Overview
Cerebral reperfusion, or hyperperfusion, syndrome is a rare but serious complication that can occur following rapid revascularization of a partially or completely occluded artery with successful thrombolytic therapy, successful thrombectomy, carotid enterectomy (CEA), or carotid artery stenting (CAS).
Studies suggest that the incidence of cerebral reperfusion syndrome ranges from approximately 0.2% to 1.9%, though the exact incidence is unknown, in part due to variability in clinical definitions. [1, 2, 3, 4, 5, 6]
The clinical presentation of cerebral reperfusion syndrome varies but generally includes components of the following triad: 1) ipsilateral headache, 2) contralateral focal neurologic deficits, and/or 3) seizures. These symptoms can present independently or as sequelae of cerebral edema and/or symptomatic intracerebral hemorrhage (sICH).
Outcomes are dependent on timely recognition and prevention of precipitating factors, the most important of which is uncontrolled hypertension. When susceptible patients are identified and treated early, the prognosis is better and the risk of sICH is reduced. [7] The prognosis following symptomatic hemorrhagic transformation is poor. Mortality in such cases is 36%–63%, and 80% of survivors have significant morbidity. [8, 9, 10]
Nomenclature
The terms reperfusion and hyperperfusion are often used interchangeably. The former implies normalization of flow, while the latter suggests excessive flow. [11, 12] Hyperperfusion is defined as a major increase in ipsilateral cerebral blood flow (CBF) that is well above the metabolic demands of the brain tissue. Quantitatively, hyperperfusion is a 100% or greater increase in CBF compared with baseline. [11]
Symptom severity is not necessarily proportional to degree of reperfusion – both reperfusion and hyperperfusion can result in cerebral injury with similar clinical presentations. For example, while patients with hyperperfusion may be completely asymptomatic, patients with only moderate rises in CBF can have devastating outcomes. Since reperfusion is necessary, but hyperperfusion is not required, some authors prefer use of the broader term reperfusion syndrome. [12]
Symptoms of Cerebral Reperfusion Syndrome
Cerebral reperfusion syndrome presents with ipsilateral headache, contralateral focal neurologic deficits, and/or seizures, but a complete triad is not required for diagnosis. [11]
Headache is the most common symptom (62%). [13] Typically, the headache is migrainous in quality, characterized by severe, ipsilateral, pounding frontal/retroorbital head pain. Facial pain has also been reported.
Focal neurologic deficits are usually due to cortical injury or irritation and depend on the vascular territory involved. Symptoms can include hemiplegia, aphasia, and visual field deficit and neglect and range in severity from worsening of deficits caused by the initial ischemic injury to new symptoms localizing to a larger area within the same vascular territory.
Seizures can be focal or secondarily generalized. [11, 14]
Patients are usually symptomatic within the first week, [11, 8, 14] but the time of symptom onset can range from immediately after restoration of blood flow to up to one month later. Presentation may occur earlier after CAS when compared to CEA. [15]
Causes of Cerebral Reperfusion Injury
Pathophysiology
Several mechanisms have been proposed for the pathogenesis of cerebral reperfusion injury. Each theory is complex and none are widely accepted.
The extent of reperfusion injury likely depends on the extent of initial hypoperfusion, the time to collateral collapse, the volume of irreversible tissue damage, and the degree of autoregulatory and pro-inflammatory responses.
Impairment of cerebral autoregulation
Cerebral autoregulation protects the brain against sudden changes in systemic blood pressure. In the normally perfused brain, cerebral blood flow remains constant despite changes in systemic blood pressure. Conversely, in the chronically hypoperfused brain, autoregulation is impaired, and cerebral blood pressure varies with changes in systemic blood pressure. A sudden drop in systemic blood pressure could lead to cerebral ischemia, while, on the other hand, a sudden rise in systemic blood pressure could lead to edema or hemorrhage.
In the chronically hypoperfused brain, increased production of carbon dioxide and nitric oxide result in vasodilation and endothelial dysfunction. [16]
Collateral arterioles dilate to maintain flow. [17] A robust collateral circulation may be able to sustain adequate cerebral perfusion to maintain normal neurological function for a period of time. The capacity of the collateral circulation to do so is highly patient-specific and is dependent on patient risk factors, discussed later.
