Overview
Anencephaly is a serious developmental defect of the central nervous system in which the brain and cranial vault are grossly malformed. The cerebrum and cerebellum are reduced or absent, but the hindbrain is present. Anencephaly is a part of the neural tube defect (NTD) spectrum. This defect results when the neural tube fails to close during the third to fourth weeks of development, leading to fetal loss, stillbirth, or neonatal death. [1, 2, 3, 4]
Anencephaly, like other forms of NTDs, generally follows a multifactorial pattern of transmission, with interaction of multiple genes as well as environmental factors, although neither the genes nor the environmental factors are well characterized. In some cases, anencephaly may be caused by a chromosome abnormality, or it may be part of a more complex process involving single-gene defects or disruption of the amniotic membrane. Anencephaly can be detected prenatally with ultrasonography and may first be suspected as a result of an elevated maternal serum alpha-fetoprotein (MSAFP) screening test. Folic acid has been shown to be an efficacious preventive agent that reduces the potential risk of anencephaly and other NTDs by approximately two thirds. [5, 6, 7, 8]
Pathophysiology
In the normal human embryo, the neural plate arises approximately 18 days after fertilization. During the fourth week of development, the neural plate invaginates along the embryonic midline to form the neural groove. The neural tube is formed as closure of the neural groove progresses from the middle toward the ends in both directions, with completion between day 24 for the cranial end and day 26 for the caudal end. Disruptions of the normal closure process give rise to NTDs. Anencephaly results from failure of neural tube closure at the cranial end of the developing embryo. Absence of the brain and calvaria may be partial or complete.
Most cases of anencephaly follow a multifactorial pattern of inheritance, with interaction of multiple genes as well as environmental factors. The specific genes that are most important in NTDs have not yet been identified, although genes involved in folate metabolism are believed to be important. One such gene, methylenetetrahydrofolate reductase (MTHFR), has been shown to be associated with the risk of NTDs. In 2007, a second gene, a membrane-associated signaling complex protein called VANGL1, was also shown to be associated with the risk of neural tube defects. [9]
A variety of environmental factors appear to be influential in the closure of the neural tube. Most notably, folic acid and other naturally occurring folates have a strong preventive effect. Folate antimetabolites, maternal diabetes, maternal obesity, mycotoxins in contaminated corn meal, arsenic, and hyperthermia in early development have been identified as stressors that increase the risk of NTDs, including anencephaly.
Causes
Anencephaly is usually an isolated birth defect and not associated with other malformations or anomalies. The vast majority of isolated anencephaly cases are multifactorial in their inheritance pattern, implicating multiple genes interacting with environmental agents and chance events.
Inadequate folic acid
Adequate folic acid consumption during pregnancy is protective against anencephaly. [10] Exposure to agents that interfere with normal folate metabolism during the critical period of neural tube development (up to 6 weeks after last menstrual period) increases the likelihood of an NTD.
Valproic acid, an anticonvulsant, and other antimetabolites of folic acid have been shown to increase the chance of an NTD when exposure occurs in early development. While these induced NTDs are usually spina bifida, the chance of anencephaly is probably increased as well. [11]
Since the United States began fortifying grains with folic acid, there has been a 28% decline in pregnancies affected by neural tube defects (spina bifida and anencephaly). [12]
Insulin-dependent diabetes mellitus
Maternal type 1, or pregestational insulin-dependent diabetes mellitus (IDDM), confers a significant increase in the risk for NTDs, and it also delays production of alpha-fetoprotein (AFP) during pregnancy. [13, 14] Maternal serum AFP is used as a screening test to detect NTDs, and adjustment of the expected values for AFP in maternal serum must be made if the patient is known to have IDDM. Presumably, well-controlled IDDM confers a lower risk for NTDs, while gestational diabetes does not appear to be associated with any significant increase in NTD risk. The degree of diabetic control is generally monitored using hemoglobin A1c levels.
Maternal hyperthermia
Maternal hyperthermia has been associated with an increased risk for NTD; therefore, pregnant women should avoid hot tubs and other environments that may induce transient hyperthermia. Similarly, maternal fever in early gestation also has been reported as a risk factor for anencephaly and other NTDs. [15]
Genetics
While most NTDs are associated with a multifactorial model of inheritance, rare cases of NTDs are transmitted in an autosomal dominant or autosomal recessive manner in certain families. Such families may have children or fetuses with spina bifida, anencephaly, or other subtypes of NTDs. In families with a pedigree suggestive of autosomal dominant inheritance, reproduction is clearly only possible for the individuals with spina bifida, since death occurs early in the life of individuals with anencephaly.
