Overview
The electroencephalogram (EEG) provides useful information that reflects the function of the neonatal brain. [1, 2, 3] The EEG may assist in determining brain maturation and identifying focal or generalized abnormalities, existence of potentially epileptogenic foci, or ongoing seizures. [4] It is useful in assessing prognosis for neonates at risk for neurological sequelae. Basic guidelines for visual analysis and techniques of recording and interpretation of the EEG in infants younger than 44 weeks are discussed in this article.
Patient Education
For excellent patient education resources, see eMedicineHealth's patient education article Electroencephalography (EEG).
Technique
While the principles of electroencephalography are the same in neonates as in older children and adults, successfully recording and interpreting neonatal EEGs require additional skills. Considerable experience and patience are required to obtain technically satisfactory EEGs from neonates in a busy intensive care nursery. Sick neonates often have multiple organs being monitored by a variety of devices that can lead to unusual artifacts and may make reaching the infant's head difficult.
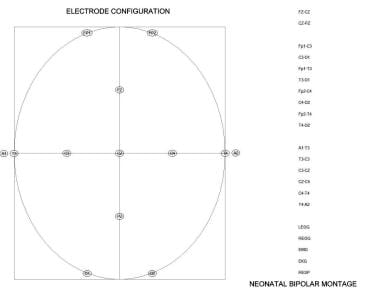
The number of electrodes used in recording the neonatal EEG is reduced owing to the small head circumference of the newborn. A standard 10-20 system is used with a single combined longitudinal and transverse montage. The frontal-temporal, frontal-central, temporal-occipital, and central-occipital longitudinal measurements are double-distance recordings. Instead of each interelectrode distance being 20% of the diameter of the head, they are 40%. Transverse and midcentral interelectrode distances are the standard 20% distances. See the image below.
Additional physiological leads that consist of 2 eye-movement monitors, electromyogram (EMG), and ECG and respiratory monitor electrodes (chest wall and/or nasal airflow) are placed to aid in determination of state. One eye electrode is placed 0.5 cm below the outer canthus, and the other electrode is placed 0.5 cm above the outer canthus of the other eye. Differential field effects of the retina-to-cornea dipoles recorded in these opposing electrodes provide data on the types of eye movements. EMG electrodes are useful in differentiating subcortical or peripheral myoclonus from movements associated with epileptiform activity recorded at the surface of the brain.
Recording settings are chosen to acquire the necessary spectrum of data and to eliminate artifact. A time constant of 0.3 seconds typically is chosen to record the slower frequencies present in the neonatal EEG. A low-pass filter of 70 Hz is recommended. Filtering of high-frequency muscle artifact with 35 Hz (or other low-pass filter) is not recommended because EEG activity can be distorted and may be misinterpreted as sharp activity. Sensitivity typically is set at 7 µV/mm and adjusted, if necessary. ECG, EMG, and eye electrodes are set at sensitivities of 50 µV/mm, 50 µV/mm, and 7 µV/mm, respectively, and adjusted as necessary.
The EEG test typically is run for 60 minutes or more to ensure the recording of at least one change in sleep state (a full sleep cycle in the neonate is typically 50-60 min).
Traditionally, neonatal EEGs were performed on paper EEG writers, and a consensus regarding paper speed was not established. Recording speed often was chosen to be half the speed of the typical adult record (ie, 15 mm/s instead of 30 mm/s).
Detection of asymmetries or asynchronies may be enhanced by slow speed. This becomes less of an issue as digital EEG acquisition and review allows for postrecording change of the time base of the recording. Review at different speeds is available, when necessary. Unfortunately, waveform morphology is different in records that are recorded at different paper speeds. At 15 mm/second, sharp waves and spikes are half the width in the EEG record and look sharper. Sharp waves and spikes must stand out from the background, be sharply contoured, and be 70-200 milliseconds (ms) and 20-70 ms in duration, respectively.
Visual Analysis
A 5-step process should be performed when analyzing a neonatal EEG. The 5 steps consist of the following:
Knowledge of the postconceptional age and topography of the infant's head
Identification of artifacts in the EEG
Identification of sleep and wake states
Feature extraction
Classification of the record as normal or abnormal and the clinical correlation provided to the clinician
Postconceptional age and topography
An accurate estimate of postconceptional age is required because features in the EEG vary with the age of the newborn. Postconceptional age is defined as gestational age (in weeks) plus the number of weeks since birth. Gestational age is the number of weeks/months the child was in the womb. Legal age is the age of the child since birth. Prematurity (PT) is defined as birth at a gestational age of less than 38 weeks; full term (FT) is defined as birth at a gestational age of 38-42 weeks.
As in the adult, a description of skull and scalp topography is necessary. Scalp swelling and other forms of trauma can increase interelectrode resistance and attenuate the recording. Meningocele or subdural or epidural fluid collections can alter interelectrode resistance as well. Skull fractures provide a low-resistance pathway for electric fields and result in increased voltage. Distorted cranial vaults (common after birth trauma) also alter the topography of the EEG.
Artifact rejection
As with the older child, all artifacts must be identified before a visual analysis of the EEG can begin so that they are not considered part of the background or paroxysmal activity. The neonate should lie still with the head in the midline position to minimize artifacts. The technician must try to determine the nature of artifacts at the time they occur because, once the child is disconnected from the EEG, identification of artifacts is difficult.
Common artifacts seen on neonatal EEG are as follows.
-
Cardiac
ECG and pulse (See the image below.)