Eventually, the compensatory collateral circulation fails. Chronic dilation of the collateral vasculature damages smooth muscle cells comprising the vessel walls, resulting in loss of reactivity. The vessels are no longer able to change caliber in response to stimuli. Increased hydrostatic pressure and vessel permeability results in extravasation of proteins and fluid into the interstitial space and the development of cerebral edema.
Correction of a critical stenosis causes rapid and large changes in cerebral blood flow, which can lead to edema or hemorrhage. [18]
Endothelial damage
In cases of acute ischemia, endothelial damage disrupts the blood–brain barrier and promotes an inflammatory state, further contributing to the formation of edema and damage of tissue. Factors that contribute to this process include:
-
Early release of cytokines such as IL-1B, IL-6, and TNFα (peaking within the first 24 hours)
-
Increased expression of free radicals such as nitric oxide with subsequent vasodilation and increased vascular permeability
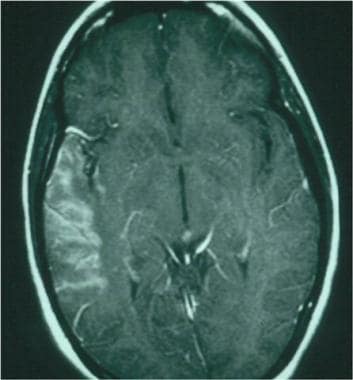 Postcontrast image 24 hours after a right middle cerebral artery stroke, demonstrating contrast extravasation through a faulty blood-brain barrier.
Postcontrast image 24 hours after a right middle cerebral artery stroke, demonstrating contrast extravasation through a faulty blood-brain barrier.
Overview of risk factors
For the time being, potential risk factors for development of cerebral reperfusion syndrome include but are not limited to the following:
-
Higher degree of vessel stenosis prior to recanalization [19]
-
Female sex [21]
-
Recent contralateral CEA (within three months) [1]
-
Intraoperative ischemia
Hypertension
Elevated blood pressure is the most common risk factor found in symptomatic patients diagnosed with reperfusion syndrome. [13, 27, 28] During acute ischemic stroke, systemic blood pressure often rises as a physiologic compensatory response to cerebral ischemia. [29] Small-caliber capillary vessels are unable to maintain structural integrity against increased pressure, thus predisposing patients to hypertension-related hemorrhagic transformation. [30] In patients with incomplete revascularization of arterial occlusion, blood pressure is allowed to remain elevated (permissive hypertension), so as not to compromise flow to the tenuous penumbra. However, in patients with restored perfusion, some studies suggest that a lower blood pressure goal may be warranted to prevent reperfusion injury.
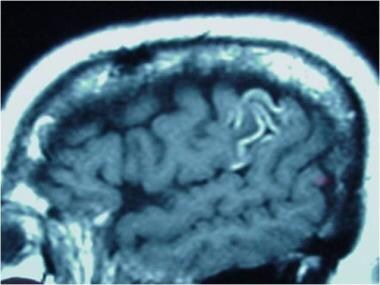 T1 sagittal image without contrast demonstrating gyriform hyperintensities. These represent subacute petechial hemorrhage around an area of subacute infarction secondary to uncontrolled hypertension.
T1 sagittal image without contrast demonstrating gyriform hyperintensities. These represent subacute petechial hemorrhage around an area of subacute infarction secondary to uncontrolled hypertension.
Risk factors for hemorrhagic transformation
While uncontrolled hypertension is the most common risk factor for hemorrhagic transformation, factors such as older age, stroke severity, hyperglycemia, and delayed revascularization are also thought to increase risk.
Assessment of Risk for Reperfusion Injury in Carotid Endarterectomy and Stenting
Patient selection based on physiologic parameters likely plays an important role in reducing late hemorrhage attributable to carotid revascularization.
