Practice Essentials
Parkinson disease (PD) is one of the most common neurologic disorders, affecting approximately 1% of individuals older than 60 years and causing progressive disability that can be slowed, but not halted, by treatment. The 2 major neuropathologic findings in Parkinson disease are loss of pigmented dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies and Lewy neurites. See the images below.
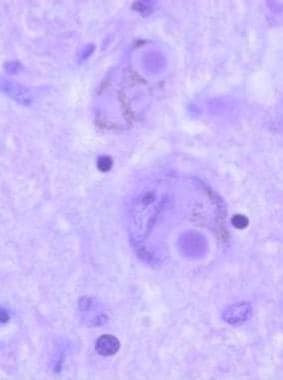 Lewy bodies are intracytoplasmic eosinophilic inclusions, often with halos, that are easily seen in pigmented neurons, as shown in this histologic slide. They contain polymerized alpha-synuclein; therefore, Parkinson disease is a synucleinopathy.
Lewy bodies are intracytoplasmic eosinophilic inclusions, often with halos, that are easily seen in pigmented neurons, as shown in this histologic slide. They contain polymerized alpha-synuclein; therefore, Parkinson disease is a synucleinopathy.
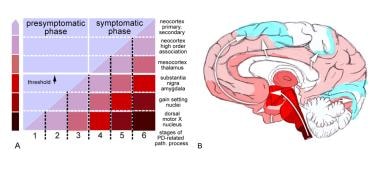 Stages in the development of Parkinson disease (PD)-related pathology (path.). Adapted from Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004 Oct;318(1):121-34.
Stages in the development of Parkinson disease (PD)-related pathology (path.). Adapted from Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004 Oct;318(1):121-34.
Signs and symptoms
Initial clinical symptoms of Parkinson disease include the following:
-
Tremor
-
Subtle decrease in dexterity
-
Decreased arm swing on the first-involved side
-
Soft voice
-
Decreased facial expression
-
Sleep disturbances
-
Rapid eye movement (REM) behavior disorder (RBD; a loss of normal atonia during REM sleep)
-
Decreased sense of smell
-
Symptoms of autonomic dysfunction (eg, constipation, sweating abnormalities, sexual dysfunction, seborrheic dermatitis)
-
A general feeling of weakness, malaise, or lassitude
-
Depression or anhedonia
-
Slowness in thinking
Onset of motor signs include the following:
-
Typically asymmetric
-
The most common initial finding is a resting tremor in an upper extremity
-
Over time, patients experience progressive bradykinesia, rigidity, and gait difficulty
-
Axial posture becomes progressively flexed and strides become shorter
-
Postural instability (balance impairment) is a late phenomenon
Nonmotor symptoms
Nonmotor symptoms are common in early Parkinson disease. Recognition of the combination of nonmotor and motor symptoms can promote early diagnosis and thus early intervention, which often results in a better quality of life.
See Clinical Presentation for more detail.
Diagnosis
Parkinson disease is a clinical diagnosis. No laboratory biomarkers exist for the condition, and findings on routine magnetic resonance imaging and computed tomography scans are unremarkable.
Clinical diagnosis requires the presence of 2 of 3 cardinal signs:
-
Resting tremor
-
Rigidity
-
Bradykinesia
See Workup for more detail.
Management
The goal of medical management of Parkinson disease is to provide control of signs and symptoms for as long as possible while minimizing adverse effects.
Symptomatic drug therapy
-
Usually provides good control of motor signs of Parkinson disease for 4-6 years
-
Levodopa/carbidopa: The gold standard of symptomatic treatment
-
Monoamine oxidase (MAO)–B inhibitors: Can be considered for initial treatment of early disease
-
Other dopamine agonists (eg, ropinirole, pramipexole): Monotherapy in early disease and adjunctive therapy in moderate to advanced disease
-
Anticholinergic agents (eg, trihexyphenidyl, benztropine): Second-line drugs for tremor only
Treatment for nonmotor symptoms
-
Sildenafil citrate (Viagra): For erectile dysfunction
-
Polyethylene glycol: For constipation
-
Modafinil: For excessive daytime somnolence
-
Methylphenidate: For fatigue (potential for abuse and addiction)
Deep brain stimulation
-
Surgical procedure of choice for Parkinson disease
-
Does not involve destruction of brain tissue
-
Reversible
-
Can be adjusted as the disease progresses or adverse events occur
-
Bilateral procedures can be performed without a significant increase in adverse events
See Treatment and Medication for more detail.
