Neonatal EEG
Neonatal electroencephalography (EEG) in infants has become valuable as a serial, noninvasive screening tool for infants at high risk of perinatal injuries. The brain dynamics and connectivity in different states (awake or asleep) can be defined, and a whole range of acute or chronic cerebral disorders can be identified. Such information often reveals presymptomatic or subclinical conditions.
The EEG prognostic value at the time of continuous development is often greater than at a later stage. EEG testing can provide reassurance to the physician and parents at a time of potential catastrophic damage.
The continuous changes that occur during early brain development are often associated with striking changes in EEG patterns over short periods. This makes it difficult to interpret EEG results, which can discourage the use of EEG testing.
Given the close relationships between certain morphological aspects of the developing brain and EEG results, gestational age can be reliably estimated (to ±1 wk) by EEG criteria. In fact, CNS development of the immature brain proceeds at about the same rate during fetal development as in the postnatal environment.
The physiological substrate for these early EEG patterns is unknown, but is probably derived from cortical generators that are strongly influenced by subcortical (primarily thalamic) afferent input. Rapid maturation of these structures (and not the corpus callosum) is most likely responsible for the interhemispheric synchrony that occurs close to full-term gestational age; in particular, rapid dendritic spine development and synaptogenesis are typical of the last month of fetal development. The complex development of cerebral sulci during this same period is probably responsible for the neonatal EEG results showing complex, more definitive patterns at term. At this age, easily recognizable and organized activity patterns appear. These continue with little change during the first month of life and are strictly characteristic of neonatal EEG.
There are several technical considerations when recording from a small (neonate) scalp. High skin resistance impedes low-resistance scalp-to-electrode contact. The state of activity (awake or quiet vs active sleep) can be selectively bound to certain aspects of pathology. It is important to annotate the tracing with particular attention to the presence and type of eye movements, facial movements, respiration (regular or irregular), sucking, crying, grimacing, and so on. Extracerebral monitors are needed in routine recordings, including at least electrooculogram (EOG), respiration rate measurement, and electrocardiogram (ECG). Only a reduced number of scalp electrodes, generally never more than the set in a 16-channel recording, are applicable. A low time constant (0.25–0.60 s) is preferable to record the low-frequency background activity. Slow paper speed maximizes the slow background and the degree of interhemispheric synchrony.
Recent technologic advances have promoted the use of amplitude integrated EEG (aEEG) to offer an accessible tool for bedside continuous cerebral monitoring. [1] However, this easy-to-use device requires nonetheless careful management and a vigilant well-trained staff. It also needs to complement standard conventional electroencephalography to be interpreted by experienced readers to avoid inappropriate conclusions and false positives, especially in the domain of neonatal seizures. As for amplitude measurements, 2-channel aEEG appears considerably more sensitive in detecting cerebral injury in the term encephalopathic infant, in comparison with a single-channel aEEG, especially in the setting of unilateral injury. [2]
For related information, see EEG Seizure Monitoring and Visual Analysis of Neonatal EEG.
The Normal Neonatal EEG
In the full-term born infant, ultradian sleep and waking cycles are well defined and easy to detect with polygraphic and behavioral criteria. On EEG, wakefulness characterized by eye opening, crying, and vigorous motor behavior accompanied by irregular vital signs on recordings (ECG, respiration) is marked by a low-amplitude activity and discontinuous 4–7 cps theta activity interspersed with low-voltage delta rhythms. Hence, the French name activité moyenne.
Active sleep (AS), the antecedent of rapid eye movement (REM) sleep, is usually indicated by irregular respiratory patterns with interspersed, brief apneic episodes that often precede clusters of eye movements. Contrary to adult physiology, prominent, subtle motor activity, especially of the face (eg, grimacing, smiling), accompanies this state. These results are often interpreted as seizure activity by the inexperienced reader. On EEG, 2 patterns are observable, as follows:
-
A continuous, low- to medium-voltage background with theta and delta activity and occasional anterior sharp-waves that occur primarily at sleep onset, or
-
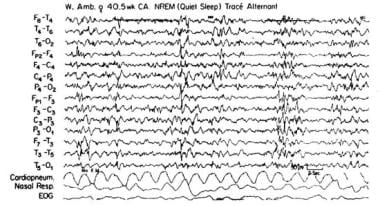
A lower amplitude, more continuous theta background is mostly seen between periods of quiet sleep (QS). The latter non-REM sleep is marked by a discontinuous pattern (tracé alternant) that is characterized by bursts of high-amplitude (50-20 mV) synchronous delta activity and separated by intervals of lower mixed activity that resemble wake or AS activity (see image below).
Sleep state cyclicity is certainly achieved after 30 weeks' postconceptional age (PCA), with stability over multiple cycles only at 36 weeks' PCA.
