Overview of Polysomnography
Nocturnal, laboratory-based polysomnography (PSG), also known as a sleep study, is the most commonly used test in the diagnosis of obstructive sleep apnea syndrome (OSAS). A sleep study can be used for diagnosing other sleep disorders as well, including periodic limb movement disorder, narcolepsy, chronic insomnia, and REM sleep behavior disorder. It is often considered the criterion standard for diagnosing OSAS, determining the severity of the disease, and evaluating various other sleep disorders that can exist with or without OSAS. PSG is non-invasive and consists of a simultaneous recording of multiple physiologic parameters related to sleep and wakefulness. See the image below. Home-based, limited-channel sleep studies are being used more often to diagnosis obstructive sleep apnea, but they have some limitations.
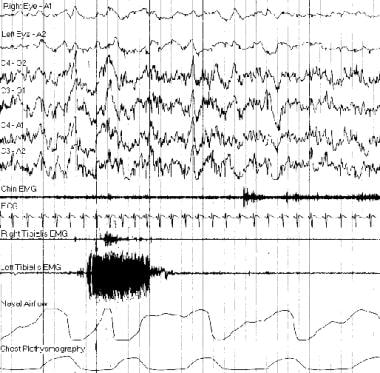 Typical polysomnogram tracing. The burst of electromyogram activity recorded from the left tibialis anterior muscle was caused by a periodic movement of sleep.
Typical polysomnogram tracing. The burst of electromyogram activity recorded from the left tibialis anterior muscle was caused by a periodic movement of sleep.
PSG or sleep study can directly monitor and quantify the number of respiratory events (ie, obstructive, central, or complex) and the resultant hypoxemia and arousals related to the respiratory events or even independent of the respiratory events. [1]
A single-night PSG is usually adequate to determine if OSAS is present and the degree of the disorder. However, night-to-night variability may exist in patients who have a high probability but a low apnea index. In addition, variability in laboratory equipment, scoring technique, and interscorer reliability may also play roles. As is well known, PSG scoring also usually varies from laboratory to laboratory.
PSG is used to evaluate abnormalities of sleep and/or wakefulness and other physiologic disorders that have an impact on or are related to sleep and/or wakefulness.
Parameters Monitored
Assessment of sleep stages requires simultaneous monitoring of three physiological activities: electroencephalography (EEG), electrooculography (EOG), and surface electromyography (EMG).
At least one EEG channel (central channel with an ear reference provides the best amplitude) is needed to monitor sleep stage. However, most laboratories use two central channels and two occipital channels, with ear references as adjuncts to help identify sleep latency and arousals. The 10- to 20-electrode placement system is used to determine the location of these channels. Additional EEG channels can be used, particularly in patients with epilepsy (ie, a full 10-20 montage).
Two EOG channels are used to monitor both horizontal and vertical eye movements. Electrodes are placed at the right and left outer canthi, one above and one below the horizontal eye axis. The electrodes pick up eye movements by tracking movement of the dipole spanning the eye; the cornea has a positive charge and the retina has a negative charge. Evaluation of the eye movements is necessary for 2 reasons. First is for documentation of the onset of rapid eye movement (REM) sleep, and second is to note the presence of slow-rolling eye movements that usually accompany the onset of sleep.
One EMG channel (usually chin or mentalis and/or submentalis) is used to record atonia during REM sleep or lack of atonia in patients with REM-related parasomnias. To assess bruxism, the EMG electrodes can be placed over the masseter. The EMG recording from other muscle groups is assessed for other sleep disorders. For example, the anterior tibialis EMG is helpful for assessing periodic limb movements during sleep and the intercostal EMG is used as adjunctive help for determining effort during respiratory events.
Two channels are used for monitoring airflow. One thermistor channel (oral and/or nasal) is used to evaluate the presence or absence of airflow. Any change in temperature as a patient inhales and exhales leads to a normal signal, so this channel is insensitive for partial flow obstruction. A thermistor is the recommended channel for evaluation of apneas. A nasal pressure transducer channel is a more sensitive measure of airflow restriction. Normal breathing has a rounded pattern, while resistance to airflow leads to a squaring off of the flow signal. A pressure transducer is the recommended channel for evaluating hypopneas. It is also used to show airflow resistance in upper airway resistance syndrome.
