Practice Essentials
The carotid-cavernous fistula (CCF) is a specific type of dural arteriovenous fistula characterized by abnormal arteriovenous shunting within the cavernous sinus. CCFs are classified as either direct or dural(indirect). Direct CCFs are high-flow fistulas with a direct connection between the internal carotid artery (ICA) and the cavernous sinus. Dural CCFs are low-flow fistulas resulting from communications of cavernous arterial branches and the cavernous sinus. [1, 2, 3, 4, 5, 6, 7]
Case reports of dural arteriovenous fistulas were first published in the 1930s. The clinical presentation was recognized, but the pathophysiology was not well understood. During the 1970s and 1980s, the anatomy was further elucidated. Barrow and associates developed the current classification system of caroticocavernous fistulas in 1985. [8, 9]
The cavernous sinus is a network of venous channels traversed by the intracranial portion of the internal carotid artery. The internal carotid artery gives rise to several intracavernous branches. These are the meningohypophyseal and inferolateral trunks. These vessels branch to provide arterial blood to the nerves and dura of the cavernous sinus and the pituitary gland. The external carotid artery provides several branches to the dura of the cavernous sinus and forms anastomoses with the branches of the internal carotid artery.
Classifications
CCFs can be classified based on the hemodynamic properties, etiology, or anatomy of the shunt. Hemodynamically, they are classified as low flow or high flow. They may occur following a trauma or spontaneously. Anatomically, they are classified as type A through type D, as follows:
-
Type A fistulas consist of a direct connection between the intracavernous internal carotid artery and the cavernous sinus. They usually are high-flow and high-pressure fistulas.
-
Type B fistulas consist of a dural shunt between intracavernous branches of the internal carotid artery and the cavernous sinus.
-
Type C fistulas consist of a dural shunt between meningeal branches of the external carotid artery and the cavernous sinus.
-
Type D fistulas are a combination of types B and C, with dural shunts between internal and external carotid artery branches and the cavernous sinus.
-
Types B, C, and D tend to be lower-flow and lower-pressure fistulas with a slower progression of signs and symptoms.
Causes
Direct CCFs result from a traumatic tear in the artery from a skull base fracture, from an acceleration-deceleration force (eg, motor vehicle accident), or from an iatrogenic injury after an endovascular intervention or a trans-sphenoidal procedure. They can also occur spontaneously after rupture of an intracranial aneurysm or weakening of the arteries. [3] Karaman et al reported on a carotid-cavernous fistula secondary to blunt trauma after functional endoscopic sinus surgery. [10]
Indirect CCFs result from a dural branch rupture of the carotid artery caused by a genetic condition or a comorbidity such as hypertension. [3]
Blunt head injury can lead to shearing of intracavernous arteries, causing the development of a fistula. Penetrating head injury can lead to fistula formation by direct laceration of intracavernous vessels.
Spontaneous fistula formation has been associated with (1) ruptured intracavernous aneurysm, (2) fibromuscular dysplasia, [11] (3) Ehlers-Danlos syndrome and other collagen vascular diseases, (4) atherosclerotic vascular disease, (5) pregnancy, and (6) straining.
Signs and symptoms
Onset is usually sudden. Direct carotid-cavernous fistulas are characterized by the triad of pulsatile proptosis, chemosis, and intracranial whistling. There is severe conjunctival congestion, hemorrhagic chemosis, ptosis, and pulsatile proptosis accompanied by a whistling. Painful ophthalmoplegia may be present, with the most common being VI nerve palsy. In cases of indirect carotid-cavernous fistulas, there is moderate ocular congestion, mild proptosis, and ocular pulsation on aplanotonometry. [2, 3, 12] Arterialization of episcleral veins is shown in the image below. Bruit and headache also may be present upon clinical presentation.
(The image below depicts a type D carotid-cavernous fistula.)
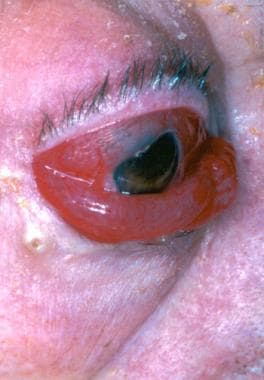 Type-D caroticocavernous fistula: the eye demonstrates proptosis, chemosis, and scleral edema. The patient is unable to close the eye, exposing the cornea to dehydration and potential trauma.
Type-D caroticocavernous fistula: the eye demonstrates proptosis, chemosis, and scleral edema. The patient is unable to close the eye, exposing the cornea to dehydration and potential trauma.
A carotid-cavernous fistula results in high-pressure arterial blood entering the low-pressure venous cavernous sinus. This interferes with normal venous drainage patterns and compromises blood flow within the cavernous sinus and the orbit, as depicted in the diagram below.
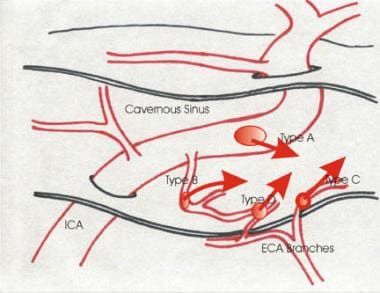 This is a diagrammatic representation of the 4 types of caroticocavernous fistulas. ICA is the internal carotid artery; ECA is the external carotid artery.
This is a diagrammatic representation of the 4 types of caroticocavernous fistulas. ICA is the internal carotid artery; ECA is the external carotid artery.
A CCF is not a life-threatening disease. The risk of visual loss and the severity of associated symptoms must be evaluated to determine the appropriate degree and timing of intervention.
Workup
Complete ophthalmologic workup includes visual acuity, pupillary function, intraocular pressure. funduscopy (direct and indirect), and gonioscopy.