Background
Parkinson disease is recognized as one of the most common neurologic disorders, affecting approximately 1% of individuals older than 60 years. There are 2 major neuropathologic findings: the loss of pigmented dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies (see the following image). Most cases of Parkinson disease (idiopathic Parkinson disease [IPD]) are hypothesized to be due to a combination of genetic and environmental factors. However, no environmental cause of Parkinson disease has yet been proven. A known genetic cause can be identified in approximately 10% of cases, and these are more common in younger-onset patients.
 Gross comparison of the appearance of the substantia nigra between a normal brain and a brain affected by Parkinson disease. Note the well-pigmented substantia nigra in the normal brain specimen on the left. In the brain of a Parkinson disease patient on the right, loss of pigmented substantia nigra due to depopulation of pigmented neurons is observed.
Gross comparison of the appearance of the substantia nigra between a normal brain and a brain affected by Parkinson disease. Note the well-pigmented substantia nigra in the normal brain specimen on the left. In the brain of a Parkinson disease patient on the right, loss of pigmented substantia nigra due to depopulation of pigmented neurons is observed.
The classic motor features of Parkinson disease typically start insidiously and emerge slowly over weeks or months, with tremor being the most common initial symptom. The 3 cardinal signs of Parkinson disease are resting tremor, rigidity, and bradykinesia. Postural instability (balance impairment) is sometimes listed as the fourth cardinal feature. However, balance impairment in Parkinson disease is a late phenomenon, and in fact, prominent balance impairment in the first few years suggests that Parkinson disease is not the correct diagnosis. (See Presentation.)
When a patient presents with tremor, the clinician evaluates the patient's history and physical examination findings to differentiate Parkinson disease tremor from other types of tremor. In patients with parkinsonism, careful attention to the history is necessary to exclude causes such as drugs, toxins, or trauma. (See Differential Diagnosis.) Other common causes of tremor include essential tremor, physiologic tremor, and dystonic tremor.
No laboratory or imaging study is required in patients with a typical presentation of Parkinson disease. Such patients are aged 55 years or older and have a slowly progressive and asymmetric parkinsonism with resting tremor and bradykinesia or rigidity. There are no red flags such as prominent autonomic dysfunction, balance impairment, dementia, or eye-movement abnormalities. In such cases, the diagnosis is ultimately considered confirmed once the patient goes on dopaminergic therapy (levodopa or a dopamine agonist) as needed for motor symptom control and exhibits a robust and sustained benefit. (See Workup.)
Imaging studies can be considered, depending on the differential diagnosis. Magnetic resonance imaging (MRI) of the brain can be considered to evaluate possible cerebrovascular disease (including multi-infarct state), space-occupying lesions, normal-pressure hydrocephalus, and other disorders.
Iodine-123–labeled fluoropropyl-2beta-carbomethoxy-3beta-4-iodophenyl-nortroptane (FP-CIT I123) (Ioflupane, DaTscan) single-photon emission computed tomography (SPECT) can be considered in cases of uncertain parkinsonism to help differentiate disorders associated with a loss of dopamine neurons (Parkinson disease and atypical parkinsonisms, including multiple system atrophy [MSA] and progressive supranuclear palsy [PSP]) from those disorders not associated with a loss of dopamine neurons (eg, essential tremor, dystonic tremor, vascular parkinsonism, medication-induced parkinsonism or tremor, psychogenic conditions). [1]
Levodopa coupled with a peripheral decarboxylase inhibitor (PDI), such as carbidopa, remains the gold standard of symptomatic treatment of motor features of Parkinson disease. It provides the greatest antiparkinsonian benefit with the fewest adverse effects in the short term. However, its long-term use is associated with the development of fluctuations and dyskinesias. Moreover, the disease continues to progress, and patients accumulate long-term disability. (See Treatment.)
