Overview
Despite decades of enormous effort, the cause of Parkinson's disease (PD) remains unclear and a preventive treatment unavailable. However, dopaminergic neuron depletion in substantia nigra led to a wide acceptance of levodopa therapy. But, the long-term complications of this therapy and a better understanding of the basal ganglia activity and interconnections (see the image below) as well as progress in surgical techniques, [1] neuroimaging, [2] and electrophysiologic recording have allowed surgical procedures to be performed more accurately [3, 4] and have furthered the interest in stereotactic surgery as a treatment for PD and the levodopa-related dyskinesias. [5]
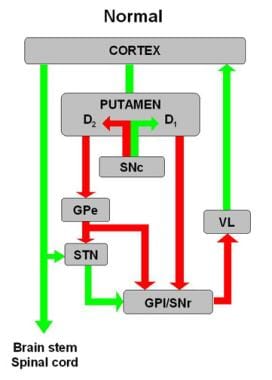 Schematic diagram of basal ganglia circuitry. Inhibitory (red arrows) and excitatory (green arrows) projections between motor cortex, putamen, globus pallidus pars externa (GPe) and globus pallidus pars interna (GPi), subthalamic nucleus (STN), substantia nigra pars reticulata (SNr) and substantia nigra pars compacta (SNc), and ventrolateral thalamus (VL) are represented. D1 and D2 indicate direct (regulated by dopamine D1 receptors) and indirect (regulated by dopamine D2 receptors) pathways, respectively.
Schematic diagram of basal ganglia circuitry. Inhibitory (red arrows) and excitatory (green arrows) projections between motor cortex, putamen, globus pallidus pars externa (GPe) and globus pallidus pars interna (GPi), subthalamic nucleus (STN), substantia nigra pars reticulata (SNr) and substantia nigra pars compacta (SNc), and ventrolateral thalamus (VL) are represented. D1 and D2 indicate direct (regulated by dopamine D1 receptors) and indirect (regulated by dopamine D2 receptors) pathways, respectively.
Based on the concept introduced by Horsley and Clarke (1908) and refined by Leksell (1949), [6] neuroablative (cryo-, radiation, radiofrequency, and electrical) stereotactic surgery techniques were widely used for numerous neurological and psychological disorders. [7] It was introduced for PD patients who have motor fluctuations and extended to patients with dyskinesia that cannot be adequately managed with pharmacologic therapy. However, since 1997 and due to a relatively low success rate, neuroablation has been replaced by deep brain stimulation (DBS). [8] Other stereotactic but mostly experimental surgical approaches include stem cell therapy, genetically engineering cells transplantation, and viral vector-based medical and gene therapies (addressed below).
Depending on the systems, a contemporary stereotactic surgery is based on the RA Brown N-Localizer device, [9] which combines imaging and real data, allowing for a precise yet "blind" targeting of the deep brain structures with minimal trauma to the tissue. The image below depicts a Cartesian system frame (x,y,z coordinates) secured to the patent's head allowing for a precise approach to any point in the brain. Today, with development of computed tomography (CT) and magnetic resonance imaging (MRI), this technique as well as ventriculography have been replaced by the N-localizer method. However, the interest in the Cartesian system has been revived with a nascent of a robotic-assisted stereotactic placement of electrodes for DBS, both frame-based and frameless, as it facilitates the targeting process and eliminates human errors while targeting deep brain structures for PD [10] as well as minimizes the necessity of analgesia and/or anesthesia. [11]
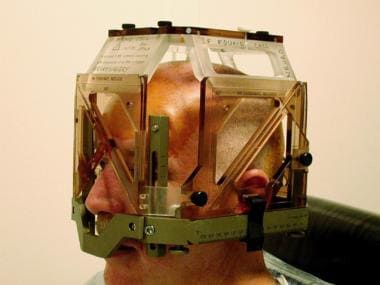 Stereotactic headframe is applied at start of surgery. MRI-localizing box is attached to frame only during targeting MRI. Localizer defines working volume of frame and provides reference coordinate system from which target coordinates are derived.
Stereotactic headframe is applied at start of surgery. MRI-localizing box is attached to frame only during targeting MRI. Localizer defines working volume of frame and provides reference coordinate system from which target coordinates are derived.