Lately, the increased survival of extremely young premature babies has allowed to assess very early expression of sleep cyclicity by combining measures of REM and EEG discontinuity [3] between 25 and 30 weeks' PCA. Early forms of "transitional" sleep akin to "seismic sleep" in the rat represents an immature form of paradoxical sleep with mixed features of active and quiet sleep. It probably corresponds to a primitive form of brain activity characterized by a low level of inhibition progressively declining toward term. [4]
Near the end of the first month, a more diffuse pattern usually appears, consisting of continuous, high-to-moderate amplitude, slow activity that is not seen in the preterm infant. A high degree of synchrony between burst and interburst activity is desirable at term. This usually confirms normal maturational patterns.
Several morphological figures may occur with variable frequency. Random sharp-waves, most commonly temporal or rolandic, are sporadically seen in QS. Nonrolandic, repetitive, highly focal spikes confined to a single location that occur during wakefulness usually indicate abnormalities. A burst of frontal delta and synchronous, frontal sharp waves are still abundant in the full-term born infant during AS. Spindle delta bursts (brushes) are seen with decreasing frequency in the full-term born infant and are usually confined to the central and temporal leads during QS. This state is the most vulnerable, being susceptible to various minor CNS insults that are only transiently apparent, depending on their expression. It is important to perform prolonged recordings, especially in stressed infants as they are likely to express less QS.
Several important milestones characterize EEG maturation patterns during the first months of life. The newborn progressively develops a circadian rhythm, resulting from the interaction of endogenous factors with external synchronizers such as light, eating, and sensory stimulation over the course of a day. At approximately the third month, sleep efficiently occurs in nocturnal intervals of at least 8 hours, reflecting mother-child interactions and the established activity of endogenous pacemakers.
With regard to EEG results, several important changes accompany this phase. From the second week of life, slow and continuous background activity (consisting of increasing amplitude delta waves whose frequency also decreases with the approaching first month of life) progressively replaces the discontinuous pattern (tracé alternant) that is typical of QS. Typical EEG characteristics disappear within the second month of life, including slow frontal biphasic spikes (encoches frontales) and negative rolandic spikes. The newborn still falls asleep in AS until the end of the third month. AS decreases from 50% to 40% by the end of the fourth month; likewise, QS progressively increases and becomes more defined due to the appearance of EEG hypnic features that are typical of adults. Vertex waves can be noted in the rolandic regions after the third month; sleep spindles appear earlier, at about the sixth week, over the central regions.
The first sleep spindling samples are slower in frequency and more anteriorly distributed in newborns compared with older infants. These infrequently appear at the beginning of QS as rudimentary, low-voltage (< 25 mV), immature, asymmetric, and asynchronous 10–16 Hz EEG waveforms. The length, amplitude, and synchrony of these spindling samples increase during the first year of life and are more prominent in females and small for gestational age newborns, especially those with neonatal respiratory distress. Spindling maturation is prognostically valuable: their absence at the third month indicates abnormal maturation (eg, hypothyroidism, severe patterns of intellectual disability). At the same time, the sawtooth waves that are typical of adult REM sleep make their first appearance in AS.
Around the sixth postnatal week, 75 mV occipital sharp waves characterize AS and increase in frequency from 2 to 4 Hz toward the end of the third month. At 3 months, a clearly defined 3–4 Hz, 50–75 mV occipital rhythm appears during wakefulness; this is interrupted by eye opening. It progressively evolves at about 5 months to a faster frequency of 6–7 Hz.
The Abnormal Neonatal EEG
Even more than in other epochs of life, in neonates the abnormal neonatal EEG has a prognostic value as opposed to a diagnostic value. Rarely, specific EEG patterns correspond to typical syndromes. Prognostic value can be increased with the following methods:
-
Early recordings, possibly within the first 48 hours of life - Markedly abnormal EEG patterns usually last for a relatively short time, followed by less abnormal or even normal patterns despite the absence of clinical resolution.
-
Prolonged recordings to include samples of different activity states - QS, for instance, is far more likely to show valuable maturation pattern abnormalities and yet is less likely to occur within short recording intervals in a compromised infant.
-
Serial EEGs obtained at short intervals to assess the rapid changes that are likely to occur in rapidly maturing, high-risk infants - Normalization of a previously abnormal pattern may indicate a minimal impact of a brain insult on maturation. Conversely, progressive deterioration of previously normal or moderately abnormal patterns favors the possibility of long-term neurological sequelae.
Because EEG abnormalities in neonates cover a broad spectrum, any classification is difficult and, in some cases, arbitrary. One possibility for classification would be to distinguish between diffuse and focal abnormalities and to categorize separately ictal and paroxysmal patterns in the presence of neonatal convulsions.