Dopamine agonists such as pramipexole (Mirapex) and ropinirole (Requip) can be used as monotherapy to improve symptoms in early Parkinson disease or as adjuncts to levodopa in patients who are experiencing motor fluctuations. Monoamine oxidase (MAO)-B inhibitors, such as selegiline (Eldepryl) and rasagiline (Azilect) provide mild benefit as monotherapy in early disease and as adjuncts to levodopa in patients with motor fluctuations. (See Medication.) Entacapone (Comtan), a catechol-o-methyltransferase (COMT) inhibitor, reduces the peripheral metabolism of levodopa, thereby making more levodopa available to enter the brain over a longer period; this agent is used as an adjunct to levodopa in patients with motor fluctuations.
Anatomy
Parkinson disease is predominantly a disorder of the basal ganglia, which are a group of nuclei situated at the base of the forebrain. The striatum, composed of the caudate and putamen, is the largest nuclear complex of the basal ganglia. The striatum receives excitatory input from several areas of the cerebral cortex, as well as inhibitory and excitatory input from the dopaminergic cells of the substantia nigra pars compacta (SNc). These cortical and nigral inputs are received by the spiny projection neurons, which are of 2 types: those that project directly to the internal segment of the globus pallidus (GPi), the major output site of the basal ganglia; and those that project to the external segment of the globus pallidus (GPe), establishing an indirect pathway to the GPi via the subthalamic nucleus (STN).
For an illustration of the subthalamic nucleus, see the image below.
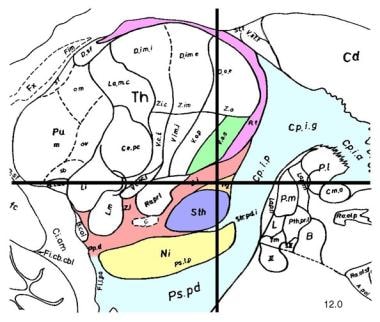 Sagittal section, 12 mm lateral of the midline, demonstrating the subthalamic nucleus (STN) (lavender). The STN is one of the preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
Sagittal section, 12 mm lateral of the midline, demonstrating the subthalamic nucleus (STN) (lavender). The STN is one of the preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
The actions of the direct and indirect pathways regulate the neuronal output from the GPi, which provides tonic inhibitory input to the thalamic nuclei that project to the primary and supplementary motor areas.
Pathophysiology
No specific, standard criteria exist for the neuropathologic diagnosis of Parkinson disease, as the specificity and sensitivity of its characteristic findings have not been clearly established. However, the following are the 2 major neuropathologic findings in Parkinson disease:
-
Loss of pigmented dopaminergic neurons of the substantia nigra pars compacta
-
The presence of Lewy bodies and Lewy neurites
The loss of dopamine neurons occurs most prominently in the ventral lateral substantia nigra. Approximately 60-80% of dopaminergic neurons are lost before the motor signs of Parkinson disease emerge.
Some individuals who were thought to be normal neurologically at the time of their deaths are found to have Lewy bodies on autopsy examination. These incidental Lewy bodies have been hypothesized to represent the presymptomatic phase of Parkinson disease. The prevalence of incidental Lewy bodies increases with age. Note that Lewy bodies are not specific to Parkinson disease, as they are found in some cases of atypical parkinsonism, Hallervorden-Spatz disease, and other disorders. Nonetheless, they are a characteristic pathology finding of Parkinson disease.
Motor circuit in Parkinson disease
The basal ganglia motor circuit modulates the cortical output necessary for normal movement (see the following image).
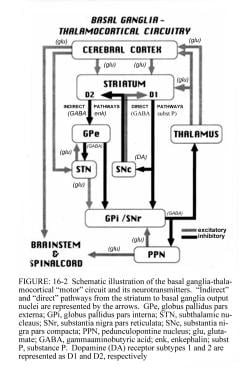 Schematic representation of the basal ganglia - thalamocortical motor circuit and its neurotransmitters in the normal state. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:240. Copyright, McGraw-Hill Companies, Inc. Used with permission.