Other parameters that can be monitored in a sleep study include the following:
-
Electrocardiography
-
Pulse oximetry
-
Respiratory effort (thoracic and abdominal)
-
End tidal or transcutaneous CO2
-
Sound recordings to measure snoring
-
Surface EMG monitoring of limb muscles (to detect limb movements, periodic or other)
-
Continuous video monitoring
Optional parameters that can be monitored in a sleep study include the following:
-
Core body temperature
-
Incident light intensity
-
Penile tumescence
-
Pressure and pH at various esophageal levels
Staging of Sleep
Standardized criteria for the staging of sleep were published first in 1968 by Rechtschaffen and Kales. A revised version was published in 2007 by the American Academy of Sleep Medicine. [2] The chief revision was the consolidation of stages 3 and 4 into a single stage N3 (slow wave sleep). Previous stages 1 and 2 were renamed N1 and N2. Both systems are reflected below.
The following definitions of EEG patterns provide the terminology used to describe sleep stages.
EEG background
Alpha EEG
-
Frequency of 8-13 Hz
-
Produced in occipital region
-
Crescendo-decrescendo appearance
Theta EEG
-
Frequency of 3-7 Hz
-
Produced in the central vertex region
-
No amplitude criteria
-
Most common sleep frequency
Delta EEG
-
EEG frequency of 0.5-2 Hz
-
Seen predominantly in frontal region
-
Amplitude of greater than 75 microvolts
Sleep spindle
See the list below:
-
Frequency of 12-14 Hz
-
Produced in central-vertex region
-
Greater than 0.5-3 seconds in duration
-
0.5-second spindles with 6-7 cycles
-
Indicative of stage 2 sleep
K complexes
See the list below:
-
Sharp, slow waves with a negative, then positive, deflection
-
No amplitude criteria
-
Duration must be at least 0.5 seconds
-
Predominantly produced in central-vertex region
-
Indicative of stage 2 sleep
-
May occur with or without stimuli
Wake stage
See the list below:
-
Greater than 50% of each epoch contains alpha activity
-
Eye blinks at a frequency of 0.5-2 Hz
-
Reading eye movements
-
Irregular conjugate rapid eye movements associated with normal or high chin tone.
Stage N1 (formerly stage 1)
See the list below:
-
Greater than 50% of the epoch contains theta activity (4-7 Hz) with slowing of the background rhythms greater than or equal to 1 Hz from those of stage wake
-
Vertex sharp waves
-
Slow-rolling eye movements in EOG channels
-
Relatively high submental EMG tone
Stage N2 (formerly stage 2)
See the list below:
-
Theta activity (4-7 Hz)
-
K-complexes and sleep spindles occur episodically
-
High tonic submental EMG
Stage N3
See the list below:
-
Greater than 20% of each epoch must contain delta activity
-
Amplitude of 75 microvolts or greater
-
Submental muscle tone may be slightly reduced
Discontinued former stage 3
See the list below:
-
Between 20-50% of each epoch must contain delta activity
-
Amplitude of 75 microvolts or greater
-
Submental muscle tone may be slightly reduced
Discontinued former stage 4
See the list below:
-
Greater than 50% of the epoch has scorable delta activity
-
Amplitude of 75 microvolts or greater
-
Submental EMG activity slightly reduced from that of light sleep
REM sleep
REM sleep is sometimes called "REM stage."
-
Rapid eye movements
-
Low amplitude, mixed frequency EEG (similar to awake pattern)
-
Atonia or the lowest tonic submental EMG
-
May see saw-tooth waves
Procedures
In 1992, the Office of Technology Assessment of the Agency of Health Care Policy and Research recommended, in an evidence-based assessment, declared two tests as having been studied sufficiently. Both tests are performed in a sleep laboratory.