Diffuse EEG Abnormalities
With regard to severity and prognosis, severe and irreversible abnormalities should be distinguished from moderate, reversible abnormalities. Severe abnormalities correspond to 2 main EEG patterns, inactive and paroxysmal, both of which are accompanied by a lack of sleep cycles and a lack of reactivity to internal or environmental stimuli.
The inactive or isoelectric pattern consists of cerebral activity below 10 mV that is continuously present throughout the record. Brief intervals of low-voltage activity, which are located over the posterior head regions, may occasionally be present. This pattern may occur in varying clinical conditions and often occurs with the following states:
-
Early severe asphyxia or massive hemorrhage
-
Severe inborn metabolic deficits
-
CNS bacterial or viral infections
-
Gross congenital malformations (see image below)
-
Drug-induced state
-
Hypothermia
-
Postictal recording
In the absence of a drug-induced state, hypothermia, or postictal recording, the prognosis is poor but not necessarily fatal.
The paroxysmal or burst suppression EEG pattern is characterized by intervals of inactive background activity (< 10–15 mV) that alternate with synchronous or asynchronous activity bursts. These include primarily high-voltage, irregular slow waves with or without sharp components (see image below). This pattern, which carries a highly unfavorable prognosis, must be clearly distinguished from a full-term newborn's tracé alternant and a preterm infant's tracé discontinue (TD), both of which are normal patterns. Serial recordings are essential to reach a reliable prognosis. Certain conditions (eg, Aicardi syndrome or uncommon dysgenetic conditions that involve the corpus callosum) rarely present as hemihypsarrhythmia.
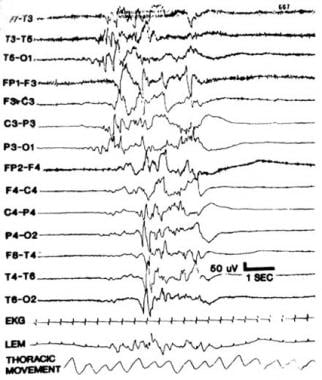 Paroxysmal or burst suppression EEG. Notice prominent bursts of paroxysmal activity interspersed with an inactive background activity.
Paroxysmal or burst suppression EEG. Notice prominent bursts of paroxysmal activity interspersed with an inactive background activity.
Severe but reversible diffuse abnormalities can occur and are exemplified by the so-called low-voltage pattern throughout the EEG record. QS and AS are only distinguishable by the slightly higher voltage in QS, where mixed frequencies under 10–50 mV are almost continuously recorded. This finding and a diffuse delta pattern with minimal theta rhythms throughout the entire EEG record hold an intermediate prognosis. When the abnormalities are compatible with these changes seen in sleep, they are generally considered moderate and reversible.
Diffuse EEG abnormalities can also be seen as irregularities in maturational indices and organizational states. In addition to the patterns of profound disruption to the ability to organize cyclic states (which are typical of the most severe abnormalities), several patterns of EEG dysmaturity can be recognized and identified. In newborns who are small for their gestational age, transient or persistent dysmaturity patterns can be distinguished by their duration. Quantification may include assessment of interhemispheric synchrony in tracé alternant, typical of QS, or the counting of premature features such as delta brushes.
Abnormalities of EEG patterns, noted in relation to sleep states and the instability of sleep-wake states during the newborn period, have some prognostic value. When different etiologies of the EEG pattern are considered, a few fundamental groups can be distinguished.
Transient metabolic disorders
Neonatal hypoglycemia can range from an asymptomatic state with a minimal EEG correlation to late-onset, idiopathic hypoglycemia accompanied by neurological symptoms and seizures. Toxemia and maternal diabetes are often encountered in high-risk pregnancies. These newborns usually present with decreased QS with a relative increase in AS. Transient hypocalcemia is often associated with barely abnormal interictal EEG and variable focal seizures (in 20% of patients). Convulsive patterns of hypomagnesemia, iatrogenic hypernatremia, and pyridoxine deficiency are described in EEG in Neonatal Seizures.
Inborn errors of metabolism
Periodic EEG patterns in newborns with uneventful deliveries strongly suggest the possibility of an inborn error of metabolism. The most frequent neurological symptoms are early movement disorders, convulsions, and cognitive dysfunction. In 1977, Mises accurately described periodic EEG patterns in methylmalonic aminoacidopathy. [5]
High interindividual variability characterizes a pattern of periodic frontal or occipital sharp waves that are interspersed with rapid rhythms. In maple syrup urine disease, EEG complexes are low-voltage and less periodic; background activity is less depressed. Comb-like rhythms during the second and third postnatal weeks are pathognomonic of this disorder.