CT-guided stereotaxis provides direct imaging of brain parenchyma without MRI image distortion; however, its gray-white resolution is inferior to that of MRI and it exposes patients to a significant amount of radiation during longer procedures. MRI provides better target resolution and 3D imaging; however, some smaller targets may not be visualized. Although the MRI distortions are usually small, they affect the precision required in targeting for functional neurosurgery. Thus, it is necessary to use intraoperative neurophysiologic monitoring to confirm the accuracy of reaching a target (see the image below). [12] A CT and MRI image fusion technique [13, 14] with support of neurophysiologic stimulation and recordings has proven to be advantageous, as a combination of these techniques improves clinical outcome and reduces morbidity. [15]
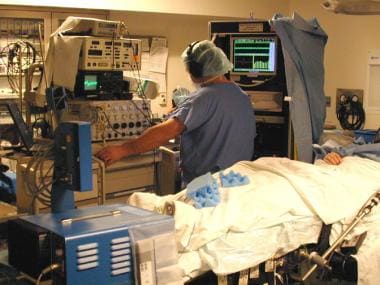 Intraoperative physiologic monitoring equipment. Surgical team, consisting of neurosurgeon, neurologist, and highly trained neurophysiologist (pictured), employs single-cell microelectrode recording to define surgical target physiologically.
Intraoperative physiologic monitoring equipment. Surgical team, consisting of neurosurgeon, neurologist, and highly trained neurophysiologist (pictured), employs single-cell microelectrode recording to define surgical target physiologically.
Deep Brain Stimulation
For years, primary stereotactic treatment of movement disorders involved neuroablative techniques that destroyed abnormally hyperactive deep brain nuclei. [16] However, the observation that high-frequency electrostimulation in the ventral lateral nucleus (VL) of the thalamus eliminates tremors in patients undergoing thalamotomy established deep brain stimulation (DBS) as an alternative to neuroablation. [8, 17, 18]
DBS is a surgical procedure that compromises stereotactic implantation of electrodes (usually bilaterally) in the deep structures of the brain through image-guided skull burr holes. Those electrodes are connected with wires to a neurostimulator placed under the skin of the chest or abdomen. Controlled by the patient, a neurostimulator generates electric impulses, which modulate a specific neuronal circuitry's activity evoking a desired clinical response. DBS has become the surgical procedure of choice for PD because (1) it is more effective in reducing both Parkinson's and levodopa-related dyskinesia symptoms; [18] (2) it minimalizes injury to the brain; (3) it is reversible; [19] (4) it can be adjusted as the disease progresses or adverse events occur; and (5) it allows for simultaneous bilateral stimulations. [15]
Continued advances of understanding of basal ganglia circuitry and PD pathophysiology has focused movement disorder surgery on 3 deep brain structures (see image below):
-
globus pallidus (GPi) - affecting tremors, slowness, rigidity, dystonia, and dyskinesia
-
thalamus - affecting tremor
-
subthalamic nucleus (STN) - affecting tremor, slowness, rigidity, dystonia, and dyskinesia
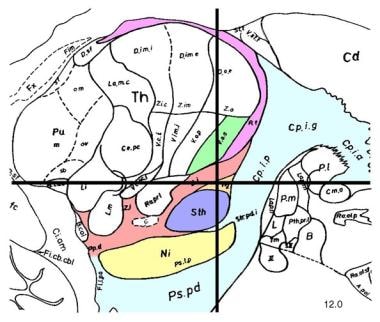 Sagittal section, 12 mm lateral to midline, demonstrating subthalamic nucleus (STN; lavender). STN is one of preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
Sagittal section, 12 mm lateral to midline, demonstrating subthalamic nucleus (STN; lavender). STN is one of preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
The most common targets are the STN and GPi, which have shown similar motor improvements, with DBS of the STN additionally reducing the dosage of levodopa and DBS of the GPi producing fewer cognitive side effects. [20]
Advances in understanding of the cerebellar influence on thalamic nuclei through the dentato-rubro-thalamic tract (cerebello-thalamic connections) in evoking tremor introduced yet another target for DBS: a posterior subthalamic/caudal zona incerta, [16] which is more effective and efficient than VIM stimulation [21] for control of tremor even if applied unilaterally. [22]
Several randomized clinical trials of patients with advanced PD found that bilateral DBS was more effective than optimal medical therapy in improving symptoms, motor function, and quality of life as well as that it was safe and provided long-lasting beneficial results. [18] However, DBS was associated with an increased risk of serious adverse events. [23, 8, 17] Those DBS complications include a clinically relevant intracerebral hemorrhage, infections, and hardware malfunction as well as neurological symptoms like paresthesias, dysarthria, dysphasia, hypophonia, and ataxia, which were more often observed in bilateral DBS. [24] Advances in the development of DBS instrumentation, programable electrodes and stimulators and, especially, MRI compatible components, further facilitated the use of DBS. Based on those findings, guidelines have been developed to help neurologists and general physicians identify PD patients who may benefit without an increased risk from DBS treatment. [25, 15, 19]
A more extensive discussion of DBS in this setting, including mechanisms of action, advantages and disadvantages, and stages of the procedure, is provided in Deep Brain Stimulation in Parkinson's Disease.