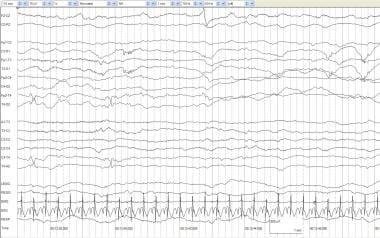
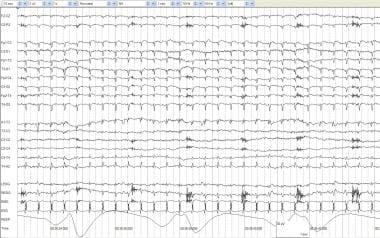 Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
Ballistocardiographic (movement of the head with heartbeat)
-
Body
Respiratory, twitches, and tremor (See the image below.)
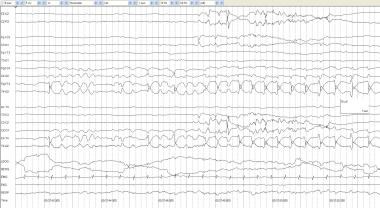
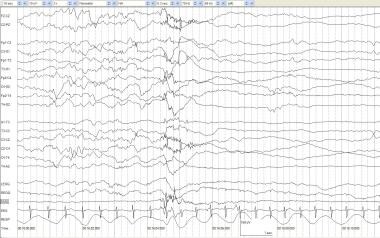 Twitching artifact. A full-term, 3-day-old infant with anoxic encephalopathy and episodes of generalized twitching that last 1-2 seconds at a time. Muscle artifacts are seen in multiple electrodes and are particularly prominent in the EMG channel. After the twitch, the background attenuates and appears to be a state change rather than an electrographic seizure correlate.
Twitching artifact. A full-term, 3-day-old infant with anoxic encephalopathy and episodes of generalized twitching that last 1-2 seconds at a time. Muscle artifacts are seen in multiple electrodes and are particularly prominent in the EMG channel. After the twitch, the background attenuates and appears to be a state change rather than an electrographic seizure correlate.
-
Head
Vertical, horizontal, and rotatory head movements
Electrode "pops" and fontanelle-related pulsations
-
Face
Sucking, glossokinesthetic, eye movements and blinks, frontotemporal muscle contraction
-
Other
60-Hz electrical
Electromechanical devices (eg, ventilators, IV drips) (See the image below.)
 Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
Identification of sleep and wake states
The observation of the infant's movements, description of the infant's behavior by the technician, and analysis of the data in the noncerebral electrodes determine the neonate's state. Table 2 below describes types of activity expected for each state. Lateral eye movements, ventilatory rate, and ECG and EMG patterns do not become reliable indicators of state, however, until 34-36 weeks postconceptional age.
EEG is a useful test in providing reliable clues for assessing postconceptional age. Quantification of various sleep states from the newborn period to adulthood show that FT infants spend more time in rapid eye movement (REM) sleep than any other age group. [5] Furthermore, as a PT baby approaches FT, the amount of nonrapid eye movement (NREM) sleep increases, while REM sleep decreases. [6] Quiet sleep is analogous to NREM sleep, and active sleep is analogous to REM sleep in the adult. Quiet sleep is characterized by an absence of lateral eye movements and increased chin EMG activity with regular respirations and ECG. See the image below.
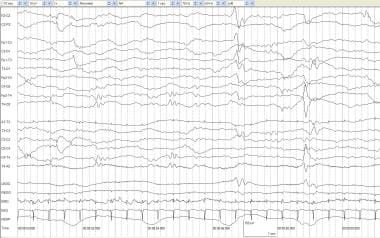
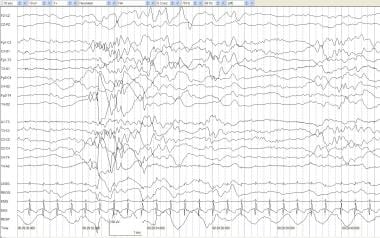 Quiet sleep and tracé alternant (TA). An infant of 39 weeks' postconceptional age with hydrocephalus and possible seizures. During quiet sleep, respirations are regular, EMG activity is low-voltage tonic with no phasic activity, and no spontaneous eye movements occur. TA is seen with medium- to high-voltage mixed frequencies alternating with periods of relative voltage attenuation.
Quiet sleep and tracé alternant (TA). An infant of 39 weeks' postconceptional age with hydrocephalus and possible seizures. During quiet sleep, respirations are regular, EMG activity is low-voltage tonic with no phasic activity, and no spontaneous eye movements occur. TA is seen with medium- to high-voltage mixed frequencies alternating with periods of relative voltage attenuation.
Neonates often enter active sleep at onset of sleep. Low-to-moderate voltage, continuous EEG pattern with rapid eye movements; irregular respirations and cardiac rate; decreased chin EMG activity; and quick irregular movements of the fingers, hand, or face characterize active sleep. By term, 2 patterns of REM may be seen if the recording continues long enough. In the first REM cycle, the EEG shows fairly continuous mixed frequencies in the delta, theta, and alpha ranges with a paucity of faster activity, and the voltages range from 40-l00 µV. In the second REM cycle, which occurs after a period of NREM sleep, the background is more continuous, voltage is lower (20-50 µV), and frequencies are faster than in the first REM period. See the image below.
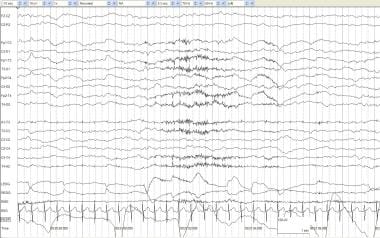 Active sleep. An otherwise healthy infant of 41 weeks' postconceptional age with episodes of arm and leg extension without EEG correlates. This segment of normal, active sleep shows irregular respirations; frequent eye movements on EOG; and a mixed pattern of delta, theta, and alpha frequency activity.