Anencephaly may be associated with the unbalanced form of a structural chromosome abnormality in some families. In these cases, other malformations and birth defects that are not usually found in isolated cases of anencephaly may be present.
Amniotic band disruption sequence
Amniotic band disruption sequence is a condition resulting from rupture of the amniotic membranes. This can cause disruption of normally formed tissues during development, including the structures of the head and brain. Anencephaly caused by amniotic band disruption sequence is frequently distinguishable by the presence of remnants of the amniotic membrane. Recurrence risk for anencephaly caused by this mechanism is lower, and the risk is not modified by the use of folic acid.
Epidemiology
Considerable geographical variation in neural tube defects (NTDs) rates exists, with noted hot spots in Guatemala, northern China, Mexico, and parts of the United Kingdom. Hispanic and non-Hispanic whites [16] are affected more frequently than women of African descent, and females are affected more frequently than males. Anencephaly is determined by the 28th day of conception and is therefore invariably present at the time of birth.
In the United States, about 1 in every 4600 babies is born with anencephaly. [12] The frequency during pregnancy is considerably higher than the birth prevalence, with estimates as high as 1 case per 1000 pregnancies. Such pregnancies often end in early pregnancy loss, spontaneous abortion, fetal death, or pregnancy termination.
Within the United States, South Carolina has historically reported the highest birth prevalence of NTDs, with a rate that has been approximately double that of the national average. The rate of NTDs in South Carolina has fallen dramatically following the introduction of aggressive campaigning for periconceptional folic acid supplementation, fortification of wheat flour, and increased periconceptional vitamin supplementation. [17] The reason for a higher occurrence of NTDs in South Carolina compared with other areas of the country is not known.
In 1990-1991, a cluster of NTDs was reported in Brownsville, Texas. [18] This primarily Hispanic population was targeted for surveillance as well as an intensive folic acid supplementation campaign directed at prevention of recurrences. Since that time, it has been generally accepted that the Hispanic population has an increased risk of anencephaly and other NTDs compared with other racial/ethnic populations in the United States, although the reasons have not been identified. [16, 19]
In families that have previously experienced a pregnancy affected with anencephaly, the use of folic acid supplements at a dose 10 times higher than what is generally advised for the general population (4 mg/day vs 400 mcg/day) is recommended. In the South Carolina study, more than 300 pregnancies have been followed from women with a prior NTD-affected pregnancy who received the higher dose of folic acid supplements as part of the follow-up program with no recurrences of NTDs observed.
Study of NTDs in the United States by the Centers for Disease Control and Prevention shows a significant reduction of anencephaly and other NTDs following the introduction of fortification of wheat flour with folic acid. During the period of 1996-2001, there was a 23% decline in spina bifida and anencephaly combined, with spina bifida declining by 24% and anencephaly by 21%. [20]
Prognosis
Anencephaly is lethal in all cases because of the severe brain malformation that is present. A significant proportion of all anencephalic fetuses are stillborn or are aborted spontaneously.
The neonate's prognosis when born alive is exceptionally poor; death of a live child is unavoidable and most often occurs during the early neonatal period.
History and Physical Examination
Anencephaly is readily apparent at birth because of the absence of the cranial vault and portions of the cerebrum and cerebellum. Facial structures are generally present and appear relatively normal. The cranial lesion occasionally is covered by skin, but usually it is not. When the lesion is covered with skin, prenatal screening using maternal serum alpha-fetoprotein (MSAFP) is ineffective. Babies are frequently stillborn, and spontaneous abortion during pregnancy is common. Although the features of anencephaly are readily evident, physical examination for anomalies not related directly to the anencephaly is indicated to evaluate the possible need for cytogenetic studies. When additional malformations are present, the likelihood of cytogenetic abnormalities is increased.
Lab Studies
Maternal serum alpha-fetoprotein (MSAFP) screening during the second trimester of pregnancy is an effective screening tool for identification of the vast majority of cases of anencephaly in women with or without a positive family history or other risk factors for neural tube defects. [21]
Amniotic alpha-fetoprotein (AFAFP) testing during the late first trimester and second trimester of pregnancy is a diagnostic biochemical test for anencephaly. False positives from AFAFP can be excluded based on the results of testing for acetylcholinesterase (ACHE), which should be clearly positive for open anencephaly.