Neuroablative Lesion Surgeries
Deep brain stimulation (DBS) has become the preferred treatment for Parkinson's disease (PD) and motor complications of its treatment. However, DBS cannot be used for patients with immunodeficiencies or infections and other medical exemptions or religious and psychological objections. Moreover, the cost of DBS can be prohibitive, particularly in less affluent countries, leaving neuroablation as a reasonable and effective alternative. [26]
Neuroablative surgeries, which involve the destruction of targeted areas of the brain to control the symptoms of PD or a treatment complicating dyskinesias, have been heralded as being completely replaced by DBS. [1] However, despite DBS becoming the treatment of choice, neuroablation continues to be performed around the world successfully when DBS is contraindicated and unavailable to address PD, levodopa-related dyskinesias, or when the symptoms are unilateral. [27] Moreover, one analysis revealed that in many situations DBS is considered a better option than neuromodulation when a cost comparison between DBS and pallidotomy is taken into account. [28]
Furthermore, PD surgery is more often used when motor symptoms (tremors) are dominant, as this intervention does not help non-motor effects such as reduced facial expression and dry skin. [29]
Neuroablation destroys a specific deep brain target using thermo- or cryo-coagulation, radiofrequency, or radiation. Those surgical procedures include thalamotomy, pallidotomy, and subthalamotomy. [5]
Thermal and cryoablation require a scalp incision, drilling skull burr hole(s), and a brain penetration, which can cause serious medical complications, such as bleeding to the brain and brain infection, as well as side effects like abnormal movements and/or sensations and confusion. [5] However, deep brain lesions can also be performed with a laser or radiofrequency without cutting the skin and/or drilling the skull. [30]
Radiofrequency is used to produce heat of the lesioning electrode; DBS and radiofrequency thalamotomy results are equally beneficial in controlling tremors. [31] Also, radiation using a Gamma Knife has been advocated for neuroablation. [32] For obvious reasons, surgical complications are more often encountered with thalamotomy than with DBS. [16]
The most commonly performed neuroablative procedures are thalamotomy and pallidotomy, with subthalamotomy increasing in popularity. [26]
Lesions from stereotactic thermocoagulation or other techniques are created in the ventral lateral thalamic nucleus (VL), the internal segment of the globus pallidus (GPi; the globus pallidus medialis), and the subthalamic nucleus (STN), respectively.
Ventrolateral thalamotomy
The VL receives afferent innervation from 2 primary sources: the GPi via the ansa lenticularis and thalamic fasciculus and the contralateral cerebellum via the superior cerebellar peduncle. These cerebellar fibers synapse primarily in the ventral intermediate (VIM) and ventral oral posterior (VOP) segments, the most posterior segments of the VL.
Cellular activity synchronous with the frequency of PD tremor has been recorded in the VL in response to oscillating excitatory input from the cerebellum, strongly suggesting a cerebellar influence on thalamic nuclei through the dentato-rubro-thalamic tract (cerebello-thalamic connections) in evoking tremor, which introduced another target for PD treatment: a posterior subthalamic/caudal zona incerta(29), which is more effective and efficient than VIM [21] for control of tremor even if applied unilaterally. [22]
VL thalamotomy was the most frequently performed procedure for movement disorders in the pre-levodopa era because of the immediate control of tremor providing easy monitoring of ablation results in the operating room, which was simpler than assessing gait, rigidity, or akinesia. [33, 32]
In this procedure, the VIM ablation provides excellent short- and long-term tremor suppression. But thalamotomy has little effect on bradykinesia, rigidity, motor fluctuations, or dyskinesia, which are successfully treated with ablation of the GPi and the STN.