Active sleep. An otherwise healthy infant of 41 weeks' postconceptional age with episodes of arm and leg extension without EEG correlates. This segment of normal, active sleep shows irregular respirations; frequent eye movements on EOG; and a mixed pattern of delta, theta, and alpha frequency activity.
Delta brushes are present in the premature infant beginning at 26 weeks' postconceptional age. Delta brushes are analogous to K complexes in the adult; however, they typically occur asynchronously in the neonate. Delta brushes consist of a mixture of medium- to high-voltage delta activity intermixed with low- to medium-voltage fast activity, commonly with 18- to 22-Hz maximum in the central region. Delta brushes are a prominent feature in active sleep by 29-33 weeks' postconceptional age, and, by 33-38 weeks' postconceptional age, they are expressed maximally in quiet sleep. [7]
Prior to 28-30 weeks' postconceptional age, the record is discontinuous, with interbursts of low voltage or inactivity alternating with higher amplitude and mixed-frequency activity, a pattern termed tracé discontinu (TD). See the image below.
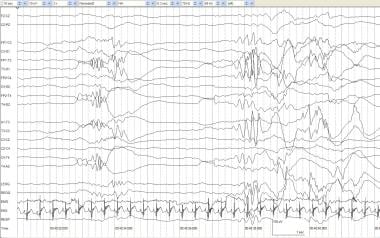 Tracé discontinu (TD). An infant of 24 weeks' gestational age at age 4 weeks with an intraventricular hemorrhage and left shoulder twitching. Periods of alternating high-voltage mixed frequencies and periods of voltage suppression are normal findings before 28-30 weeks' postconceptional age.
Tracé discontinu (TD). An infant of 24 weeks' gestational age at age 4 weeks with an intraventricular hemorrhage and left shoulder twitching. Periods of alternating high-voltage mixed frequencies and periods of voltage suppression are normal findings before 28-30 weeks' postconceptional age.
Beginning at 28-30 weeks' postconceptional age, some sleep-state differentiation occurs, with active sleep having more continuous patterns than quiet sleep. However, sleep-state differentiation may be difficult until 32-34 weeks' postconceptional age. As the infant matures, the interburst durations decrease, and the amplitude and morphologies of the interburst activity change. Beginning at approximately 34-35 weeks' postconceptional age, other physiological features become increasingly helpful in determining sleep state.
Tracé alternant (TA) is defined as the discontinuous pattern of NREM sleep in which bursts of prevalent slow activity (1-4 Hz) alternate with random, faster transients at 50-200 µV. The bursts appear about every 4-5 seconds and last 2-4 seconds. The interburst consists of low-voltage, continuous, somewhat rhythmic activity with a dominant frequency in the theta range and voltages between 20-50 µV. TA usually emerges by 36-38 weeks' postconceptional age. See the image below.
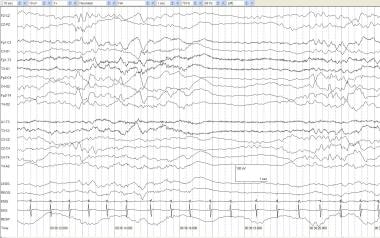 Tracé alternant (TA). An infant of 42 weeks' postconceptional age delivered via cesarean birth, with Apgar scores of 4/4/4 and left arm and leg jerking movements. TA occurs with regular respirations, low-voltage tonic EMG, and minimal eye movements. Periods of relative voltage attenuation may occur periodically during quiet sleep.
Tracé alternant (TA). An infant of 42 weeks' postconceptional age delivered via cesarean birth, with Apgar scores of 4/4/4 and left arm and leg jerking movements. TA occurs with regular respirations, low-voltage tonic EMG, and minimal eye movements. Periods of relative voltage attenuation may occur periodically during quiet sleep.
By term, the infant often has a slow wave sleep pattern (SWS) of NREM state, which consists of prominent diffuse delta with some theta rhythms. Although some brief periods of SWS can occur as early as 36 weeks' postconceptional age, the amount of SWS gradually increases until 44-48 weeks' postconceptional age, when it almost completely replaces the TA pattern.
Table 1. State-related Polygraphic Changes in the Neonate (Open Table in a new window)
Physiological Measure |
Awake/Sleep State |
||
Awake |
Active Sleep |
Quiet Sleep |
|
EMG (chin) |
Phasic and tonic |
Phasic |
Tonic |
Respiration |
Irregular |
Irregular |
Regular |
Eye movements |
Random or pursuits |
Rapid eye movements |
Absent |
Body movements |
Facial, limbs, and body |
Sucking and irregular limb movements |
None |
Neonatal EEG Feature Extraction
Feature extraction
The process of feature extraction is performed for each state that is identified in the EEG record. The types of features to be identified include amplitude, continuity of background activity, frequency, symmetry, reactivity, synchrony, and maturational and paroxysmal patterns.
-
Amplitude: The spectrum of abnormalities of background varies from the ominous pattern of electrocerebral inactivity to the more benign finding of low-amplitude activity during discontinuous sleep. Note that caput succedaneum, scalp edema, and subdural effusions or hematomas may affect the apparent amplitude of the EEG.
The most extreme abnormality in amplitude consists of electrocerebral inactivity (isoelectric/inactive) (see image below). This is a record in which no cerebral electrical activity is present. Before concluding that this pattern is present, the electroencephalographer must be certain the test was performed satisfactorily and for a long enough time to include potential sleep cycling. Increased sensitivities, long time constants, and long interelectrode distances are used. Artifacts are identified and eliminated, when possible. The technician always performs various stimulations (auditory and nociceptive) to establish lack of reactivity. This EEG pattern carries a grave prognosis in both PT and FT infants if not due to postictal state, hypothermia, acute hypoxia, or drug intoxication. Most infants with persistent electrocerebral inactivity either die or have severe neurological sequelae. See the image below.
 Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
A second abnormality of amplitude occurs when the low-voltage undifferentiated pattern consists of background activity between 5-15 µV in all states. Distinct state changes typically do not occur. This background pattern is easier to recognize in PT infants because discontinuity and high-voltage bursts are normal, and a decrease or absence of delta brushes, which should be quite prominent at this age, is common. The low-voltage undifferentiated pattern is associated with poor outcomes, particularly when the pattern persists beyond the first weeks of life. Low-voltage records can be observed in a variety of neonatal encephalopathies, including hypoxia-ischemia, congenital hydrocephalus, and severe intracranial hemorrhages such as large subdural hematomas. They also may occur in various toxic or metabolic disturbances. A low-voltage record shortly after a hypoxic or ischemic event is less concerning than one obtained days after the event.
-
Continuity: One of the most striking features of the neonatal EEG is its discontinuity (ie, periods of higher voltage activities followed by periods of lower ones that occur during portions of the recording). Degrees and morphologies of discontinuity vary significantly in healthy neonates according to their postconceptional age (most dramatic in young PT and almost nonexistent in healthy FT infants reaching 3-4 weeks of postnatal life). These features also vary according to the newborn's states.
No absolute criteria currently exist that can be used to determine whether records are excessively discontinuous. However, recent studies do provide some guidance. Hahn et al studied interburst intervals (IBIs). Conservatively stated, the maximum IBI duration should be less than 40 seconds in infants younger than 30 weeks' postconceptional age; by term, the IBIs should be less than 6 seconds in duration.
The most obvious abnormality of continuity is burst-suppression (BS). This pattern consists of bursts of high-voltage activity lasting 1-10 seconds. BS is composed of various features (eg, spikes, sharp waves, theta, delta), which are followed by periods of marked background attenuation (voltage < 5 µV). The bursts (highly synchronous between hemispheres) contain no age-appropriate activity. In the most austere form, this pattern is invariant, minimally altered by stimuli, and persistent throughout awake and sleep states. This pattern is easy to differentiate from the discontinuous features normally seen during NREM sleep in infants older than 34-36 weeks' postconceptional age when TA begins to emerge clearly. See the image below.
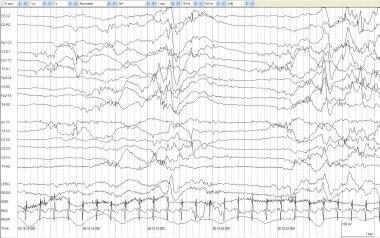 Burst suppression. An infant of 42 weeks' postconceptional age with asphyxia. An alternating pattern of high-voltage mixed frequency activity and voltage attenuation in a term infant indicates severe diffuse cerebral dysfunction. Compare this to tracé discontinue (TD), in which similar activity may be considered normal prior to 30 weeks' gestational age.
Burst suppression. An infant of 42 weeks' postconceptional age with asphyxia. An alternating pattern of high-voltage mixed frequency activity and voltage attenuation in a term infant indicates severe diffuse cerebral dysfunction. Compare this to tracé discontinue (TD), in which similar activity may be considered normal prior to 30 weeks' gestational age.
The discontinuous EEG pattern of TA is distinguished from BS by the presence of higher amplitude interburst activity, reactiveness to stimulation, and containment of EEG features normal for postconceptional age (ie, delta brushes, temporal theta, or frontal sharp transients). Most importantly, cycling of the discontinuous pattern of TA with the continuous pattern of REM sleep is prevalent.
Problems may arise in young PT infants (34 weeks' postconceptional age and younger) owing to discontinuous periods of practically absent activities between bursts, which are typical at this age. [8] Unlike the normal discontinuous pattern of TD, BS usually is invariant and not associated with other features characteristic of the EEGs of neonates of various postconceptional ages. Testing for reactivity, usually but not invariably absent in BS, is also helpful. In young PT infants, serial recordings are advisable before reaching the dire prognosis generally attached to BS. However, the most reliable clue to distinguish the pathological pattern of BS from the ontogenetically normal TD is an accurate postconceptional age of the infant.
-
Frequency: Records that are excessively slow or fast are unusual in neonates. Rarely, a neonatal record consists of diffuse delta activity in both waking and sleep states, minimal theta or faster frequencies, and poor reactivity. When these conditions persist longer than 2 weeks in FT neonates, the prognosis is poor.
-
Symmetry: Neonatal records with voltage asymmetries of 25% or more are abnormal. Noncerebral hemisphere abnormalities should be excluded when evaluating records for amplitude abnormalities. These noncerebral hemisphere abnormalities include unilateral cephalhematomas, subgaleal or scalp edema, and technical faults (eg, asymmetric placement of electrodes, sweating, smearing of electrode paste). Because of the nature of their lesions, neonates exhibiting this pattern often develop seizures. Ictal EEG discharges can be seen over the depressed side, and sustained focal discharges superimposed on this abnormal background represent the rare occurrence of focal ictal events in direct proximity with underlying anatomical lesions.
Transient interhemispheric asymmetry is likely a normal variant. A sudden, markedly exaggerated TA pattern approaching a BS pattern involving only one hemisphere while the other hemisphere maintains normal features is seen occasionally. This finding may be indicative of a focal lesion.
Persistent attenuation of voltage involving only one scalp region can be associated with focal or lateralized neuropathological lesions. The technician must be aware that improper placement of electrodes or localized scalp edema can result in an EEG pattern that resembles focal attenuation. See the image below.