Schematic representation of the basal ganglia - thalamocortical motor circuit and its neurotransmitters in the normal state. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:240. Copyright, McGraw-Hill Companies, Inc. Used with permission.
Signals from the cerebral cortex are processed through the basal ganglia-thalamocortical motor circuit and return to the same area via a feedback pathway. Output from the motor circuit is directed through the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). This inhibitory output is directed to the thalamocortical pathway and suppresses movement.
Two pathways exist within the basal ganglia circuit, the direct and indirect pathways, as follows:
-
In the direct pathway, outflow from the striatum directly inhibits the GPi and SNr; striatal neurons containing D1 receptors constitute the direct pathway and project to the GPi/SNr
-
The indirect pathway contains inhibitory connections between the striatum and the external segment of the globus pallidus (GPe) and between the GPe and the subthalamic nucleus (STN); striatal neurons with D2 receptors are part of the indirect pathway and project to the GPe
The STN exerts an excitatory influence on the GPi and SNr. The GPi/SNr sends inhibitory output to the ventral lateral nucleus (VL) of the thalamus. Dopamine is released from nigrostriatal (substantia nigra pars compacta [SNpc]) neurons to activate the direct pathway and inhibit the indirect pathway. In Parkinson disease, decreased striatal dopamine causes increased inhibitory output from the GPi/SNr via both the direct and indirect pathways (see the following image).
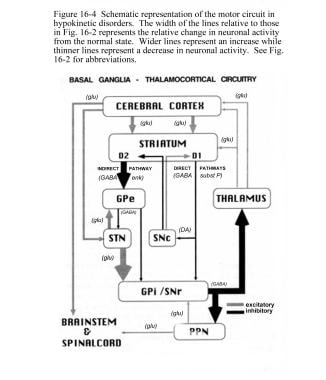 Schematic representation of the basal ganglia - thalamocortical motor circuit and the relative change in neuronal activity in Parkinson disease. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:241. Used with kind permission. Copyright, McGraw-Hill Companies, Inc.
Schematic representation of the basal ganglia - thalamocortical motor circuit and the relative change in neuronal activity in Parkinson disease. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:241. Used with kind permission. Copyright, McGraw-Hill Companies, Inc.
The increased inhibition of the thalamocortical pathway suppresses movement. Via the direct pathway, decreased striatal dopamine stimulation causes decreased inhibition of the GPi/SNr. Via the indirect pathway, decreased dopamine inhibition causes increased inhibition of the GPe, resulting in disinhibition of the STN. Increased STN output increases GPi/SNr inhibitory output to the thalamus.
Etiology
Although the etiology of Parkinson disease is still unclear, most cases are hypothesized to be due to a combination of genetic and environmental factors. Currently known genetic causes of Parkinson disease account for approximately 10% of cases.
Environmental causes
Environmental risk factors commonly associated with the development of Parkinson disease include use of pesticides, living in a rural environment, consumption of well water, exposure to herbicides, and proximity to industrial plants or quarries. [2]
A meta-analysis of 89 studies, including 6 prospective and 83 case-control studies, found that exposure to pesticides may increase the risk for PD by as much as 80%. [3, 4] Exposure to the weed killer paraquat or to the fungicides maneb or mancozeb is particularly toxic, increasing the risk for PD about 2-fold. Many of the agents studied are no longer used in the United States and Europe; however, some are still found in developing parts of the world. [3, 4]
In case-control studies, PD was associated with exposure to any type of pesticide, herbicide, insecticide, and solvent, with risks ranging from 33% to 80%. [3, 4] Increased PD risk was also associated with proxy conditions of exposure to organic pollutants, such as farming, well-water drinking, and rural living. In addition, risk seemed to increase with length of exposure. [3, 4]
The National Institutes of Health-AARP Diet and Health Study, as well as a meta-analysis of prospective studies, found that higher caffeine intake was associated with lower risk of Parkinson disease in both men and women. A similar association was found for smoking and Parkinson disease risk. [5] The biological mechanisms underlying the inverse relationship between caffeine or smoking and Parkinson disease risk are not well elucidated.