Therefore, thalamotomy is indicated for PD patients who are disabled by a medically refractory tremor. Nevertheless, the outcome benefit of tremor reduction or elimination might be unsatisfactory when bradykinesia and rigidity reduce dexterity. Eighty to 90% of patients with PD who undergo thalamotomy have significant improvement in a tremor of the limbs contralateral to the side of the lesion. Bilateral thalamotomy is avoided because of the increased risk of surgical complications.
The mortality for thalamotomy due almost exclusively to an intraparenchymal hemorrhage ranges from 0.5% to 1%.
The morbidity for thalamotomy is reported to range from 9% to 23%. The predominant complication is speech impairment with dysarthria and hypophonia. The risk of speech abnormalities is 30% after unilateral thalamotomy and greater than 60% after bilateral thalamotomy. Other complications include cognitive impairment, memory loss, contralateral hemiparesis, and rarely, hemineglect, dystonia, hemiballismus, athetosis, and dyspraxia. [18]
Preoperative memory and language evaluation can help identify patients who are at greatest risk for postoperative dysfunction.
Pallidotomy
Although Svennilson et al described ventral posterior pallidotomy back in the 1960s, [34] their report was largely overlooked. The original pallidotomy target was in the medial and anterodorsal parts of the nucleus. This so-called medial pallidotomy effectively relieved rigidity but inconsistently improved tremor.
Leksell subsequently moved the target to the posteroventral and lateral GPi, achieving sustained improvement in as many as 96% of patients. In 1992, Laitinen et al reported reduced tremor, rigidity, akinesia, and levodopa-induced dyskinesia in 38 patients treated with pallidotomy, prompting a reappraisal of the procedure performed with more modern techniques. [35]
The negative symptoms of PD (ie, rigidity and bradykinesia) are caused, in part, by excessive inhibitory output from the GPi to the VL thalamic nucleus. Lesioning of the sensorimotor region of the GPi, which lies ventral and posterior in the nucleus, decreases this hyper-inhibition of the motor thalamus. [27] Radiofrequency and radiosurgery pallidotomy were used to control the tremor in patients who were poor candidates for DBS, and the interest in using this target for those patients further increased with the introduction of radiofrequency (focused ultrasound) technology, which eliminates skin, skull, and brain openings. [1]
Pallidotomy involves the destruction of a part of the GPi. Pallidotomy studies have demonstrated significant improvements in each of the cardinal symptoms of PD (tremor, rigidity, bradykinesia), as well as a significant reduction in dyskinesia. [28] However, the tremor improvement is less consistent than that seen with thalamotomy. [5]
The most serious and frequent (3.6%) adverse effect of pallidotomy is a scotoma in the contralateral lower-central visual field. This complication occurs when the GPi lesion extends into the optic tract, which lies immediately below the GPi. The risk of the visual-field deficit is reduced greatly by accurate delineation of the ventral GPi border by microelectrode recording.
Less frequent complications (< 5%) include injury to the internal capsule, facial paresis, and intracerebral hemorrhage (1–2%). Abnormalities of speech, swallowing, and cognition may also be observed. Bilateral pallidotomy is not recommended, because complications, including speech difficulties, dysphagia, and cognitive impairment, are relatively common. [28]
Subthalamotomy
Hyperactivity of the excitatory projections of the STN to the GPi is a crucial physiologic feature of PD. Subthalamotomy involves the destruction of a part of the STN. Although lesioning of the STN usually has been avoided because of concerns that hemiballismus might develop, results of some studies showed that subthalamotomy is safe and reverses parkinsonism dramatically with significant improvements in all features of PD, as well as reductions of motor fluctuations and dyskinesias. [26]
Evaluation of Patients for Stereotactic Surgery
Good surgical outcomes from stereotactic surgery for Parkinson's disease (PD) begin with careful patient selection and end with attentive, detail-oriented postoperative care. The authors believe that this level of care is best provided by a multidisciplinary team comprising a movement disorder neurologist, a neurosurgeon who is well versed in stereotactic technique, a neurophysiologist, a psychiatrist, and a neuropsychologist. Additional support from neuroradiology and rehabilitation medicine is also important. But, as a European-based survey revealed, [36] having the proper clinical support is only one of the challenges that stereotactic surgery for PD faces. There are also issues of regulatory, technical, scientific, and intellectual property rules as well as public perception challenges that have to be overcome.