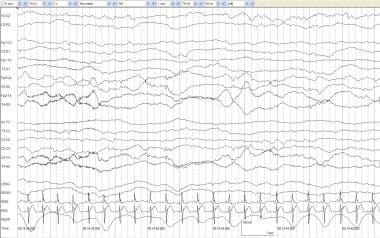 Voltage attenuation, focal. An infant of 40 weeks' postconceptional age with a left middle cerebral artery infarction and intermittent posturing and hyperextension of the neck. Background activity demonstrates attenuation of delta and fast activity on the left indicative of a structural lesion on the left.
Voltage attenuation, focal. An infant of 40 weeks' postconceptional age with a left middle cerebral artery infarction and intermittent posturing and hyperextension of the neck. Background activity demonstrates attenuation of delta and fast activity on the left indicative of a structural lesion on the left.
-
Synchrony: Synchronous activity is defined as simultaneous EEG activity in homologous regions of each hemisphere. Synchrony is assessed during TA and NREM sleep. In well-established TA in healthy FT infants, the bursting activities between hemispheres occur almost simultaneously. In younger infants (32-38 weeks' postconceptional age), these bursts occur with less synchrony. In even younger PT infants (27-28 weeks' postconceptional age), the bursts of TD are synchronous between hemispheres.
-
Sleep-state transitions: While observation of sleep-state changes provides the electroencephalographer with a considerable amount of information regarding the status of the infant's brain, those interpreting neonatal EEGs must be cautioned that considerable variation occurs during a routine 1-hour EEG, not only in different neonates but also in the same infant when tested at intervals of a few days. These marked individual fluctuations often overlap with those observed in impaired newborns. Care must be taken in interpreting an abnormal record solely based on sleep features. As with other abnormalities, persistence of the abnormality on serial records adds to the strength of the predictive value of the EEG.
Records in which no recognizable sleep states exist, despite lengthy recordings, are abnormal. In infants older than 30 weeks' postconceptional age, the lack of sleep-state differentiation usually is readily apparent (continuous activity typically appears in active sleep by 30 weeks' postconceptional age). In addition to the lack of cyclical states, the EEGs often contain other abnormalities such as persistent low voltage or excessive discontinuity, monotonous moderate theta or alpha range frequencies, and even interictal or ictal discharges. Disruption in the ability to develop cyclic states often is observed in infants in a coma state due to a variety of causes. In these patients, the following factors should be ruled out: reversible toxic factors, hypothermia, and the use of drugs for controlling seizures or minimizing brain damage due to anoxic-ischemic insults. Lack of distinct sleep states is associated with poor outcomes.
Transitional or indeterminate sleep is used by some to indicate periods of sleep in which a lack of concordance between criteria does not allow their classification as either REM or NREM. An excessive amount of transient sleep is considered abnormal. A similar pattern, excessive labile sleep states, refers to rapid changes of sleep state, with the infant spending only a few minutes or even seconds in one sleep state before entering another state.
Both excessive transient sleep state and excessive labile sleep state have been observed in infants born to mothers on drugs such as alcohol.
-
Maturation: EEG maturation from PT to FT and beyond occurs in a predictable time-linked fashion. The observation that the maturation of the EEG is primarily dependent on the postconceptional age renders the EEG a valuable tool in assessing central nervous system physiological maturation.
A number of indices are used in determining whether the EEG is appropriate for the infant's postconceptional age. These indices include the percentages of interhemispheric synchrony during TA, amount of delta brushes in both REM and NREM states, and sleep-state transitions including concordance between EEG and other bioelectric and behavioral parameters.
The neonatal record is considered to have a pattern of dysmaturity when the background patterns lag behind postconceptional age by at least 2 weeks. Dysmaturity may be either transient or persistent.
A constellation of electroencephalographic and physiological information goes into determining whether a record is mature or not. The following criteria may be helpful in determining when an EEG is maturationally delayed:
By 32-34 weeks' postconceptional age, the background activity should have some periods of continuous activity as periods of better-organized REM and NREM states begin to emerge. The average interburst interval should be less than 20 seconds. The range of interhemispheric synchrony between bursts of the emerging TD varies between 50% and 70%. Delta brushes still abound, tending to localize more over rolandic and occipital areas. The high-voltage temporal theta of younger PT infants is less frequent, while lower voltage, multifocal, sharp transients occur between bursts. In the absence of an isoelectric reading, distinguishing immature records in this age group from those with more severe disturbances of background activity may be difficult.
By 34-36 weeks' postconceptional age, clear EEG distinction between REM and NREM states should be observed. REM should consist of a continuous pattern with mixed delta and theta waves of variable voltage (usually 30-100 µV). Delta brushes are present more frequently in NREM than in REM sleep. Interhemispheric synchrony of bursts of NREM TD ranges from 70-85%.
By 36-38 weeks, TD prevails in NREM sleep, and activity >20 µV is present during most of the interburst interval. Brief periods (< 3 s) of lower voltage activity still can be seen during the interburst interval. During REM sleep, abundant clusters of rapid eye movements, frequent body movements, decreased muscle tone, and irregular respiration are present. In NREM sleep, only rare eye movement, general body quiescence, increased muscle tone, and regular respirations (in the absence of pulmonary disease) are present. Interhemispheric synchrony of bursts in TD ranges from 85-95%. Delta brushes still occur but are diminished during NREM sleep, with an average of approximately 20 during every 5 minutes of REM sleep.
By 38-40 weeks' postconceptional age, the infant should cycle through clear sleep states. TA occurs during NREM sleep. Bursts are 90-100% synchronous (< 2 s difference in time of onset). Bursts and interburst intervals are of similar duration. Occurrence of delta brushes decreases, with only 1 or 2 during every 5 minutes of REM sleep.