MPTP interference with mitochondrial function
Several individuals were identified who developed parkinsonism after self-injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). These patients developed bradykinesia, rigidity, and tremor, which progressed over several weeks and improved with dopamine replacement therapy. MPTP crosses the blood-brain barrier and is oxidized to 1-methyl-4-phenylpyridinium (MPP+) by monoamine oxidase (MAO)-B. [6]
MPP+ accumulates in mitochondria and interferes with the function of complex I of the respiratory chain. A chemical resemblance between MPTP and some herbicides and pesticides suggested that an MPTP-like environmental toxin might be a cause of Parkinson disease, but no specific agent has been identified. Nonetheless, mitochondrial complex I activity is reduced in Parkinson disease, suggesting a common pathway with MPTP-induced parkinsonism.
Oxidation hypothesis
The oxidation hypothesis suggests that free radical damage, resulting from dopamine's oxidative metabolism, plays a role in the development or progression of Parkinson disease. The oxidative metabolism of dopamine by MAO leads to the formation of hydrogen peroxide. Normally, hydrogen peroxide is cleared rapidly by glutathione, but if hydrogen peroxide is not cleared adequately, it may lead to the formation of highly reactive hydroxyl radicals that can react with cell membrane lipids to cause lipid peroxidation and cell damage. In Parkinson disease, levels of reduced glutathione are decreased, suggesting a loss of protection against formation of free radicals. Iron is increased in the substantia nigra and may serve as a source of donor electrons, thereby promoting the formation of free radicals.
Parkinson disease is associated with increased dopamine turnover, decreased protective mechanisms (glutathione), increased iron (a pro-oxidation molecule), and evidence of increased lipid peroxidation. This hypothesis has raised concern that increased dopamine turnover due to levodopa administration could increase oxidative damage and accelerate loss of dopamine neurons. However, there is no clear evidence that levodopa accelerates disease progression.
Genetic factors
If genetic factors are important in a particular disease, concordance in genetically identical monozygotic (MZ) twins will be greater than in dizygotic (DZ) twins, who share only about 50% of genes. Early Parkinson disease twin studies generally found low and similar concordance rates for MZ and DZ pairs.
However, genetic factors in Parkinson disease appear to be very important when the disease begins at or before age 50 years. In a study of 193 twins, overall concordance for MZ and DZ pairs was similar, but in 16 pairs of twins in whom Parkinson disease was diagnosed at or before age 50 years, all 4 MZ pairs, but only 2 of 12 DZ pairs, were concordant. [7]
The identification of a few families with familial Parkinson disease sparked further interest in the genetics of the disease. In one large family in Salerno, Italy, 50 of 592 members had Parkinson disease; linkage analysis incriminated a region in bands 4q21-23, and sequencing revealed an A-for-G substitution at base 209 of the alpha-synuclein gene. [8] Termed PD-1, this mutation codes for a substitution of threonine for alanine at amino acid 53. These individuals were characterized by early age of disease onset (mean age, 47.5 years), rapid progression (mean age at death, 56.1 years), lack of tremor, and good response to levodopa therapy. [8] Five small Greek kindreds were also found to have the PD-1 mutation.
In a German family, a different point mutation in the alpha-synuclein gene (a substitution of C for G at base 88, producing a substitution of proline for alanine at amino acid 30) confirmed that mutations in the alpha-synuclein gene can cause Parkinson disease. [9] A few additional familial mutations in the alpha-synuclein gene have been identified and are collectively called PARK1. It is now clear that these mutations are an exceedingly rare cause of Parkinson disease.