The first is overnight polysomnography (PSG) or sleep study, which is an overnight recording of the patient's sleep. Typically for a baseline study, the patient is observed sleeping naturally without any treatment, but if a significant amount of sleep-disordered breathing (AHI> 20–30 events per hour) is seen in the first hours of the study, a split-night study is performed during which positive airway pressure (PAP) is started. Titration studies may also be done with initiation of PAP from the start of the study to determine optimal settings.
The second is multiple sleep latency testing (MSLT), which records multiple naps throughout a day. Maintenance of wakefulness testing (MWT) can also be performed, which determines how long wakefulness can be maintained.
Standard sleep studies usually use the overnight PSG (may be repeated over several nights if the initial study is uninformative, and there is a high suspicious for an abnormality). If daytime sleepiness is an issue and cannot be fully explained by the overnight study results, an MSLT should be performed the next day. Limitations usually stem from the fact that recording conditions may not reflect what happens during a regular night in the patient's home.
Although diagnosing a sleep problem on the basis of a recording over a single night is common practice, some authorities caution that more than one night of recording may be necessary so the patient can become comfortable with unfamiliar surroundings and sleep more naturally. This effect is greatest on the first night in the sleep laboratory (ie, first-night effect).
Sporadic events may be missed with a single-night PSG. External factors that disturb the subject's sleep may be present in the home but absent from the controlled environment of the sleep laboratory.
Patient preparation is important so that the patient sleeps naturally. Patient instructions include the following:
-
Maintain regular sleep-wake rhythm.
-
Alcohol and sleeping pills may alter the PSG results, but if they are part of the patient's normal routine, they should not be abruptly stopped.
-
Avoid stimulants, including medications for narcolepsy.
-
Avoid strenuous exercise on the day of the PSG.
-
Avoid naps on the day of the sleep study.
Daytime PSG can be useful for patients who typically sleep during the day. Simplified sleep studies with limited subsets of monitored parameters, such as PAP-NAPs, can be used to help the patient with acclimatization and finding optimal settings.
High costs and long waiting lists have prompted the exploration of alternative methods of evaluation and many insurance companies are requiring home-based, limited-channel sleep studies prior to in-laboratory PSG. Instead of in-laboratory titration, many patients with obstructive sleep apnea can be started on automatically adjusting continuous positive airway pressure (CPAP) and then have the settings adjusted and response monitored through data collected by the device.
Multiple sleep latency test
Multiple sleep latency testing (MSLT) is used to assess the degree of daytime sleepiness and to evaluate for possible narcolepsy. MSLT should be performed after a full-night polysomnogram to ensure that at least 6 hours of sleep precede the test and that no other causes for excessive daytime sleepiness are present. A sleep log should be kept for at least 1 week prior to the study, and all medications taken for the 2 weeks prior to the study should be noted. Urine drug testing is often done to evaluate for drugs that may affect study results. Stimulant medications, nicotine, and caffeine can affect the mean sleep latency, and medications (especially selective serotonin reuptake inhibitors [SSRIs]) can affect sleep-onset rapid eye movement (REM) periods. [3] In general, SSRIs and stimulants need to be discontinued at least 2 weeks prior to the test. Small amounts of caffeine do not usually need to be discontinued.
The patient is given 20 minute opportunities to nap every 2 hours for 4 or 5 naps. The first nap should begin within 1.5-3 hours after waking. If the patient falls asleep, he or she is allowed to sleep for 15 minutes. Sleep latency is the time to the first epoch with over 15 seconds of any stage of sleep. The mean sleep latency is determined. A mean sleep latency of 10-15 minutes is consistent with mild sleepiness, 5-10 minutes is consistent with moderate sleepiness, and less than 5 minutes is consistent with severe sleepiness. A series of 2 sleep-onset REM periods (SOREMP) is consistent with the diagnosis of narcolepsy; however, only 80% of patients with narcolepsy and 6.6% of patients without narcolepsy had 2 or more SOREMPs in a review of over 2000 MSLTs.