By 40-42 weeks, no delta brushes are present. If present, delta brushes occur only during NREM sleep, at most 4 per 5-minute epoch. Complete interhemispheric synchrony between bursts of TA is observed. The infant should cycle through clear sleep states.
By 44 weeks, continuous slow-wave pattern should be predominant during NREM sleep.
By 48 weeks, TA should be minimal with NREM sleep dominated by high-voltage, slow-wave activity. Some sleep spindles also should emerge.
-
Paroxysmal patterns: These include interictal discharges (positive rolandic sharp waves, frontal and temporal sharp transients), ictal discharges (focal spikes and sharp waves, pseudo delta, theta, alpha, and beta), and low-frequency discharge patterns.
Interictal patterns
While spikes or sharp waves are defined the same way in neonates, older children, and adults, their significance may be different. In many cases, spikes and sharp waves are normal features, especially in PT infants. Spikes and sharp waves are common during the bursting phase of TD or TA and may be observed over the temporal region during the inactive phase.
In healthy FT neonates, sporadic spikes predominate over frontal areas (usually synchronously) and are incorporated within the bursts of TA. They often coexist with rhythmic slow waves, misnamed "anterior slow dysrhythmia." These transients may shift between hemispheres; they begin to appear clearly at approximately 34-35 weeks' postconceptional age, persisting with diminished frequency into the beginning of the early infancy period. Because these spikes and sharp waves can be ontogenetically normal in spite of their epileptiform morphology, they have been labeled frontal sharp transients to defuse implications of an epileptiform signature.
Positive rolandic sharp waves are surface-positive, broad-based, sharp transients with duration up to 500 ms, localized over the rolandic areas (C3-C4). In spite of their paroxysmal features that might suggest an epileptiform signature, positive rolandic spikes do not correlate with ictal phenomena. Although these waves can be observed in the context of intracerebral hemorrhages, the present consensus is that they are associated more directly with pathologies that induce deep white matter lesions. Their underlying generator remains obscure, especially since explaining them simply based on white matter necrosis is difficult.
Positive temporal sharp waves are EEG transients with morphology and polarity similar to those of positive rolandic sharp waves; however, they occur over the midtemporal areas. They occur in neonates with intracranial hemorrhages or a history of perinatal asphyxia. See the image below.
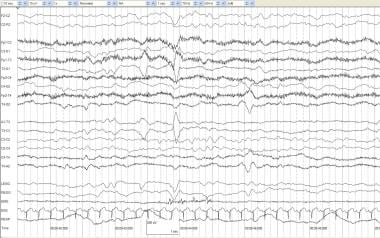 Positive temporal sharps. An infant of 41 weeks' postconceptional age with a fever (temperature, 102°F) and 3 episodes of right arm and leg jerking with eye deviation that last 5-10 seconds each. Left positive temporal sharps are seen in the 4th and 5th seconds at T3 and independently at T4 during the 8th second.
Positive temporal sharps. An infant of 41 weeks' postconceptional age with a fever (temperature, 102°F) and 3 episodes of right arm and leg jerking with eye deviation that last 5-10 seconds each. Left positive temporal sharps are seen in the 4th and 5th seconds at T3 and independently at T4 during the 8th second.
A clear differentiation between normality and abnormality for spikes and sharp waves may be difficult because the boundaries still are not distinct and are a matter of controversy. Spikes and sharp waves that occur over frontal, rolandic, and temporal areas are abnormal if they are excessively frequent for the postconceptional age, appear in short runs, are consistently unilateral, occur frequently during the attenuated phase of TA, or persist during the more continuous patterns of REM sleep or wakefulness. See the image below.
The significance of excessive numbers of spikes and sharp waves is not clear. While excessive numbers of spikes and sharp waves are more common in neonates with seizures than in neonates without them, the correlation is tenuous. Many neonates with electroencephalographic and/or behavioral seizures may have few interictal spikes or sharp waves. Conversely, the presence of excessive spikes or sharp waves can be seen in neonates without a history of seizures.
Ictal patterns
A generally accepted definition of what constitutes an ictal discharge has not been established. Some authors classify a discharge as ictal if it lasts at least 10 seconds, while other authors require a duration of at least 20 seconds. However, documented clinical and electrical seizures long have been described as lasting only a few seconds. With increasing age, electrical seizure activity becomes more frequent and longer in duration. Some have proposed that any rhythmic discharge accompanied by clinical changes be considered an ictal event, while rhythmic discharges lasting 10 seconds or longer be considered electroencephalographic events, regardless of whether behavioral changes occur.
Electrographic seizure discharges typically consist of rhythmic theta, alpha, delta, or beta waves or sharp waves or spikes that are focal or multifocal and vary in frequency and amplitude as the seizure progresses. The electroencephalographer must be aware that a variety of artifacts may resemble electroencephalographic seizures. Changing location, waveform morphology, or frequency suggests that the rhythmic activity is cerebral. The cerebral hemispheres in the neonate function relatively autonomously, so that, even when seizures spread, they may not spread to the other hemisphere. In cases when seizures do spread to the opposite hemisphere, the discharges often alternate from side to side. See the images below.
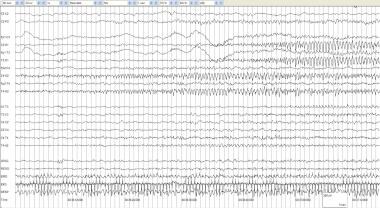 Alternating seizure 1. An infant of 32 weeks' gestational age at 40 weeks' conception with serratia meningoencephalitis. Surgery for drainage and dural repair was performed. This seizure begins at 00:36:12 in the right posterior quadrant with sharply contoured rhythmic delta at 1.5-2 Hz. Note the compressed nature of the time base, with each gradation representing 1 second. By 00:36:50, the spread to the left posterior quadrant is evident. The seizure continues in the next figure.