Preoperative transcranial Doppler ultrasonography
Transcranial Doppler (TCD) ultrasonography measures cerebral blood flow in major cerebral arteries. Low preoperative distal carotid artery pressure (< 40 mm Hg) and increased peak blood flow velocity have been found to be predictive of postoperative hyperperfusion. [18, 22] Preoperative and postoperative TCD can be used to select patients for aggressive postprocedure observation for hyperperfusion and expedited management.
Preoperative acetazolamide SPECT scanning
Cerebrovascular reactivity (CVR) to carbon dioxide can be utilized to test cerebral hemodynamic reserve. Normally, administration of acetazolamide (a carbonic anhydrase inhibitor that causes a local increase in carbon dioxide) induces a rapid increase in CBF. [31] This iatrogenic CBF surge is measured using single-photon emission computed tomography (SPECT) scanning.
In chronic cerebral ischemia, the vasculature is maximally dilated, resulting in reduced CVR. Patients with low preoperative CVR are at increased risk for developing hyperperfusion and subsequent parenchymal injury. [7, 32]
Risks of Specific Revascularization Treatments
In the absence of revascularization therapy, asymptomatic hemorrhagic transformation is a common and natural consequence of infarction. [33, 34]
In the setting of revascularization therapy, symptomatic hemorrhagic transformation rates are increased, regardless of modality (ie, intravenous lytics, intra-arterial lytics, antithrombotics, or mechanical devices). [35]
Reperfusion injury after thrombolytic therapy
In revascularization with thrombolytics, the question remains as to whether increased rates of hemorrhagic transformation are due to reperfusion or if they are specific consequences of the lytic state itself.
Rates of sICH with intravenous thrombolysis are often quoted at 6.4% per NINDS (and as high as 8.8% in ECASS-II). [36, 37, 38] Higher rates of sICH were seen in intra-arterial thrombolytic trials (eg, 10% in PROACT-II). [39]
Early trials evaluated the efficacy and safety of IV-thrombolysis utilized agents such as urokinase and alteplase. Results of the recent EXTEND-IA TNK trial suggest that, in patients undergoing endovascular mechanical thrombectomy, an alternative thrombolytic agent, tenecteplase (tNK) is more effective at acheiving TICI2b or better at the time of first run when compared to alteplase. Complications of sICH were equivalent in both groups. [40]
Reperfusion injury after endovascular mechanical thrombectomy
The use of readily available perfusion imaging to screen for potentially salvageable brain tissue, faster door-to-recanalization times, and the introduction of next-generation stent retriever devices led to the completion and publication of six positive endovascular mechanical thrombectomy trials in 2015 and 2016, making it the new standard of care for acute ischemic strokes resulting from large artery occlusion in the anterior circulation. [41, 42, 43, 44, 45, 46]
Acute ischemic strokes due to proximal cerebral artery occlusions can have large territories that are highly vulnerable to reperfusion injury following revascularization. Longer periods of hypoperfusion to these areas, particularly with extension of the therapeutic treatment window beyond 6 hours, could result in increased areas of tissue susceptible injury.
The rates of symptomatic ICH following revascularization with a device are lower than with thrombolytic therapy and range from 2% to 4% with the Solitaire and Trevo stent retriever systems, respectively. [47, 48]
While somes studies have demonstrated that higher blood pressure goals following thrombectomy correlated with worse clinical outcomes, [49, 50] perhaps in part due to the negative effects of reperfusion injury, results of CATIS-II and BEST-II suggested that a lower target conferred limited benefit and might worsen long-term disability. Further studies are needed to inform best practices.
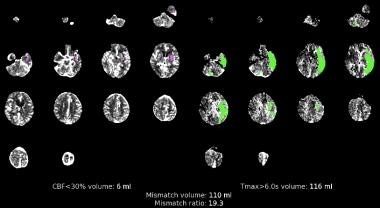 CT perfusion scan. Matched image sets with irreversible injury shown in magenta on the left and at-risk tissue (penumbra) shown in green on the right.
CT perfusion scan. Matched image sets with irreversible injury shown in magenta on the left and at-risk tissue (penumbra) shown in green on the right.