Maintenance of wakefulness test
Maintenance of wakefulness testing (MWT) is used to determine how long a patient is able to maintain wakefulness. The patient should be in a dim room in a semirecumbent position. The 20-min MWT includes 5 20-min tests of wakefulness, and the 40-min MWT includes 4 40-min tests of wakefulness every 2 hours. The first nap should begin within 1.5-3 hours after waking. A preceding PSG is not always necessary. The patient should be instructed to sit still and remain awake as long as possible. The test ends after 20 or 40 minutes if no sleep occurs or if the patient achieves unequivocal sleep, which is determined by either 3 consecutive epochs of stage 1 sleep or 1 epoch of any other stage of sleep. Sleep latency is the time to the first epoch with over 15 seconds of any stage of sleep. The mean sleep latency is determined.
Sleep Monitoring Guidelines
Medicare guidelines
In 2008, Medicare approved the use of unattended home sleep monitoring devices of types II, III, or IV (with at least 3 channels) if the patient received a complete clinical evaluation and does not have atypical or complicated symptoms and the studies were read by a trained sleep specialist. [4, 5] The guidelines for using a portable monitor unattended home sleep study device for continuous positive airway pressure (CPAP) therapy include the following:
-
Type II device: This type of device has a minimum of 7 channels (eg, EEG, EOG, EMG, ECG-heart rate, airflow, respiratory effort, oxygen saturation). This type of device monitors sleep staging so the apnea-plus-hypopnea index (AHI) can be calculated.
-
Type III device: This device has a minimum of 4 channels, including ventilation or airflow (at least 2 channels of respiratory movement or airflow), heart rate or ECG, and oxygen saturation.
-
Type IV device: This type of device does not meet requirements for other types, and many measure only 1-2 parameters (eg, oxygen saturation or airflow). For Medicare reimbursement, these devices, including WatchPAT (Itamar Medical Ltd, Caesarea, Israel) can be used if they have a minimum of 3 channels.
American Academy of Sleep Medicine guidelines
The American Academy of Sleep Medicine (AASM) evaluated the literature on unattended sleep monitoring devices to develop their clinical guidelines, published in 2007. [6] These guidelines include the following recommendations and cautions:
-
Portable monitoring (PM) may be indicated for the diagnosis of obstructive sleep apnea (OSA) in patients for whom in-laboratory PSG is not possible by virtue of immobility, safety, or critical illness. PM may also be indicated to monitor the non-CPAP treatments of sleep apnea including oral appliances, weight loss, and upper airway surgery.
-
PM is not appropriate for diagnostic evaluation of patients who may have comorbid sleep disorders including central sleep apnea, periodic limb movements, insomnia, parasomnias, circadian rhythm disorders, or narcolepsy.
-
PM is not appropriate for the diagnosis of OSA in patients with significant comorbid medical conditions that may degrade the accuracy of PM. This includes, but is not limited to, severe pulmonary disease, [7] neuromuscular disease, or congestive heart failure. PM is not indicated in the absence of a comprehensive sleep evaluation.
-
PM is not appropriate for general screening of asymptomatic patients.
-
At minimum, PM must record airflow, respiratory effort, and blood oxygenation.
-
The PM device must allow for display of raw data with the capability of manual scoring or editing of automated scoring by a qualified sleep technologist.
-
A board-certified sleep specialist or an individual who fulfills eligibility criteria for the sleep medicine certification examination must review the raw data from PM using scoring criteria consistent with current published AASM standards.
-
False negative rates may be as high as 17% in unattended PM studies. If the PM test is technically inadequate or does not provide the expected result, in-laboratory polysomnography should be performed.
-
AASM does not support type IV devices for home sleep testing.
In 2012, the AASM also published evidence-based practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. [8] PSG is indicated for children suspected of having periodic limb movement disorder (PLMD) for diagnosing PLMD. Children with frequent NREM parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for the presence of comorbid sleep disorders and polysomnography should be performed if there is a suspicion for sleep-disordered breathing or PLMD.
Because of the lack of EEG monitoring, Type III devices may underestimate the severity of sleep-disordered breathing. Typically, events must be associated with 3% desaturations to be scored, so patients with events primarily causing arousals may be missed. Additionally, the apnea/hypopnea index (AHI) is calculated by the number of apneas and hypopneas per hours of test rather than hours of sleep, which can also underestimate severity. For these reasons, if a home study is normal in a patient with suspected sleep apnea, an in-laboratory PSG is recommended.