A total of 18 loci in various genes have now been proposed for Parkinson disease. Mutations within 6 of these loci (SNCA, LRRK2, PRKN, DJ1, PINK1, and ATP 13A2) are well-validated causes of familial parkinsonism. [10] Inheritance is autosomal dominant for SNCA and LRRK2 (although LRRK2 mutations exhibit variable penetrance). Inheritance is autosomal recessive for PRKN, DJ1, PINK1, and ATP13A2. In addition, polymorphisms within SNCA and LRRK2, as well as variations in MAPT and GBA, are risk factors for Parkinson disease. [10]
(For more information on genes/loci underlying monogenic parkinsonism and susceptibility genes/loci for Parkinson disease, see Tables 1 and 2, respectively, in The Genetics of Parkinson Disease. [10] )
In one study of 953 patients with Parkinson disease with age at onset of 50 years or younger, 64 patients (6.7%) had a PRKN mutation, 1 patient (0.2%) had a DJ1 mutation, 35 patients (3.6%) had an LRRK2 mutation, and 64 patients (6.7%) had a GBA mutation. [11] . Mutations were more common in patients with age at onset of 30 years or younger (40.6%) than in those with age at onset between 31 and 50 years (14.6%); more common in patients of Jewish ancestry (32.4%) than in non-Jewish patients (13.7%); and more common in patients reporting a first-degree family history of Parkinson disease (23.9%) than in those without such a family history (15.1%). [11]
Although the mechanisms by which genetic mutations cause Parkinson disease is not known, evidence to date converges on mechanisms related to abnormal protein aggregation, defective ubiquitin-mediated protein degradation, mitochondrial dysfunction, and oxidative damage.
Alpha-synuclein conformational changes and aggregation
Abnormally aggregated alpha-synuclein is the major component of Lewy bodies and Lewy neurites, which are characteristic pathologic findings in Parkinson disease. Missense mutations and multiplications in the SNCA gene that encodes alpha-synuclein, although rare, cause autosomal dominant Parkinson disease. However, genome-wide association studies have also demonstrated a link between SNCA and sporadic Parkinson disease.
Dysfunction of alpha-synuclein appears to play a central role in the pathogenesis of Parkinson disease, and understanding its relationship to the disease process holds major promise for the development of a cure.
Alpha-synuclein is a 140-amino-acid protein that is unfolded at neutral pH. However, when bound to membranes or vesicles containing acidic phospholipids, it takes on an alpha-helical structure. Normally, alpha-synuclein is found mainly in neuronal presynaptic terminals and may play a role in assembly and function of SNARE (soluble N-ethylmaleimide-sensitive factor activating protein receptor) proteins that are involved in neurotransmitter release.
Under certain conditions, alpha-synuclein aggregates into oligomers that are gradually converted to the beta–sheet-rich fibrillary structures that form Lewy bodies and neurites in Parkinson disease. Most evidence currently suggests that it is the intermediate soluble oligomers that are toxic to neurons.
Multiple mechanisms have been suggested as to how abnormally aggregated alpha-synuclein could exert neurotoxicity. [12] One hypothesis suggests that oligomeric alpha-synuclein can promote formation of ion-permeable pores on neuronal membranes, leading to increased calcium influx. Aberrant pore formation could also lead to neurotransmitter leaks from synaptic vesicles into the cytosol. In addition, overexpression of alpha-synuclein has been demonstrated to impair mitochondrial complex I activity, and oligomeric alpha-synuclein may have a direct effect on mitochondrial membranes. Other lines of evidence suggest that oligomerization of alpha-synuclein could cause cytoskeletal disruption, possibly by an effect on the microtubule-stabilizing protein, tau. [13]
Elevated levels of alpha-synuclein promote abnormal aggregation. levels are normally regulated by a balance between synthesis and degradation. SNCA multiplications lead to increased synthesis of alpha-synuclein and can cause Parkinson disease. Alpha-synuclein appears to be degraded by the ubiquitin proteasome system and the autophagy-lysosome pathway. Several genetic mutations associated with Parkinson disease may lead to decreased alpha-synuclein degradation. For example, increased risk of Parkinson disease in carriers of GBA (beta-glucocerebrosidase gene) mutations, which encode for the lysosomal enzyme glucocerebrosidase, may be due to lysosomal dysfunction and consequent alpha-synuclein accumulation and oligomerization.