In addition to being medically evaluated, patients are evaluated for surgery in the movement disorder centers by a neurologist, a neurosurgeon/neuro-radiotherapist, and a psychiatrist/psychologist.
A neurologist with expertise in movement disorders evaluates the patient to assure they are a good candidate for a successful subthalamic nucleus deep brain stimulation (STN-DBS) to confirm [37] a diagnosis of idiopathic PD, positive response to levodopa, absence of atypical parkinsonian features, and assess the advancement of disease as unmanageable with dopaminergic medications. Other requirements include:
-
Relatively young age; however, advanced age (> 75 y) is not an absolute contraindication to surgery (if a patient otherwise meets the selection criteria for a procedure and the quality of life is predicted to improve substantially, surgery should be offered)
-
Normal cognition
-
Absence of active psychiatric disease
-
Good social support and access to programming
Potential surgical candidates then are evaluated by the neurosurgeon, who determines whether the patient is a good candidate for surgical treatment and decides which procedure(s) would benefit the patient the most. Close collaboration between the neurologist and the neurosurgeon aids the decision-making process minimizing patient confusion and stress. If the neurologist and neurosurgeon agree that the patient is a good surgical candidate, further workup includes:
-
Magnetic resonance imaging (MRI) of the brain to rule out comorbid conditions and to assess the degree of brain atrophy; significant atrophy may increase the risk of perioperative hemorrhage
-
Detailed neuropsychological testing to rule out subtle cognitive impairment, which can be exacerbated by the surgical procedure
-
Consultation with a psychiatrist with expertise in psychiatric complications of movement disorders to rule out active psychiatric disease and screen for relevant past psychiatric history that may pose a contraindication to surgery (eg, major depression or suicidal thoughts)
-
Fluorodopa positron emission tomography (PET) in the unusual circumstance that an alternative diagnosis of multiple-system atrophy cannot be ruled out clinically
-
Medical evaluation to determine the patient’s general fitness for surgery
Surgery is reserved for patients with medically refractory PD who have disabling problems. Currently, most of the treatment centers adopt the following surgical recommendations for patients with medically refractory PD:
-
Unilateral pallidotomy is offered to patients with asymmetric PD who develop fluctuations in their response to levodopa, including disabling dyskinesias and off-state dystonia
-
Bilateral pallidotomy is avoided, though investigations are underway to evaluate contralateral globus pallidus pars interna deep brain stimulation (GPi-DBS) in patients who have undergone a successful pallidotomy and are experiencing disease progression in the untreated side
-
Thalamotomy or thalamic DBS is offered to the minority of patients with PD who have predominant and disabling tremor (more commonly, this procedure is performed on patients with disabling essential tremors)
-
Thalamic DBS is preferred to thalamotomy, particularly in young patients with PD who are disabled solely by tremor early in the course of their disease, because it gives the option of removing the stimulator if more effective therapies are developed or if symptom progression necessitates DBS at another target (eg, the STN)
-
Bilateral STN-DBS is offered to patients with advanced PD who have bilateral levodopa-induced dyskinesia, significant gait disturbances and axial symptoms, or medically refractory rigidity and akinesia; STN-DBS has a variable effect on speech, but it ultimately results in the deterioration of speech intelligibility [38]
Before surgery, the patient should be informed that these procedures do not cure PD and that disease progression is still to be expected. [18]
Advanced Medicinal Products for Parkinson's Disease
For the last couple of decades several new and exciting technologies (tissue engineering, cell- and gene-based grafting) have been investigated to address a plethora of brain diseases, including Parkinson's disease (PD), with positive experimental results. However, these advances have lead to the development of medical tourism to obtain unproven or disapproved therapies (stem cell transplants). [39]
Some mostly experimental and unapproved therapies include:
-
grafting of stem cell-derived dopaminergic neurons into the striatum
-