Alternating seizure 1. An infant of 32 weeks' gestational age at 40 weeks' conception with serratia meningoencephalitis. Surgery for drainage and dural repair was performed. This seizure begins at 00:36:12 in the right posterior quadrant with sharply contoured rhythmic delta at 1.5-2 Hz. Note the compressed nature of the time base, with each gradation representing 1 second. By 00:36:50, the spread to the left posterior quadrant is evident. The seizure continues in the next figure.
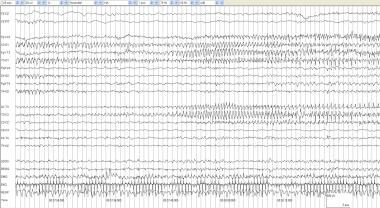 Alternating seizure 2. In the first 10 seconds of the page, the electrographic seizure pattern in the right posterior quadrant attenuates, leaving behind the ongoing left posterior quadrant discharge. By 00:37:32, spread to T3 is evident. Seizures in neonates often alternate or "ping pong" from one side to the other.
Alternating seizure 2. In the first 10 seconds of the page, the electrographic seizure pattern in the right posterior quadrant attenuates, leaving behind the ongoing left posterior quadrant discharge. By 00:37:32, spread to T3 is evident. Seizures in neonates often alternate or "ping pong" from one side to the other.
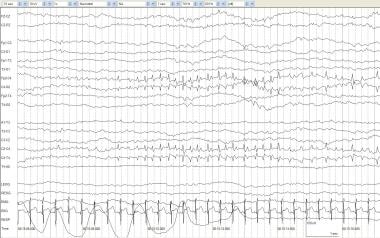 Electrographic seizure, surface positive. An infant of 40 weeks' postconceptional age with partial seizures and hypocalcemia. An asymptomatic surface-positive electrographic seizure occurs at C4 with rhythmic spikes at 4 Hz.
Electrographic seizure, surface positive. An infant of 40 weeks' postconceptional age with partial seizures and hypocalcemia. An asymptomatic surface-positive electrographic seizure occurs at C4 with rhythmic spikes at 4 Hz.
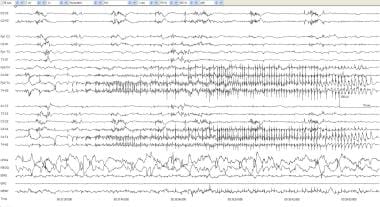 Electrographic seizure, focal 1. An infant of 40 weeks' postconceptional age with meningitis. This page contains 2 minutes of compressed EEG to demonstrate the entire extent of the seizure. At 00:37:32, repetitive T4 spikes begin, and, over the next 10 seconds, they become rhythmic spikes. This spreads centrally, and, by 00:39:05, the discharge begins to end with slow spike discharges at T4 and then completely attenuates.
Electrographic seizure, focal 1. An infant of 40 weeks' postconceptional age with meningitis. This page contains 2 minutes of compressed EEG to demonstrate the entire extent of the seizure. At 00:37:32, repetitive T4 spikes begin, and, over the next 10 seconds, they become rhythmic spikes. This spreads centrally, and, by 00:39:05, the discharge begins to end with slow spike discharges at T4 and then completely attenuates.
Focal spikes or sharp wave discharges consist of trains of rhythmic spikes or sharp waves that erupt focally out of the background activity, usually abruptly. While initially the amplitude may be low, it often increases as frequency decreases. Frequency of discharge varies most commonly in the delta to alpha bands (from 4-10 Hz), with the most frequent location in the rolandic areas. Discharge may spread to the adjacent cortex, but usually at a much slower pace in neonates than in older patients. The discharge may appear in homotopic areas of the other hemisphere. In general, these types of focal patterns correlate well with the clinical manifestations of seizures that are usually clonic.
Ictal focal EEG discharges do not necessarily indicate an underlying lesion because they often occur in the context of transient metabolic disorders following a mild hypoxic-ischemic event or a subarachnoid hemorrhage. In these situations, the background activities and state organizations usually are normal, and prognosis is good. Similar focal ictal discharges also may occur in the presence of acquired or congenital brain lesions. In these infants, the EEG often exhibits abnormalities of background, such as interhemispheric amplitude asymmetry and focal attenuation.
Pseudo-delta, -theta, -alpha, and -beta activity consists of runs of rhythmic monomorphic waves ranging from 0.5-15 Hz, therefore resembling normal background activities. Their amplitude varies from a low of 20-30 µV to a high of 200 µV, generally being higher for the slower frequencies. They may occur with a generalized distribution or a focal distribution. They can appear in all states, but, because they often occur in severely compromised neonates, state organization may be disrupted and background activities may be undifferentiated. These discharges can be seen (1) after severe hypoxic-ischemic insults, (2) after intraventricular hemorrhages, (3) in neonates with inborn metabolic defects, or (4) in infants with various chromosomal and dysgenetic brain abnormalities. See the image below.
Low-frequency discharge patterns
Low-frequency discharge patterns are stereotyped, repetitive, rhythmic, or quasi-rhythmic paroxysmal discharges consisting of sharp, broad-based waves that occur at low frequencies (around 0.5-1 Hz). These discharges may involve focal or multifocal areas or involve an entire hemisphere. This pattern is similar to the periodic lateralized epileptiform discharges (PLEDs) seen in older children.
