after injection of dye on 67-year-old woman showing pigment epithelial detachments.
-
A later frame of the angiogram demonstrating the absence of dye leakage outside the lesion, with staining of the areas of atrophy (window defects) in the macular region.
-
High definition optical coherence tomography right eye demonstrating retinal pigment epithelium atrophy and changes in the deeper layers of retina. The absence of intraretinal cysts, subretinal fluid, or sub-retinal pigment epithelium fluid indicates the absence of wet age-related macular degeneration.
-
Spectral domain optical coherence tomography (SD-OCT) analysis: OCT B-scans show the presence of pigment epithelial detachment bilaterally in a patient with previously diagnosed dry age-related macular degeneration (AMD), which appeared relatively stable in comparison to previous scans. No subretinal fluid or retinal edema was detected.
-
Fluorescein angiography: Fundus angiography in the same patient shows staining of drusen and window defects only in each eye. No active neovascularization was detected in either eye.
-
Optical coherence tomography (OCT) shows an active neovascular network in the right eye as opposed to the nonvascularized pigment epithelial detachment found in the left eye. The spectral domain optical coherence tomography (SD-OCT) images in the lower panels confirm pigment epithelial detachment formation in each eye.
-
Optical coherence tomography (OCT) shows an active neovascular network in the right eye as opposed to the nonvascularized pigment epithelial detachment found in the left eye. The spectral domain optical coherence tomography (SD-OCT) images in the lower panels confirm pigment epithelial detachment formation in each eye.