Diagnostic tests and imaging for a CCF include the following [3] :
-
Cerebral angiography (see the image below), which is considered the gold standard.
-
Tonometry and pneumotonometry, which may show increased ocular pulse amplitude on the side of the fistula.
-
B-scan or color Doppler ultrasonography, which will identify a dilated superior ophthalmic vein or orbital congestion.
-
CT or MRI, which may reveal a dilated superior ophthalmic vein, orbital congestion, or enlargement of the extraocular muscles.
-
CT angiography or MR angiography, which can detect visual symptoms of CCFs.
Lab studies include routine preangiography workup to evaluate coagulation and renal function prior to delivering contrast dye (CBC count, platelets, prothrombin time [PT], and partial thromboplastin time [PTT]), electrolytes, blood urea nitrogen (BUN), and creatinine.
(An angiogram of a carotid-cavernous fistula is shown below.)
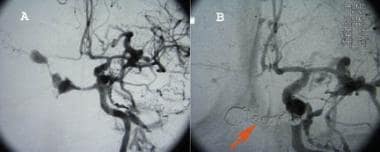 Panel A is an angiogram of caroticocavernous fistula showing filling of the cavernous and circular sinuses. Panel B shows a post-Guglielmi detachable coil, ie, coiling of the fistula. The red arrow points to coils within the cavernous and circular sinuses after obliteration of the fistula.
Panel A is an angiogram of caroticocavernous fistula showing filling of the cavernous and circular sinuses. Panel B shows a post-Guglielmi detachable coil, ie, coiling of the fistula. The red arrow points to coils within the cavernous and circular sinuses after obliteration of the fistula.
In a study of time-resolved magnetic resonance angiography (MRA) in 6 patients with CCFs, typical morphologica findings (including enlargement of the superior ophthalmic vein, exophthalmos) were found in all cases. [13]
A study of 98 suspected cases of CCF evaluated with thin-section MR imaging reported overall accuracy, sensitivity, and specificity of 88.8%, 97.4%, and 83.3%, respectively. Abnormal contour of the cavernous sinus, internal signal void of the cavernous sinus, prominent venous drainage flow, and orbital/periorbital soft tissue swelling were proposed as possible predictive findings. [14]
Treatment
Various options are available for the management of CCFs depending on the flow rate. The goal is to achieve complete occlusion of the fistula while preserving normal ICA flow. In cases of indirect, low-flow fistulas, spontaneous closure is possible.
Endovascular intervention is the first-line treatment of CCFs. For direct, high-flow CCFs, the transarterial route is preferred. Surgical options include suturing or clipping the fistula, packing the cavernous sinus, or ligating the ICA. Radiosurgery is not an option for urgent cases, because it can take months to achieve complete obliteration. For low-flow fistulas, compression treatment is the least invasive opton, consisting of compression a number of times a day for 4-6 weeks to achieve fistula thrombosis. [3]
Type A fistulas rarely resolve spontaneously. Treatment is recommended for intolerable bruit, progressive visual loss, and the cosmetic effects of proptosis. Types B, C, and D fistulas have a higher incidence of spontaneous resolution.
Complications of untreated lesions may include visual loss, cranial nerves paralysis, and the cosmetic concerns of proptosis.
Type A fistulas usually are approached through the internal carotid artery. A detachable balloon or endovascular coil can then be positioned to occlude the fistula while maintaining patency of the internal carotid artery. Venous approaches through the internal jugular vein and the petrosal sinus may allow access to the fistula from the venous side. Guglielmi detachable coils also may be used and are becoming increasingly popular. Types B, C, and D fistulas have smaller fistulous connections and usually are not amenable to the aforementioned treatment approaches.
Patients with CCFs generally have a good prognosis. Persistent lesions respond well to intervention. The risk of nonophthalmologic neurologic complications is not significant; however, persistent untreated lesions may cause significant visual complications.
Complications of treatment include the standard complications of cerebral angiography. Arterial and venous compromise also may occur, yielding cerebral or retinal ischemia and resultant infarction.
Prognosis
Although direct carotid-cavernous sinus fistulae rarely reopen after closure using a detachable balloon technique, it is not unusual for dural carotid-cavernous sinus fistulae to recanalize or form new abnormal vessels after embolization. The ocular pulse amplitude should be checked postoperatively in all patients, preferably using a pneumotonometer.
Once a fistula is closed, symptoms and signs usually begin to improve within hours to days. The rate and extent of improvement are associated with the severity of the signs and the length of time the fistula was present.
Preexisting ocular bruit, ocular pulsations, and thrill generally disappear immediately after the surgery.
Eyelid engorgement, conjunctival chemosis, dilated conjunctival vessels, stasis retinopathy, disc swelling, and elevated intraocular pressure generally return to normal within weeks to months.
Most patients with dural carotid-cavernous sinus fistulae are healthy within 6 months after treatment, but patients with direct carotid-cavernous sinus fistulae may not experience complete resolution of proptosis, ophthalmoparesis, and visual loss. [15]
-
Type-D caroticocavernous fistula: the eye demonstrates proptosis, chemosis, and scleral edema. The patient is unable to close the eye, exposing the cornea to dehydration and potential trauma.
-
Panel A is an angiogram of caroticocavernous fistula showing filling of the cavernous and circular sinuses. Panel B shows a post-Guglielmi detachable coil, ie, coiling of the fistula. The red arrow points to coils within the cavernous and circular sinuses after obliteration of the fistula.
-
This is a diagrammatic representation of the 4 types of caroticocavernous fistulas. ICA is the internal carotid artery; ECA is the external carotid artery.