Laboratory studies are not performed postnatally in most cases of isolated anencephaly. Cytogenetic testing can exclude trisomy 13 as well as unbalanced structural chromosome abnormalities. [22]
Imaging Studies
Prenatal 2-dimensional ultrasonography has steadily improved over the years and has superseded maternal serum alpha-fetoprotein measurements as a screening tool. Since ossification of the cranial vault is not consistently apparent prior to the completion of the 12th week of pregnancy, anencephaly should not be diagnosed by ultrasonography any earlier than this.
In the first trimester, absent calvarium, reduced crown-rump length, absent or exposed neural tissue with lobular appearance (exencephaly), and absence of the normal head contour geometry with the orbits demarcating the upper border of the face (coronal view) are associated with anencephaly. Later in pregnancy, polyhydramnios may arise as a result of reduced swallowing of the amniotic fluid.
Postnatal MRI findings have included absence of the cranial vault, supretentorial structures, and the cerebellum. [23]
Treatment & Management
Because anencephaly is a lethal condition, heroic measures to extend the life of the infant are contraindicated. The physician and medical care team should focus on providing a supportive environment in which the family can come to terms with the diagnosis and make preparations for their loss.
Families who are not aware of the diagnosis of anencephaly prior to birth or for whom the diagnosis is still fresh probably will need extra emotional support and possibly grief counseling. Families who have had some time to adjust to the diagnosis prior to delivery and who have had an opportunity to begin the grieving process ahead of time may seem well prepared, but they also will need adequate time to grieve and come to closure. The presence of family, friends, or clergy may be helpful in many cases.
Families often want to hold the baby after delivery, even if the baby is stillborn, and families wanting photographs of the baby with the family are not unusual. A cap or head covering of some sort is useful to minimize the visual impact of the malformation. Some families want to see the lesion, and this may help to dispel mental pictures, which are often worse than the actual malformations. In most cases, direct personal contact with the baby may help the parents to actualize the medical information they have been given and may help in the process of grief resolution.
If parents have chosen a name for the baby, they may be comforted if the doctor refers to the baby by name.
Feelings of guilt are normal responses of parents of a baby with serious birth defects. The involvement of genetic counselors, if available, may be particularly useful to parents in this situation because of their experience in dealing with a wide range of birth defects.
With timely prenatal diagnosis of this lethal disorder, the option of pregnancy termination should be presented to the couple. For couples who elect to continue the pregnancy, the possibilities of preterm labor, polyhydramnios, failure to progress, and delayed onset of labor beyond term also should be discussed.
Families commonly inquire about organ donation after the diagnosis of anencephaly. This cannot practically be arranged without crossing the lines of ethical care. Patients should be affirmed in their desires to see something meaningful come from the tragedy of having a pregnancy affected with anencephaly.
Pregnancy care
All patients diagnosed prenatally with a fetus affected by anencephaly should be offered a consultation with a care provider who is skilled in delivering grave information and is knowledgeable about recurrence risk, prevention, screening, and diagnostic testing options for future pregnancies.
Although a geneticist or genetics counselor is an ideal source and may be best suited for exploring family history, an experienced maternal fetal medicine physician or properly trained obstetrician may provide requisite information, especially in regions of the United States where there are inadequate numbers of geneticists or genetic counselors. Specific information related to the management of an ongoing pregnancy should be discussed during this consultation.
Once a diagnosis of anencephaly is made, pregnancy management varies according to the gestational age at diagnosis. At pre-viable gestational ages, the option of pregnancy termination should be among those discussed. The gestational age limits for this procedure are state specific and subject to the training and skill of the physician available to perform the pregnancy termination.
When patients choose not to proceed with pregnancy termination or when the pregnancy has progressed to a viable gestational age such that pregnancy termination is no longer an option (except in rare locations throughout the United States), attention should be focused on whether the labor will be induced or spontaneous and, if the former, at what gestational age.
Due to the physical stresses of pregnancy compounded by the emotional stress of carrying a fetus with a lethal birth defect, or because of the identification of medical conditions (eg, preeclampsia) that may complicate any pregnancy, labor induction may be considered.
Focused discussions directed at neonatal resuscitation efforts should be held in advance of labor. These discussions should include a discussion of neonatal procedures used to resuscitate neonates, the cost of these measures, and alternatives to aggressive resuscitation. It is often best to include a neonatologist in these discussions. Clear documentation of these discussions is warranted. When delivery is planned in a hospital setting, labor and delivery nurses, obstetric care providers, and pediatric/neonatal attendants should be informed of the patient’s wishes for her child.
Because of the lethal nature of this condition, tocolysis (medical management to reduce uterine contractions) in an effort to prevent preterm birth is not a reasonable option, nor is cesarean delivery.