The highly peculiar EEG pattern of non-ketotic hyperglycemia distinguishes it from other forms. During the first 10 postnatal days, these infants, who present with hypotonia, respiratory distress, and myoclonic seizures, have EEGs characterized by periodic, highly stereotyped 1–3 Hz complexes with 4- to 18-second interburst intervals. Frontal, high-voltage slow waves are associated with characteristic rolandic and occipital early alpha rhythms.
Pyridoxine dependence (not to be confused with pyridoxine deficiency) is inherited as an autosomal recessive trait and is accompanied by severely abnormal EEGs and refractory seizures that only respond to pyridoxal supplementation.
CNS Infections
An important distinction must be made between prenatal and postnatal infections. No specific or typical EEG patterns exist for the first group. Severity and extent of CNS involvement is more significant compared to noninfectious etiologies. Rubella and toxoplasmosis are the most common causative agents.
Infants with congenital rubella and cataracts present with the most consistent EEG abnormalities in this group (ie, prolonged subclinical seizures expressed as paroxysmal bursts without interburst intervals or alpha-like activity). In the occipital areas, there is marked asynchrony between burst and interburst intervals. Slow anterior dysrhythmia with excessive frontal sharp waves is present. Sleep states are not well defined, given the absence of recorded REM and the paucity of QS.
Fetal toxoplasmosis is less disruptive, at least in terms of ultradian sleep cycle organization. It is associated with more variable EEG patterns. In cytomegalovirus, the EEG is frequently inactive.
For postnatal infections (usually meningitis), the prognostic value of EEG is linked to the severity and extent of the abnormalities rather than their specificity. In most cases, they are associated with early status epilepticus (SE) or single seizures with a definite interval following birth that is unlike postanoxic SE. Consistently abnormal recordings (rather than merely an initial abnormal recording) are linked to an unfavorable prognosis.
Three distinctive patterns are associated with type 1 and type 2 herpes simplex virus (HSV) encephalitis. For pregnant women in many countries, HSV is still the most common (and preventable in neonates by means of cesarean section) genital infection. HSV is easily transmitted to the newborn during vaginal delivery. One type of EEG abnormality consists of continuous, sharply contoured unifocal or multifocal delta activity with a typical asynchronous distribution across several foci (each with a specific rate). Foci are predominantly temporal, frontal, or central in distribution. In older infants, hemispheric, monomorphic slow waves appear interspersed on a low-voltage or suppressed background. They may recur as periodic lateralized epileptiform discharges (PLEDs) within several seconds. Typical electroencephalographic seizures are associated with positive, multifocal sharp waves on a background that is characterized by significant interhemispheric voltage asymmetries and asynchrony.
Focal Acute Neurological Abnormalities
The following conditions may cause focal neurologic abnormalities: trauma, primary subarachnoid hemorrhage, intraventricular hemorrhage (IVH), intraparenchymal hemorrhage, and cerebral infarction. EEG abnormalities include an interhemispheric amplitude asymmetry pattern that is mainly seen with intraparenchymal hemorrhage or with a prenatal or postnatal ischemic insult. A wider criterion (>50%) is usually applied to preterm (PT) infants in whom significant hemispheric voltage alteration has been found to strongly correlate with contralateral hemiparesis. In 1984, Challamel reported that transient hemispheric asymmetries were a normal variant in infants with no CNS-related ailments who later resumed fully developed normal EEG patterns. [6, 7]
The focal attenuation pattern refers to a single scalp region with a persistent voltage attenuation. This pattern has an inconsistent association with neuropathological lesions; both false-positive and false-negative correlations have been observed. Focal slowing is highly unusual and may be a sign of ongoing seizures.
Nonictal paroxysmal patterns include the following:
-
Midline rhythmic bursts offer no diagnostic or prognostic clue when observed during non-REM sleep. These may represent the normal maturation ebouché of primitive sleep figures such as vertex waves.
-
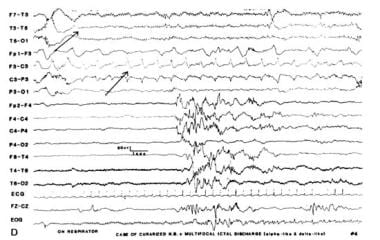
Positive rolandic sharp waves (PRWs), first described by Cukier in 1972 as pathognomonic of IVH [8] , were considered poor prognostic indicators. Later studies by Tharp [9] and Lombroso were not as conclusive about the long-term clinical implications. Although PRWs are seen in 30–50% of PT infants with IVH, they also have been detected in infants with periventricular leukomalacia without hemorrhage as well as in intraparenchymal or subarachnoid bleeding. Therefore, PRWs are probably related to deep white matter lesions, although the underlying cause is still undetermined (see image below).