Reperfusion injury after carotid endartectomy and stenting
As mentioned previously, hyperperfusion syndrome may present earlier in patients who have undergone CAS when compared to those who have undergone CEA. Results of meta-analyses comparing the risk of hyperperfusion syndrome after CEA versus CAS are conflicting, and there are multiple potential confounders to consider, including differences in age of studies included (CEA studies are older), variations in disease definition, patient selection (patients undergoing CAS are often selected for high-risk features), and post-procedural medication protocols (dual antiplatlet therapy (DAPT) post stenting). Trends toward increased risk of ICH with CAS may neglect the requirement for post-procedural DAPT. [51, 52]
Prevention of Reperfusion Injury
Blood pressure control
The most important factor in preventing reperfusion syndrome is early identification and control of hypertension. [8, 9, 10] This is important even in normotensive patients, since delayed hypertension can occur. [14]
Patients should be observed postoperatively in an intensive care unit (ICU) and blood pressure should be managed in this setting until stabilized.
Transcranial Doppler (TCD) ultrasonography can be utilized to identify patients who are at especially high risk for cerebral reperfusion syndrome. It is used preoperatively to identify patients with reduced CVR and postoperatively to identify patients with increased CBF. [7, 32] Blood pressure should be controlled aggressively if CBF elevates.
Specific guidelines for an optimal blood pressure target in patients with cerebral reperfusion syndrome are not well established and a patient-centered approach factoring in individualized risk factors is generally recommended. It may be reasonable to extrapolate a target from the following two guidelines:
-
The 2019 Guidelines for Management of Acute Ischemic Stroke state that in patients who undergo mechanical thrombectomy, it is reasonable to maintain the BP at ≤ 180/105 mm Hg during and for 24 hours after the procedure (COR IIa, LOE B-NR) and may be reasonable to target an even lower goal in cases of successful recanalization, with the DAWN protocol suggesting a target SBP < 140 mm Hg. [53]
-
The 2022 Guideline for the Management of Spontaneous Intracerebral Hemorrhage states that acute lowering of SBP to a target of 140 mm Hg with the goal of maintaining in the range of 130–150 mm Hg is safe and may be reasonable for improving functional outcomes in patients with spontaneous ICH of mild to moderate severity presenting with SBP 150–220 mm Hg (COR IIb, LOE B-R). [54]
Regardless of the specific goal, pressures should be reduced smoothly and gradually, using antihypertensives that do not increase CBF or cause excessive vasodilatation. Preferred medications include labetalol (Normodyne, Trandate) and nicardipine (Cardene). Less favored medications include intravenous angiotensin-converting enzyme (ACE) inhibitors, calcium-channel blockers, and vasodilators such as nitroprusside. [7, 55]
-
Postcontrast image 24 hours after a right middle cerebral artery stroke, demonstrating contrast extravasation through a faulty blood-brain barrier.
-
A. Schematic representation of the process of endothelial-dependent leukocyte adhesion. Endothelial cells activated by histamine or thrombin rapidly translocate P-selectin to their surfaces (also E-selectin, not shown), tethering leukocytes to the endothelial cell. This tethering does not require an active response from the leukocyte. Once tethered, other factors, including platelet-activating factor and cytokines, are released to stimulate a leukocyte activation response. This response includes shape-changing and increased surface expression of CD-11/CD-18. CD-11/CD-18 then binds to the corresponding intercellular adhesion molecule 1 (ICAM-1) receptor on the endothelial cell, leading to firm endothelial attachment. This attachment may produce direct obstruction of the microcirculation or lead to infiltration into the surrounding brain parenchyma. B. Schematic representation showing that through the use of monoclonal antibodies directed against the ICAM-1 receptor, the CD-11/CD-18 to ICAM-1 attachment is prevented. This, in turn, prevents subsequent microvascular obstruction and leukocyte infiltration.
-
T1 sagittal image without contrast demonstrating gyriform hyperintensities. These represent subacute petechial hemorrhage around an area of subacute infarction secondary to uncontrolled hypertension.
-
CT perfusion scan. Matched image sets with irreversible injury shown in magenta on the left and at-risk tissue (penumbra) shown in green on the right.