How the Parkinson disease process begins is not known. Once it is initiated, however, it may propagate by a prionlike process in which misconformed proteins induce the templated misfolding of other protein molecules. In Parkinson disease, synuclein pathology begins in the lower brainstem and olfactory bulb, ascends up the midbrain, and eventually affects the neocortex. One set of observations in support of a prionlike process comes from experience with fetal dopaminergic grafts transplanted into the striata of patients with Parkinson disease, because these grafts develop Lewy bodies, suggesting host-graft transmission of disease. [14]
Preventing the propagation of abnormal alpha-synuclein aggregation may be the key to slowing or stopping Parkinson disease progression.
Melanoma
For years, there has been speculation about a relationship between PD and melanoma. Initially, it was theorized that the drug levodopa led to an increased risk of skin cancer, but studies did not confirm this. However, subsequent trials have since found an increased risk for melanoma in patients with PD. One particular study conducted in 2017 found that Parkinson patients have about a 4-fold increased risk of having preexisting melanoma. [15, 16] Another study found the risk to be 7-fold. [17]
Diabetes
In a large cohort study, researchers found that individuals with type 2 diabetes had a 32% increased risk of developing later Parkinson's disease than those without diabetes. The study involved 2 million people with type 2 diabetes and compared them to a reference cohort of 6,173,208 people without diabetes and results showed significantly elevated rates of Parkinson's disease in the type 2 diabetes cohort (hazard ratio [HR], 1.32, 95% confidence interval [CI], 1.29 - 1.35; P < .001). The relative increase was greater in patients with diabetic complications and in younger individuals with type 2 diabetes aged 25 to 44 years. [18]
Epidemiology
Parkinson disease is recognized as one of the most common neurologic disorders, affecting approximately 1% of individuals older than 60 years. The incidence of Parkinson disease has been estimated to be 4.5-21 cases per 100,000 population per year, and estimates of prevalence range from 18 to 328 cases per 100,000 population, with most studies yielding a prevalence of approximately 120 cases per 100,000 population. The wide variation in reported global incidence and prevalence estimates may be the result of a number of factors, including the way data are collected, differences in population structures and patient survival, case ascertainment, and the methodology used to define cases. [19]
The incidence and prevalence of Parkinson disease increase with age, and the average age of onset is approximately 60 years. Onset in persons younger than 40 years is relatively uncommon. Parkinson disease is about 1.5 times more common in men than in women.
Prognosis
Before the introduction of levodopa, Parkinson disease caused severe disability or death in 25% of patients within 5 years of onset, 65% within 10 years, and 89% within 15 years. The mortality rate from Parkinson disease was 3 times that of the general population matched for age, sex, and racial origin. With the introduction of levodopa, the mortality rate dropped approximately 50%, and longevity was extended by many years. This is thought to be due to the symptomatic effects of levodopa, as no clear evidence suggests that levodopa stems the progressive nature of the disease. [20, 21]
The American Academy of Neurology notes that the following clinical features may help predict the rate of progression of Parkinson disease [22] :
-
Older age at onset and initial rigidity/hypokinesia can be used to predict (1) a more rapid rate of motor progression in those with newly diagnosed Parkinson disease and (2) earlier development of cognitive decline and dementia; however, initially presenting with tremor may predict a more benign disease course and longer therapeutic benefit from levodopa
-
A faster rate of motor progression may also be predicted if the patient is male, has associated comorbidities, and has postural instability/gait difficulty (PIGD)
-
Older age at onset, dementia, and decreased responsiveness to dopaminergic therapy may predict earlier nursing home placement and decreased survival
Patient Education
Patients with Parkinson disease should be encouraged to participate in decision making regarding their condition. [23] In addition, individuals and their caregivers should be provided with information that is appropriate for their disease state and expected or ongoing challenges. [21] Psychosocial support and concerns should be addressed and/or referred to a social worker or psychologist as needed.
Prevention of falls should be discussed. The UK National Institute for Health and Clinical Excellence has several guidance documents including those for patients and caregivers.
Other issues that commonly need to be addressed at appropriate times in the disease course include cognitive decline, personality changes, depression, dysphagia, sleepiness and fatigue, and impulse control disorders. Additional information is also often needed for financial planning, insurance issues, disability application, and placement (assisted living facility, nursing home).
For patient education information, see the Brain & Nervous System Center, as well as Parkinson's Disease Dementia.