-
Positive temporal sharp waves (PTWs) are noted in the records of infants with hypoxic-ischemic damage and are thought to carry a poor prognosis. As with PRWs, the implications of the presence PTWs are still inconclusive.
-
Although rarely associated with ictal discharges, occipital spikes/sharp waves, whether isolated or in unilateral brief runs, are usually found in a population of high-risk infants and considered to be abnormal regardless of age.
EEG In Neonatal Seizures
Seizures frequently occur in newborns (14 per 1000), often causing death or permanent neurological sequelae. The prognosis largely depends on etiologic factors and the duration of convulsive activity. It should be noted that generalized tonic-clonic seizures are not seen in the immature brain.
Seizure patterns can be distinguished into subtle, classic, tonic, and myoclonic types. Among these, some have a fairly consistent EEG correlation. For example, tonic spasms, unlike those that occur in older children, are often associated with rhythmic delta wave activity. Clonic seizures frequently correspond to repetitive spike discharges. Myoclonic seizures, which are often erratic or fragmented and which should be distinguished from fragmentary neonatal sleep myoclonus, are often associated with other seizure types (eg, tonic or clonic) and with a burst suppression pattern with or without ictal correlation.
Yoshinaga et al reported abnormal fast activity along with localized occipital spikes at the time of appearance of paroxysmal downward gaze, way before hypsarrhythmia and tonic spasms subsequently appeared with fast activity distribution over the same areas. [10]
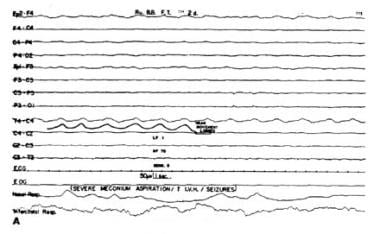
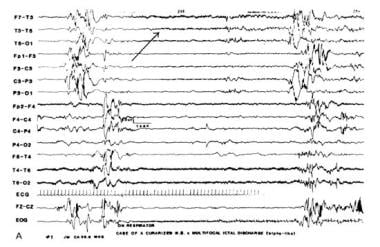
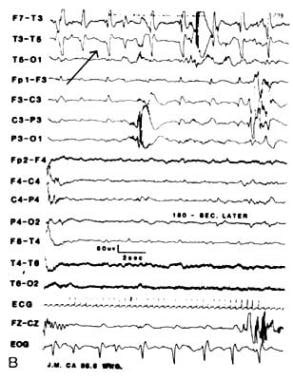
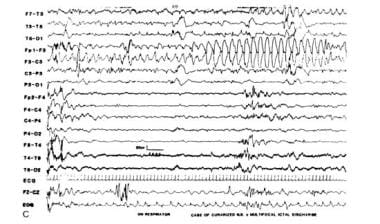
Ictal intervals, apnea, or respiratory disturbances often correlate with alphalike EEG patterns. These may be ictal discharges without clinical seizures that are not limited to iatrogenically paralyzed infants (see images below). These occult or electrographic seizures without clinically detectable signs may result from iatrogenic loading, causing serious neurological injury that disables the effector structures or silent cortical areas, which might be more generalized in newborns than in older patients.
Conversely, clinical seizures in the absence of EEG discharges suggest nonepileptic events that should be closely monitored to avoid misdiagnosis. Minimal seizure behavior, uncoupled to ictal EEG patterns, can be seen in healthy neonates and especially in encephalopathic neonates whose brains are seriously compromised by hypoxic-ischemic insults. Severe neurological injury seen in these cases causes severe background EEG abnormalities.
Perinatal asphyxia is still the major cause for the overall incidence of neonatal seizures (44.4%), especially in preterm infants with very low birth weight before 5 days of life. The second major cause is metabolic abnormalities (23.33%). Hypoxic-ischemic encephalopathy, depending on severity, yields the most abnormal results with 66.66% background activity suppression for HIE III. [11]
Several ictal discharge patterns have been identified and reported, including the following patterns:
-
Focal spikes or sharp wave discharges of progressively increasing amplitude over the course of the seizure - These discharges correspond to contralateral jerking and occur predominantly in the rolandic and temporal regions.
-
Multifocal spike and sharp wave discharges, which are often erratic with independent frequencies in multiple foci, are associated with variable seizure types. The underlying cause may range from benign conditions to CNS infection to various hypoxic-ischemic injuries. The prognosis is dependent on the background EEG abnormalities and the specific underlying etiology.