In 2017 The AASM updated its guidelines regarding the use of overnight polysomnography (oPSG) in the diagnosis of OSA. [9] Some of these recommendations include:
-
Clinical tools, questionnaires, and prediction algorithms should not be used to diagnose OSA in adults, in the absence of PSG or home sleep apnea testing.
-
PSG or home sleep apnea testing with a technically adequate device should be used for the diagnosis of OSA in uncomplicated adult patients presenting with signs and symptoms that indicate an increased risk of moderate-to-severe OSA.
-
If a single home sleep apnea test is negative, inconclusive, or technically inadequate, PSG should be performed for the diagnosis of OSA.
-
PSG, rather than home sleep apnea testing, should be used for the diagnosis of OSA in patients with significant cardiorespiratory disease, potential respiratory muscle weakness due to neuromuscular condition, awake hypoventilation or suspicion of sleep related hypoventilation, chronic opioid medication use, history of stroke or severe insomnia.
-
If clinically appropriate, a split-night diagnostic protocol, rather than a full-night diagnostic protocol for PSG, should be used for the diagnosis of OSA.
-
When the initial PSG is negative and clinical suspicion for OSA remains, a second PSG should be considered for the diagnosis of OSA.
In 2021 the AASM published clinical guideline statements regarding the use of PSG and home sleep apnea tests (HSATs) for the longitudinal management of OSA in adults. [10] They do not recommend followup PSG or HSAT for routine reassessment of asymptomatic patients with OSA on PAP therapy. However, they do indicate that followup PSG or HSAT can be used to reassess patients with recurrent or persistent symptoms, despite good PAP adherence. The AASM guidance also recommends that PSG or HSAT be used to assess response to treatment with non-PAP interventions, and to reassess sleep-related hypoxemia and/or sleep-related hypoventilation following initiation of treatment for OSA. PSG or HSAT can be used if clinically significant weight gain or loss has occurred since diagnosis of OSA or initiation of its treatment. They may also be used in patients being treated for OSA who develop or have a change in cardiovascular disease. Finally, followup PSG may be used in patients with unexplained PAP device-generated data.
It is the clinician's responsibility to choose the appropriate treatment based on factors including the patient's individual circumstances, available diagnostic tools, accessible treatment options, and resources.
Respiratory Events and Leg Movement Scoring
Basic rules
In 2015, the AASM updated scoring rules, making changes to scoring of apneas, hypopneas, Cheyne Stokes respiration, and hypoventilation.
The event duration starts at the nadir preceding the first breath that is clearly reduced to the beginning of the first breath that approximates the baseline breathing amplitude. Events terminate if there is a clear and sustained increase in breathing or if there is a desaturation, when there is a re-saturation of at least 2%.
Polysomnography reports should report an apnea/hypopnea index (AHI), which, for an in-lab study, is the number of apneas and hypopneas per hour of sleep. For portable studies, the AHI is the number of apneas and hypopneas per hour of test. It is important to know what criteria was used for the events as there have been many changes to the scoring criteria for hypopneas, which may have led patients who were scored with desaturation criteria only to have significantly underestimated sleep apnea. In general, an AHI > 5 is considered significant sleep apnea. Some polysomnography report respiratory disturbance index (RDI), which is typically the number of apneas plus hypopneas plus respiratory-related arousals.
Studies without sleep time often use a respiratory event index (REI) defined as the number of events/recording time, or the number of events/(recording time-movement time).