The pregnancy management of a child with lethal and nonlethal birth defects can be complicated by available resources. In addition to having a wealth of experience in dealing with grieving patients, some delivering hospitals are vastly more experienced in the management of pregnancies complicated by known lethal fetal birth defects. For this reason we recommend that babies with anencephaly be delivered at such centers, when possible.
Consultations
Every couple with a child who has anencephaly should consult with a geneticist and/or a genetic counselor to obtain information regarding recurrence risks, prevention, screening, and diagnostic testing options for future pregnancies and to assess the family history. Ideally, a genetic counselor should be consulted prenatally and should remain involved, as needed, until the family comes to closure after the conclusion of the pregnancy. Genetic counselors are trained and are generally skillful in helping a family work through the complex psychosocial issues that are commonly encountered in a new diagnosis of anencephaly.
Diet
Folic acid supplementation and/or a folate-enriched diet prior to and during future pregnancies are recommended. Obtaining enough folates from diet alone to effectively prevent recurrences in future pregnancies is extremely difficult. The U.S. Public Health Service recommends that women capable of becoming pregnant consume 400 µg of folic acid daily for NTD prevention. [24]
Prevention
The recurrence risk for NTDs, in general, is 2-4% in subsequent pregnancies. For families with multiple occurrences of NTDs, recurrence risks may be higher and must be determined on a case-by-case basis.
Folic acid supplementation has been shown to be an effective means of lowering recurrence risks for future pregnancies. For women who desire pregnancy and have had a child with an NTD with their current partner, supplementation with 4 mg of folic acid daily is indicated, beginning at least 3 months prior to conception.
For all other women and girls of reproductive age, regardless of family history, 0.4 mg (or 400 mcg) per day of folic acid supplementation is appropriate; this amount of folic acid is found in most over-the-counter multivitamins. Folic acid supplementation at these levels is estimated to prevent two thirds of both recurrent and new cases of NTD.
Increased folate intake also may be achieved through diet; however, the bioavailability of natural folates in foods is often lower than that of folic acid. In the United States, wheat flour is fortified with a small amount of folic acid, but it is not enough to achieve maximal preventive benefits against NTD for a woman with an average diet.
Because of the large number of pregnancies that are not actively planned and the early gestational age at which neural tube development occurs, folate supplementation should be encouraged for all girls, beginning at puberty, in order to establish this practice before entering the childbearing years.
Prenatal ultrasound and amniocentesis should be offered to any couple with a prior pregnancy affected with an NTD. Maternal serum prenatal screening with AFP is available throughout the United States and most developed countries for identification of NTDs. Positive serum screening should be followed with diagnostic testing to exclude the presence of NTDs. Since 90-95% of NTDs occur in families without a positive history, such screening is appropriate for all pregnant patients and should not be reserved only for those with a positive history.
Anencephaly cannot be treated in utero; thus, pregnancy termination is the only intervention available to prevent the birth of a child with anencephaly that has been diagnosed prenatally. Supportive care should be provided for families, irrespective of the option they choose.
Complications
Anencephaly is uniformly fatal. Polyhydramnios is a common complication during pregnancy, and patients may experience significant discomfort from the abdominal distention that accompanies this condition. Risk of preterm labor is increased.
Because the pituitary gland may be absent in persons with anencephaly, spontaneous precipitation of labor may be delayed; therefore, the risk of the pregnancy progressing into the postterm period is significant. Labor may need to be induced in these cases. The rate of abnormal fetal presentations during delivery is increased.
Patient Education
Parents of babies with anencephaly should be educated about preventive measures for future pregnancies. Consultation with a genetic counselor may be helpful.
A number of resources may assist families who are dealing with the loss of a child with anencephaly. These include the Spina Bifida Association of America (SBAA) and the March of Dimes (MOD). Contact the SBAA at (800) 621-3141.
Families may wish to participate in one of several ongoing studies of anencephaly or NTDs as a part of the healing process.
-
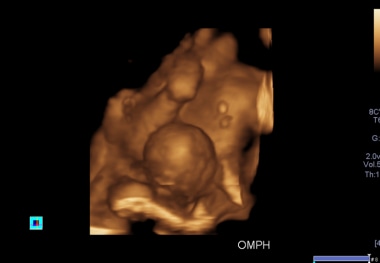
A 3D antennal ultrasound scan shows an omphalocele in one of the conjoined twins, associated with anencephaly in the first trimester, allowing termination of pregnancy.