-
Schematic representation of the basal ganglia - thalamocortical motor circuit and its neurotransmitters in the normal state. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:240. Copyright, McGraw-Hill Companies, Inc. Used with permission.
-
Schematic representation of the basal ganglia - thalamocortical motor circuit and the relative change in neuronal activity in Parkinson disease. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:241. Used with kind permission. Copyright, McGraw-Hill Companies, Inc.
-
Parkinson disease diary. The patient or caregiver should place 1 check mark in each half-hour time slot to indicate the patient's predominant response during most of that period. The goal of therapeutic management is to minimize off time and on time with troublesome dyskinesia. Copyright Robert Hauser, 1996. Used with permission.
-
Stages in the development of Parkinson disease (PD)-related pathology (path.). Adapted from Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004 Oct;318(1):121-34.
-
Schematic diagram of the basal ganglia circuitry. Represented are the following: inhibitory (red arrows) and excitatory (green arrows) projections between the motor cortex, the putamen, the globus pallidus pars externa (GPe) and globus pallidus pars interna (GPi), the subthalamic nucleus (STN), the substantia nigra pars reticulata (SNr) and substantia nigra pars compacta (SNc), and the ventrolateral thalamus (VL). D1 and D2 indicate the direct (regulated by dopamine D1 receptors) and indirect (regulated by dopamine D2 receptors) pathways, respectively.
-
Sagittal section, 12 mm lateral of the midline, demonstrating the subthalamic nucleus (STN) (lavender). The STN is one of the preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
-
The deep brain stimulating lead is equipped with 4 electrode contacts, each of which may be used, alone or in combination, for therapeutic stimulation.
-
Axial, fast spin-echo inversion recovery magnetic resonance image at the level of the posterior commissure. The typical target for placing a thalamic stimulator is demonstrated (cross-hairs).
-
Implantation of the deep brain stimulation (DBS) lead.
-
Insertion of an electrode during deep brain stimulation for Parkinson disease.
-
Postoperative coronal magnetic resonance image (MRI) demonstrating desired placement of bilateral subthalamic nuclei-deep brain stimulation (STN-DBS) leads.
-
Radiograph of the skull depicting a deep brain stimulator and leads in a patient with Parkinson disease.
-
Lewy bodies in the locus coeruleus from a patient with Parkinson disease.
-
A: Schematic initial progression of Lewy body deposits in the first stages of Parkinson disease (PD), as proposed by Braak and colleagues. B: Localization of the cluster of significant volume reduction in PD compared with health control subjects. The significant cluster located in the medulla oblongata/pons is superimposed as a red blob on the mean normalized anatomic scan of all participants. The axial and sagittal sections are centered on the peak of significance (–1; –36; –49). This image using voxel-based morphometry (VBM), which searched for regional atrophy in idiopathic PD by comparing a group of subjects with the disease and a group of healthy controls. Jubault T, Brambati SM, Degroot C, et al. Regional brain stem atrophy in idiopathic Parkinson's disease detected by anatomical MRI. PLoS ONE. 2009;4(12):e8247.
-
Gross comparison of the appearance of the substantia nigra between a normal brain and a brain affected by Parkinson disease. Note the well-pigmented substantia nigra in the normal brain specimen on the left. In the brain of a Parkinson disease patient on the right, loss of pigmented substantia nigra due to depopulation of pigmented neurons is observed.
-
Lewy bodies are intracytoplasmic eosinophilic inclusions, often with halos, that are easily seen in pigmented neurons, as shown in this histologic slide. They contain polymerized alpha-synuclein; therefore, Parkinson disease is a synucleinopathy.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Symptomatic Therapy, Early Disease
- Symptomatic Therapy, Advanced Disease
- Putative Neuroprotective Therapy
- Deep Brain Stimulation
- Neuroablative Lesion Surgeries
- Preoperative Evaluation
- Neural Transplantation
- Gene Therapy
- Management of Psychiatric Comorbidities
- Exercise and Physical Therapy
- Speech Therapy
- Dietary Considerations
- Consultations
- Long-Term Monitoring
- Future Treatments for Parkinson Disease
- Show All
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References