-
Prehypsarrhythmic or hypsarrhythmic patterns can be seen early in compromised newborns, representing the most severe examples of the previous pattern, and are usually associated with anarchic and refractory seizures. A separate group may be the brief ictal discharge pattern and questionable EEG ictal discharges. Decremental discharges, which sometimes accompany neonatal tonic seizures, must be distinguished from the normal arousal response that follows postural change or stimulation.
The expression of ictal activity in relation to sleep stages (REM/NREM) may have age-dependent mechanisms in the developing brain. Schmutzler et al tried to assess the relationship between ictal activity and sleep stages in the newborn EEG, finding the highest association with unrecognizable sleep states where sleep organization is already disrupted. [12] Ictal activity predominates otherwise in REM sleep (p=0.01) with longer duration of discharges, contradicting findings in adults with epilepsy. However, the mechanisms responsible for increased seizures during NREM in adults (synchronous EEG oscillations promoting electrographic seizure propagation and asynchronous discharge patterns reducing seizures during REM sleep) cannot be extrapolated to the immature developing brain.
There is in fact an age-related differential regional distribution of GABA with excitatory and not inhibitory roles [13] in subcortical areas like the substantia nigra that could facilitate the release of focal discharges during REM in newborns. Furthermore, an immaturity of REM-related inhibitory systems at a peripheral level have been shown in infants, which might affect the cortex influencing the frequency of ictal discharges during REM sleep in newborns.
Tekgul et al compared the yielding power of a reduced montage (9 electrodes) with the full 10/20 electrode montage to detect and characterize neonatal seizures and background EEG features. The sensitivity and specificity of the reduced montage for electrographic seizure detection was 96.8% and 100%, respectively, and only in 1 patient's record (over 31 pts) the single seizure was missed altogether. For assessing background abnormalities, the sensitivity and specificity of the reduced montage was 87% and 80%, respectively. The authors conclude that a reduced montage proves to be a sensitive tool for identification of neonatal seizures and grading of background EEG features in newborns. [14]
Specific syndromes of neonatal seizures include the following:
-
Early myoclonic encephalopathy (EME) was first proposed by Aicardi in 1978 to describe neonates who had myoclonic jerks and a burst-suppression EEG pattern. [15] The main criteria include severe neurological abnormalities in otherwise healthy neonates with early fragmentary erratic myoclonia and a burst-suppression pattern. A microdysgenesic malformation or metabolic disorder may be discovered later.
-
Early infantile epileptic encephalopathy (EIEE) was proposed by Otahara in 1976 and includes infants who, within 3 months after birth, develop refractory tonic spasm, developmental delay, and a burst-suppression EEG pattern. [16] Most of these infants later develop a full-blown hypsarrhythmia in the context of West syndrome. Unlike EME, burst-suppression accompanies wakefulness as well as sleep. It is rarely familial but may be linked to cerebral malformations.
-
Benign idiopathic neonatal convulsions (BINC), also known as fifth day convulsions, can be seen in both symptomatic and cryptogenic infants and are associated with a favorable outcome. BINC are associated with a specific EEG parameter (even with the variability of clinical manifestations) of alternating sharp-theta bursts that are observed in the interictal period. Occasionally, seizures may be described as GTCS consisting in tonic extension of the arms and legs, cyanosis, and clonic jerks. [17] The EEG correlate to these events, however, despite being described as generalized, points to a unilateral frontocentral onset on either side as a sign of focal seizure with secondary generalization.
-
In 1966, Rett first described benign familial neonatal convulsions (BFNC), which are transmitted through autosomal dominant inheritance. [18] The gene is localized on the long arm of chromosome 20 with regular penetration but variable expression. The incidence (0.9-2.1%) as well as the prevalence of epilepsy at later stages of life is low. EEG patterns are nonspecific. Seizure phenotype is highly variable as well as EEG ictal expressions, occasionally demonstrating generalized suppression and seizure onset followed by symmetric rhythmic slow wave and diffuse spikes in infants exhibiting tonic-clonic seizures. [19] GTCS in infants younger than 2 years are extremely rare [20] probably due to the immaturity of the developing brain lacking substantial synchronization mechanisms in the absence of sufficient myelination and mature interhemispheric connections.
-
Benign myoclonic epilepsy in infants (BMEI) is a rare epileptic syndrome characterized only by generalized myoclonic seizures occurring in normal children during the first 2 years of life. A review by Auvin found a high incidence of a positive family history of febrile seizures or epilepsy suggesting the importance of the genetic factor in the pathogenesis of BMEI. [21] Reflex myoclonic seizures are frequently observed. VPA therapy is effective. BMEI may be followed by JME and, despite a favorable neuropsychological outcome, intellectual disability can be observed.
