Background
In 1889, Doyne first described angioid streaks in a patient with retinal hemorrhages secondary to trauma. [1] Angioid streaks, also known as Knapp striae, are irregular jagged dehiscences in the mineralized, degenerated, brittle Bruch membrane that typically form alongside force lines exerted by intrinsic and extrinsic ocular muscles that radiate in a centrifugal pattern emanating from the optic disc. [2] Knapp named them angioid streaks because of their resemblance to blood vessels. [2]
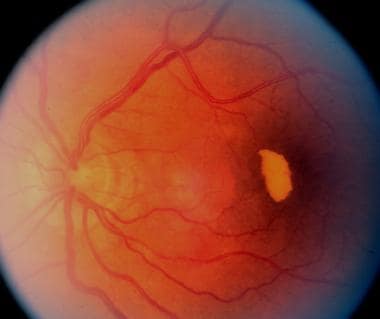 Same eye as in previous image, 11 months later. Partial resolution of subretinal blood. Notice the old subretinal hemorrhage under the fovea and color change to white-yellow.
Same eye as in previous image, 11 months later. Partial resolution of subretinal blood. Notice the old subretinal hemorrhage under the fovea and color change to white-yellow.
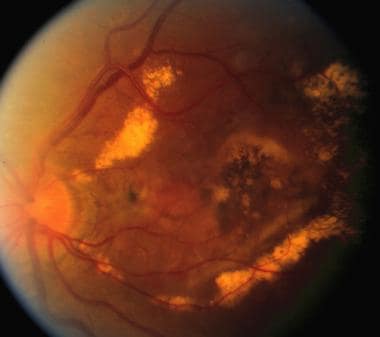 Late complication of choroidal neovascularization in angioid streaks. Same eye as in previous images, 5 years later. Notice the extensive scarring and subretinal exudates and dehemoglobinized blood.
Late complication of choroidal neovascularization in angioid streaks. Same eye as in previous images, 5 years later. Notice the extensive scarring and subretinal exudates and dehemoglobinized blood.
Pathophysiology
Controversy about the pathophysiology of angioid streaks exists. In some diseases, including pseudoxanthoma elasticum (PXE) and Paget disease, the Bruch membrane may become calcified and brittle with subsequent development of cracks. However, cytoimmunochemistry and x-ray analysis had shown that the earliest abnormality in PXE was abnormal accumulation and metabolism of polyanions (ie, glycosaminoglycans, glycoproteins) within the Bruch membrane.
The lines of force within the eye resulting from the pull of intrinsic and extrinsic ocular muscles on the relatively fixed site of the optic nerve have been studied. Those lines of forces had the same configuration as the peripapillary interlacement and radial extensions of angioid streaks. Such forces acting on the Bruch membrane undoubtedly account for the configuration of the breaks. However, in sickle cell disease, Bruch membrane calcification is not a common part of the pathology.
It is believed that the pathology may be a combination of diffuse elastic degeneration of the Bruch membrane, iron deposition in elastic fibers from hemolysis with secondary mineralization, and impairment of nutrition because of sickling, stasis, and small vessel occlusion. Klien proposed a dual mechanism as a cause of these cracks in general, as follows: a primary abnormality of fibers of the Bruch membrane, and an increase in availability of metal salts or a tendency for their deposition, resulting in a secondary brittleness of the membranes. [3]
Epidemiology
Frequency
United States
Not known
International
Not known
Mortality/Morbidity
Moderate-to-severe central visual loss is mainly related to foveal involvement with a dehiscence of the underlying Bruch membrane or a neovascular membrane formation under the retina. Choroidal neovascularization (CNV) is the major cause of vision loss and affects 70-86% of patients with angioid streaks.
Race
White people are affected most. Two studies showed similar results: of all patients with angioid streaks, 66.2% of patients were white, compared with 29% of Asian origin and 3.7% of black people. [4, 5]
Sex
No sexual predilection exists.
Age
The age of onset is variable with the underlying etiology. In one study, the age of onset of 50 patients with angioid streaks was reported as follows:
-
Patients with PXE present in the third decade with a mean age of 51.7 years.
-
Patients with sickle cell disease tend to be in their second and third decades with a mean age of 41.7 years.
-
Patients with Paget disease tend to be older at the time of diagnosis with a mean age of 67 years.
-
Angioid streaks in patients with no systemic disease or with rare etiologies tend to present late in life with a mean age of 65.7 years. Rare etiologies include patients with peptic ulcer, diabetes, hypertension, arthritis, breast cancer, metastatic cancer, rheumatoid spondylitis, and heart disease.
Prognosis
A high risk of serious complications, such subretinal hemorrhage and serous detachment, exists. Bilateral involvement is the rule, although it may not be symmetrical. Among individuals in whom CNV begins to develop, 50% will develop CNV in the fellow eye within 18 months. [5, 6, 7] Families with affected individuals need screening and regular eye examinations for early detection of any progression.
Patient Education
Patients should be instructed to return if visual acuity decreases. Signs of decreased central visual acuity may include central blurred vision, difficulty in depth perception, and distortion of lines and objects.
Families and patients will benefit from using an Amsler grid to detect early changes in asymptomatic but high-risk individuals.
More emphasis should be placed on safety measures to avoid trauma even if trivial. Protective goggles are useful for young patients who participate in sports.
-
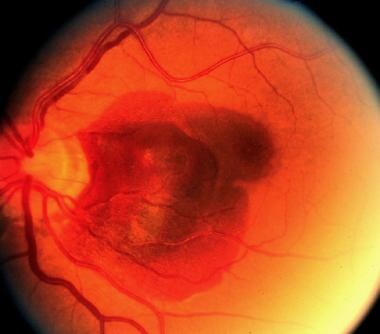
Progression of angioid streaks. Large subretinal hemorrhage.
-
Same eye as in previous image, 11 months later. Partial resolution of subretinal blood. Notice the old subretinal hemorrhage under the fovea and color change to white-yellow.
-
Late complication of choroidal neovascularization in angioid streaks. Same eye as in previous images, 5 years later. Notice the extensive scarring and subretinal exudates and dehemoglobinized blood.
-
Red-free photograph of the optic nerve and posterior pole showing the cracks in the Bruch membrane. Notice the retinal arteries and veins crossing over the dark red streaks.
-
Early fluorescein angiography showing the early hyperfluorescence, window defect, of the angioid streaks.
-
Late fluorescein angiography of the same eye as in Media file 2. Notice the staining of the edges of the streaks. Also, staining in the center of the macula is present due to extension of the Bruch membrane crack. When compared to early fluorescein angiography, no active leakage is present.
-
Right eye, midphase arteriovenous, showing choriocapillaris atrophic changes. This 45-year-old patient underwent 3 injections of Avastin and one session of half-time photodynamic therapy.
-
Same patient as in previous image, a few months before the Avastin injection and half-time photodynamic therapy.
-
A 50-year-old man with a 2-month history of blurring vision in the left eye. The color photograph showed subretinal blood and large membrane, extrafoveal in location.
-
Early fundus fluorescein angiography showing the hyperfluorescence of the choroidal neovascular membrane of the left eye of the same patient in the previous image.
-
Late fundus fluorescein angiography confirming the active choroidal neovascular membrane of the left eye.