Obstructive apnea
See the list below:
-
Greater than 90% reduction in oronasal thermistor amplitude for more than 10 seconds
-
The duration of the 90% drop is greater than or equal to 10 seconds
-
Often associated with increasing respiratory effort; usually seen as paradoxical
Hypopnea
AASM recommendation
-
Reduction in nasal pressure amplitude by greater than 30% lasting for at least 10 seconds
-
Associated with SaO2 drop of at least 3% from pre-event baseline or an arousal
-
Obstructive hypopneas can be scored if any of the following are present: Snoring, increased inspiratory flattening of the nasal pressure, or associated thoracoabdominal paradox during the event but not pre-event
-
Central hypopneas can be score if none of above findings is present
Medicare definition
-
Reduction in pressure amplitude greater than 30% of baseline value
-
Asocciated with SaO2 decrease of greater than 4%
Mixed apnea
See the list below:
-
Greater than 90% reduction in thermistor flow for greater than 10 seconds
-
Total absence of respiratory effort at the beginning of the event, followed by a gradual increase in effort, which eventually breaks the apnea (usually paradoxical)
Central apnea
See the list below:
-
Greater than 90% reduction in thermistor flow for greater than 10 seconds
-
Complete absence of respiratory effort
Respiratory effort–related arousal
See the list below:
-
Greater than 10 breaths with increasing respiratory effort or flattening of the nasal pressure followed by an arousal
-
Does not meet criteria for hypopnea or apnea
Cheyne-Stokes respiration
See the list below:
-
At least 3 consecutive cycles
-
Cyclical crescendo and decrescendo change in breathing amplitude
-
Either 5 per hour of sleep or duration greater than 10 minutes
-
Cycle length > 40 seconds
Sleep-related hypoventilation
See the list below:
-
Increase in CO2 levels (ETCO2 or TCCO2) to > 55 mmHg for greater than or equal to 10 minutes or
-
Increase in CO2 levels (ETCO2 or TCCO2) by at least 10 mmHg (from awake supine value) to > 50 mmHg for greater than or equal to 10 minutes
Periodic limb movement
See the list below:
-
Each jerk more than 0.5 seconds but less than 10 seconds in duration
-
Minimum amplitude is an 8 mV increase in EMG voltage above resting EMG
-
Must have 4 jerks separated by no less than 5 seconds and no more than 90 seconds
-
No associated respiratory event within 0.5 seconds
EEG arousal
See the list below:
-
Abrupt shift of EEG frequency including alpha, theta, and/or frequencies greater than 16 Hz (but not spindles)
-
Greater than 3 seconds of changed frequency on EEG
-
At least 10 seconds of stable sleep preceding the change
-
In REM sleep, increase in submental EMG for at least 1 second
Bruxism
See the list below:
-
At least twice the amplitude of baseline chin EMG activity
-
At least 3 elevations of 0.25-2 seconds of increased chin EMG activity
-
One elevation of greater than 2 seconds of increased chin EMG activity
Disorders Evaluated With Polysomnography
Several different types of sleep disorders can be evaluated and diagnosed using polysomnography or sleep study.
Dyssomnias (disorders of initiating or maintaining sleep)
See the list below:
-
Circadian rhythm disorders
-
Narcolepsy
-
Idiopathic hypersomnia
-
Inadequate sleep hygiene
-
Sleep-wake misperception
-
Sleep-related respiratory disorders
Sleep apnea syndrome
Upper airway resistance syndrome
Obesity hypoventilation syndrome
Central sleep apnea
Parasomnias
See the list below:
-
Disorders of arousal
-
Disorders of sleep-wake transition
-
Disorders that occur during REM sleep
Nightmares
REM behavior disorder
-
Medical-psychiatric sleep disorders
Medical - Sleep-related asthma
Psychiatric - Depression, panic disorder
Neurologic - Sleep-related epilepsy
-
Others
Bruxism [11]
Restless legs syndrome and periodic limb movement disorder
Summary of Sleep Monitoring
Standard analysis still consists of reviewing each of the parameters recorded. Overnight parameters (eg, times of lights on/off, total time in bed, total sleep time, sleep latency, REM latency) are collected. The overnight recording is divided into epochs of approximately 30 seconds. The standard EEG, EMG, and EOG recordings are evaluated, and the predominant stage of sleep (according to the AASM 2007 scoring manual) is then assigned to the entire epoch. In 2015, the AASM changed the recommended scoring criteria of most events including now recommending using 3% desaturation or arousal for criteria for hypopneas. Medicare guidelines, however, still use the not-recommended 4% desaturation criteria that can miss many patients with signficant obstructive sleep apnea.