-
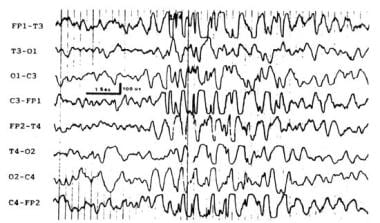
Neonatal pyridoxine dependency is a rare autosomal recessive disorder, defined by the empirical resolution of all symptoms with pyridoxine administration. It is attributed to GAD deficiency in GABA synthesis, but no gene defect has been yet identified. The EEG pattern consists of repetitive runs of 1-4 Hz high-amplitude waves and spikes that are similar to the typical spike and wave discharges that are usually seen only in older children (see image below).
In addition to neonatal seizures and reported infantile spasms from Japan, the phenotypic presentation for pyridoxine-responsive seizures includes epileptic encephalopathy of infancy and childhood and should be confirmed by pyridoxine challenge during a therapeutic EEG test. Normalization of follow-up EEGs after seizures response to pyridoxine therapy is the rule. [22] A report by Teune suggests that IV pyridoxine affects EEG background activity with reduction in amplitude and total power in infants younger than age 1 year with therapy-resistant seizures as a nonspecific effect. [23]
Follow-up studies on the incidence of epilepsy following clinical neonatal seizures yield different results. According to Ronen et al, results from a population-based study with more than 10 years follow-up, show the risk being highest for premature babies. [24] The remote symptomatic epilepsies are usually accompanied by motor and cognitive comorbidities, mortality being seen only among patients whose epilepsy was associated with intellectual disability and cerebral palsy. An adverse outcome (ie, death, severe developmental delay, CP) at age 2 years was strongly associated to seizures in preterm babies of very early gestational age and low birth weight.
In the context of neonatal seizures, besides background EEG activity, some ictal findings present on first EEG may hold a prognostic value and predict neurodevelopmental outcome. Among them, the spread of ictal discharge to the contralateral hemisphere and the ictal fraction (total duration of the seizures/EEG duration/hour) might suggest significant prognostic informations since the early hours of life. [25]
Neonatal EEG as a Diagnostic and Prognostic Tool
Although most neonatal EEG patterns are nonspecific and cannot provide the diagnosis when used alone, it is conversely true that they should cue the clinician to order neuroimaging tests. This is especially true for interhemispheric or regional asymmetries; specific EEG features may suggest infectious causes or deep matter necrosis, genetic brain malformations, or an inborn error of metabolism.
EEG is also an excellent tool to help diagnose subclinical seizures or to avoid a misdiagnosis of seizures in the presence of atypical nonictal neonatal behaviors. When used selectively through serial recordings, neonatal EEG's greatest value is its potential for prediction of short- and long-term prognosis.
Video-polysomnography (PSG) in preterm infants weighing less than 1800 g is indicated to rule out sleep-related respiratory disorders that may endanger the baby once he or she is discharged from the neonatal unit. The most common indication for video-PSG in these babies is the observed presence of apneas and oxygen desaturations. The most frequently associated conditions is hyaline membrane disease, [26] cardiorespiratory impairment being detected in 65% with a 32% rate of immature EEG activity and 13% of abnormal patterns. The authors underline that based on their study, a requirement for home monitoring was alerted in 69% of babies, with a quarter of them needing oxygen at home.
In premature infants younger than 30 weeks gestational age, "dysmature" and "disorganized" are the EEG patterns mostly and persistently found over serial recordings in newborns with severe or moderate sequelae as for cognition and cerebral palsy, with a sensitivity for psychomotor outcome of 83.3%, a specificity of 88%, and a positive predictive value of 90%. [27]
In particular in preterm babies to detect periventricular leukomalacia (PVL), EEG recordings are recommended within 48 hours from birth to detect acute-stage abnormalities and in the first 2 weeks of life to assess chronic-stage abnormalities. In fact, the latter are observed most frequently and most severe between days 5 and 14 and resolved in all survivors, persisting longer only in babies with extensive cystic PVLs. [28]
The EEG background, static abnormalities, and EEG maturational indices are the best prognostic factors. EEG may reflect the severity of brain injury in neonatal asphyxia, the degree of abnormalities usually being consistent with the clinical grading of HIE. [29] Therefore, in neonatal asphyxia, EEG background pattern especially is a valuable predictor of future neurologic outcome in combination with clinical data such as gestational age, birth weight, imaging, and HIE severity. Low birth weight amplitude of brain electrical activity, duration of interburst intervals, and sleep-wake cycle disturbance were among the most significant indexes of a poor long-term prognosis. [30]