transfection of cells in the striatum with dopamine gene
-
neurorestorative therapies, which use gene- or cell-based therapies to release certain growth factor(s) [40]
Those transplantation techniques are a potential treatment for PD for the following reasons:
-
The neuronal degeneration is site- and type-specific (striatum, dopaminergic)
-
The target area is well defined (striatum)
-
Postsynaptic receptors are relatively intact
-
The neurons provide tonic stimulation of the receptors and appear to serve a modulatory function
In double-blind studies, neither transplantation of autologous adrenal medullary cells nor transplantation of fetal porcine cells were effective; thus, both were abandoned. Although open-label studies of fetal dopaminergic cell transplantation yielded promising results, 3 randomized, double-blind, sham-surgery–controlled studies found no benefit. In addition, some patients receiving these transplants developed a potentially disabling form of dyskinesia that persisted even after the withdrawal of levodopa. [41]
Gene delivery to the brain was also attempted, but despite initial enthusiasm that it was safe and evoked the desired protein response, clinical trials have been discouraging. [42]
Not being curative, as they do not address some PD features like gait dysfunction, freezing, falling, and dementia caused by non-dopaminergic pathology, [43] those new technologies are seen as PD-modifying and affecting its natural history. [44]
In long-term transplant recipients, Lewy body-like inclusions were found in grafted nigral neurons. They stained positively for alpha-synuclein and ubiquitin and had reduced immunostaining for dopamine transporter, which suggested that PD might affect grafted cells. [45] This led to yet another treatment pathway using immunotherapy: antibodies targeting a-synuclein of Lewy bodies. Yet again, however, despite some early promises, it did not deliver in clinical trials. [46]
Human retinal pigment epithelial cells produce levodopa, and retinal pigment epithelial cells in gelatin microcarriers have been implanted into the putamen in preliminary studies with a phase II, double-blind, randomized, multicenter, sham-surgery–controlled trial, [47] which, again, was not successful. [48]
Despite those clinical failures, there have been reports of some promising long-term effects, [49, 50] which strongly support advancing a transplant technology for the treatment of PD.
-
Schematic diagram of basal ganglia circuitry. Inhibitory (red arrows) and excitatory (green arrows) projections between motor cortex, putamen, globus pallidus pars externa (GPe) and globus pallidus pars interna (GPi), subthalamic nucleus (STN), substantia nigra pars reticulata (SNr) and substantia nigra pars compacta (SNc), and ventrolateral thalamus (VL) are represented. D1 and D2 indicate direct (regulated by dopamine D1 receptors) and indirect (regulated by dopamine D2 receptors) pathways, respectively.
-
Sagittal section, 12 mm lateral to midline, demonstrating subthalamic nucleus (STN; lavender). STN is one of preferred surgical targets for deep brain stimulation to treat symptoms of advanced Parkinson disease.
-
Intraoperative physiologic monitoring equipment. Surgical team, consisting of neurosurgeon, neurologist, and highly trained neurophysiologist (pictured), employs single-cell microelectrode recording to define surgical target physiologically.
-
Medtronics Activa Tremor control system consists of 3 components: (1) stimulating lead, which is implanted to desired target; (2) extension cable, which is tunneled under scalp and soft tissues of neck to anterior chest wall; and (3) pulse generator, which is programmable source of electrical impulses.
-
Stereotactic headframe is applied at start of surgery. MRI-localizing box is attached to frame only during targeting MRI. Localizer defines working volume of frame and provides reference coordinate system from which target coordinates are derived.
-
Axial, fast spin-echo inversion recovery MRI at level of posterior commissure. Typical target for placing thalamic stimulator is demonstrated (crosshairs).
-
Implantation of deep brain stimulation lead.
-
Postoperative coronal MRI demonstrating desired placement of bilateral subthalamic nucleus deep brain stimulation leads.
-
Deep brain stimulation lead is equipped with 4 electrode contacts, each of which may be used, alone or in combination, for therapeutic stimulation.
-
Deep brain stimulation parameters can be adjusted at any time by using transcutaneous programmer.