Total time and relative proportion of the night spent in each of the stages and in REM and non-REM sleep are calculated. Latencies to REM and slow-wave sleep are reported.
Stages of sleep, any abnormalities noted with EEG, and periodic limb movements are reported. Respiratory activity (eg, apneic or hypopneic episodes, oxygen desaturations) is correlated with sleep stages. Other parameters, such as body position, are recorded. If needed, esophageal pH or penile tumescence can also be recorded.
Once sleep apnea is diagnosed with either a portable limited-channel stud or an in-laboratory PSG, a patient can either be started with autotitrating CPAP at home or an in-laboratory titration study can be done. In-laboratory titration is recommended if there are other breathing disorders than obstructive sleep apnea, including hypopnea, hypoventilation, or central sleep apnea, as devices other than CPAP are often needed.
Questions & Answers
Overview
What is polysomnography (PSG)?
Which studies are required in polysomnography (PSG)?
What is the role of EEG channels in polysomnography (PSG)?
What is the role of EOG channels in polysomnography (PSG)?
What is the role of EMG channel in polysomnography (PSG)?
How is airflow monitored in polysomnography (PSG)?
In addition to EEG, EOG and EMG, what other parameters are monitored during polysomnography?
Which optional parameters may be monitored during polysomnography?
What are the sleep spindle polysomnography (PSG) criteria?
What are the K complexes polysomnography (PSG) criteria?
What are the wake stage polysomnography (PSG) criteria?
What are the stage N1 polysomnography (PSG) criteria?
What are the stage N2 polysomnography (PSG) criteria?
What are the stage N3 polysomnography (PSG) criteria?
What are the discontinued stage 3 polysomnography (PSG) criteria?
What are the discontinued stage 4 polysomnography (PSG) criteria?
What are the REM polysomnography (PSG) criteria?
How do the AASM polysomnography (PSG) criteria differ from earlier Rechtschaffen and Kales criteria?
What are the EEG polysomnography (PSG) criteria?
What are the limitations of single-night polysomnography (PSG)?
What is included in patient instructions prior to polysomnography (PSG)?
When is daytime polysomnography (PSG) indicated?
When is home-based, limited-channel polysomnography (PSG) indicated?
What are the Medicare guidelines for the use of home-based, limited-channel polysomnography (PSG)?
What are the AASM guidelines for the use of home-based, limited-channel polysomnography (PSG)?
What are the AASM guidelines for polysomnography (PSG) in children?
What are the limitations of home-based, limited-channel polysomnography (PSG) type III devices?
How is multiple sleep latency testing (MSLT) performed in polysomnography (PSG)?
How is maintenance of wakefulness testing (MWT) performed in polysomnography (PSG)?
What are the AASM criteria for obstructive apnea on polysomnography (PSG)?
What are the AASM criteria for mixed apnea on polysomnography (PSG)?
What are the AASM criteria for central apnea on polysomnography (PSG)?
What are the AASM criteria for respiratory effort-related arousal on polysomnography (PSG)?
What are the AASM criteria for Cheyne-Stokes respiration on polysomnography (PSG)?
What are the AASM criteria for sleep-related hypoventilation on polysomnography (PSG)?
What are the AASM criteria for periodic limb movement on polysomnography (PSG)?
What are the AASM criteria for EEG arousal on polysomnography (PSG)?
What are the AASM criteria for bruxism on polysomnography (PSG)?
What are the AASM scoring rules for polysomnography (PSG)?
What are the AASM criteria for hypopnea on polysomnography (PSG)?
What is the Medicare definition for hypopnea on polysomnography (PSG)?
Which dyssomnias can be evaluated with polysomnography (PSG)?
Which parasomnias can be evaluated with polysomnography (PSG)?
What is included in a polysomnography (PSG) report?
How is on polysomnography (PSG) used in treatment selection?
-
Typical polysomnogram tracing. The burst of electromyogram activity recorded from the left tibialis anterior muscle was caused by a periodic movement of sleep.