In newborn infants undergoing cardiac surgery for congenital heart disease, amplitude-integrated EEG (aEEG) prior to surgery, during surgery, and 72 hours postoperatively, reviewed for seizure activity and background pattern to correlate with neurodevelopmental outcome at 2 years, indicate that perioperative seizures are common (30%), often subclinical, in this group and do not affect neurodevelopmental outcome. Instead, delayed recovery in aEEG background continuity was associated with increased risk of early mortality and worse neurodevelopment. [31]
In children with neonatal seizures, the presence of severely abnormal background EEG activity (odds ratio [OR], 9.5) and status epilepticus (OR, 6.1) were found to be predictors of postneonatal epilepsy in association with partial or no response to anticonvulsant therapy (OR, 16.7 and 47, respectively) and severely abnormal ultras and scan alterations (OR, 5.4), even if only the former seemed to be an independent predictor for subsequent epilepsy. [32]
To assess early markers of risk for neurobehavioral compromise in congenital heart disease (CHD) survivors, fetuses younger than 24 weeks gestational age have been enrolled in a pilot study performing serial Doppler assessment of the middle cerebral and umbilical arteries. The cerebral-to-placental resistance ratio (LPR) and middle cerebral artery pulsatility were correlated with postnatal high-density EEG and beta-frequency band EEG power, showing that abnormal cerebrovascular resistance in hypoplastic left-sided heart syndrome, transposition of great arteries, and tetralogy of Fallot predicted decreased neonatal EEG left frontal beta power and lower cognitive development scores at 18 months. [33]
A numerical score of background EEG, [34] with higher numerical scores reflecting greater abnormality, has recently shown reliability to improve the prognosis of neurodevelopmental impairment cerebral palsy and epilepsy, especially if correlated with neuroimaging abnormalities. Quantitative EEG [35] and prolonged bed-side amplitude-integrated EEG [36] have increased the prognostic accuracy for babies at risk of HIE after perinatal asphyxia.
Also, limited-channel EEG technology may be seen as a useful and economic alternative for longitudinal bedside monitoring in neonatal units when prolonged video-EEG monitoring is not obtainable.
Although some ictal discharges may have specific significance, a normal interictal EEG indicates the greatest chance of favorable outcome, even in the case of early, recurrent seizures. Several studies have demonstrated the prognostic role of EEG in different diseases, ranging from neonatal seizures to asphyxia and hemorrhage. [37, 9, 38] At this early developmental age, the EEG has a greater prognostic potential than any other diagnostic tool available to neonatologists. EEG is clearly preferable to a neurological examination of a neonate who inherently displays a narrow behavioral and clinical profile. Prospectively, EEG can predict in early infancy later developmental patterns such as hypsarrhythmia with infantile spasms.
Questions & Answers
Overview
What are the characteristics of a normal neonatal EEG?
How is the prognostic value of an abnormal neonatal EEG increased?
Which neonatal EEG patterns are characteristic of severe abnormalities?
What are the characteristic findings of the paroxysmal or burst suppression neonatal EEG pattern?
What are the characteristic findings of the low-voltage neonatal EEG pattern?
What is the significance of abnormal neonatal EEG patterns during sleep-wake states?
Which abnormal neonatal EEG findings are characteristic of hypoglycemia?
Which abnormal neonatal EEG findings are characteristic of inborn errors or metabolism?
What is the role of neonatal EEG in the workup of CNS infections?
Which neonatal EEG findings are characteristic of rubella?
Which neonatal EEG findings are characteristic of toxoplasmosis?
Which neonatal EEG findings are characteristic of postnatal CNS infections?
Which neonatal EEG findings are characteristic of herpes simplex virus (HSV) encephalitis?
What causes focal neurologic abnormalities on neonatal EEG?
What is the significance of a focal attenuation pattern on neonatal EEG?
What are nonictal paroxysmal patterns on neonatal EEG?
Which findings on neonatal EEG are characteristic of seizures?
Which findings on EEG are characteristic of specific neonatal seizure syndromes?
Which findings on EEG are characteristic of pyridoxine-responsive seizures?
Which neonatal seizure findings on EEG predict neurodevelopmental outcome?
What is the role of neonatal EEG as a diagnostic and prognostic tool?
-
Quiet sleep non–rapid eye movement tracé alternant.
-
Inactive or isoelectric pattern.
-
Paroxysmal or burst suppression EEG. Notice prominent bursts of paroxysmal activity interspersed with an inactive background activity.
-
Positive rolandic sharp waves R>L.
-
Multifocal electrographic seizure in a curarized infant (alphalike pattern).
-
Infant with L focal paroxysmal temporal discharges.
-
Infant with deltalike L-frontal electrographic seizure.
-
Infant with L electrographic seizure with multifocal L ictal discharges.
-
Classic seizures corresponding to multifocal polyspike pattern with shifting predominance.
-
Pyridoxine-dependent syndrome - generalized spike-wave discharges.