To fit the definition of PLEDS, these discharges cannot exhibit evolution in morphology, frequency, or field and cannot be associated with ictal manifestations. Almost invariably, these discharges occur in the context of abnormal backgrounds, usually of invariant, inactive, or low-voltage patterns.
Computerized seizure detection in newborns recently has been described with seizure detection rates at greater than 70% accuracy, with a false detection rate of 1.7/hour. A confirmatory study revealed similar findings. This may be a helpful tool in long-term monitoring of the newborn. Investigations into the nonlinear dynamics of neonatal EEG suggest different measurable levels of complexity of activity during different stages of sleep and with maturation. This may aid in state and maturation classification. [9]
Classification And Impression
Classification of the record as normal or abnormal is the most important step in interpreting a neonatal record. [10, 11, 12] If the record is abnormal, a list of abnormal features is reported.
Impression takes into account the classification made, abnormal features, and patient's history (including use of medications and findings prior to EEG testing). Even in neonates, barbiturates and benzodiazepines may cause diffuse beta activity and should not be interpreted as a seizure or abnormal record.
Follow-up examinations may be suggested if the examination is technically difficult or technically unsatisfactory, maturation of the neonate is inconsistent with the EEG findings, or results of therapeutic interventions are analyzed.
Table 2. Interpretation of the Neonatal EEG (Open Table in a new window)
Classification of Features |
Impression |
Normal features |
Normal for gestational age |
Immature pattern |
Cerebral dysfunction or gestational age not calculated correctly |
Multifocal abnormalities |
Multifocal or diffuse cerebral dysfunction |
Background abnormalities |
Multifocal or diffuse cerebral dysfunction |
Focal abnormalities |
Focal cerebral dysfunction |
Electrographic seizures |
Focal or multifocal epileptogenic regions |
Prognosis based on EEG findings may also be helpful to relay in the impression. For a description of prognostication see the reference by Holmes and Lombroso. [13]
Analyzing A Neonatal EEG – 5-step Approach
See the list below:
-
Postconceptional age and topography
Determination of postconceptional age
Topography
Confirm head position.
Confirm the location of scars or intravenous lines.
Confirm any edema.
-
Artifacts
Locate artifacts.
Describe artifacts.
-
Identification of sleep and wake states
Determine cycling through awake, quiet, and active sleep states.
Analyze the distinctness of the changes in sleep state.
Determine and evaluate the duration of each sleep state.
Determine the presence of a quiet sleep pattern, TD, TA, or high-voltage slow wave.
Evaluate noncerebral physiological patterns and determine whether they are consistent with quiet sleep.
Determine whether the EEG pattern is appropriate for postconceptional age.
Determine whether REM sleep is continuous.
Determine whether noncerebral physiological patterns are concordant with quiet sleep.
Determine whether the pattern is appropriate for postconceptional age.
-
Feature extraction
Determine whether abnormalities in quiet sleep, active sleep, or wakefulness are present.
Determine amplitude.
Determine continuity.
Determine frequency.
Determine synchrony.
Determine maturation.
Note any paroxysmal abnormalities.
Interictal
Ictal
-
Classification of the record
Determine whether normal or abnormal.
Determine the clinical correlation.
Determine the need for follow-up.
Questions & Answers
Overview
What is the role of electroencephalogram (EEG) in neonatal medicine?
How is neonatal electroencephalogram (EEG) performed?
What are the steps of neonatal electroencephalogram (EEG) analysis?
How are artifacts identified in the visual analysis of neonatal electroencephalogram (EEG)?
What are the common artifacts seen on neonatal electroencephalogram (EEG)?
Which features are identified during neonatal electroencephalogram (EEG)?
How is amplitude identified in the visual analysis of neonatal electroencephalogram (EEG)?
How is continuity identified in the visual analysis of neonatal electroencephalogram (EEG)?
How is frequency identified in the visual analysis of neonatal electroencephalogram (EEG)?
How is symmetry identified in the visual analysis of neonatal electroencephalogram (EEG)?
How is synchrony identified in the visual analysis of neonatal electroencephalogram (EEG)?
How is maturation identified in the visual analysis of neonatal electroencephalogram (EEG)?
How are ictal patterns identified in the visual analysis of neonatal electroencephalogram (EEG)?
What is the accuracy of computerized seizure detection of neonatal electroencephalogram (EEG)?
How is the neonatal electroencephalogram (EEG) record classified?
What is included in the visual analysis of a neonatal electroencephalogram (EEG)?
-
Electrode map and montage.
-
Artifacts and a low-voltage record. A full-term infant aged 4 days with anoxia at birth and seizures. No spontaneous respirations occur. The record is low voltage with no activity of cerebral origin greater than 2 mV. All other activity can be accounted for by physiologic or nonphysiologic artifacts. ECG artifacts are diffuse. Pulse artifact is time locked to the ECG. An IV artifact occurs in multiple electrodes every 1.75 seconds and is most prominent in the right electrooculogram (REOG). The RESP channel demonstrates mechanical ventilation artifact.
-
Twitching artifact. A full-term, 3-day-old infant with anoxic encephalopathy and episodes of generalized twitching that last 1-2 seconds at a time. Muscle artifacts are seen in multiple electrodes and are particularly prominent in the EMG channel. After the twitch, the background attenuates and appears to be a state change rather than an electrographic seizure correlate.
-
Quiet sleep and tracé alternant (TA). An infant of 39 weeks' postconceptional age with hydrocephalus and possible seizures. During quiet sleep, respirations are regular, EMG activity is low-voltage tonic with no phasic activity, and no spontaneous eye movements occur. TA is seen with medium- to high-voltage mixed frequencies alternating with periods of relative voltage attenuation.
-
Active sleep. An otherwise healthy infant of 41 weeks' postconceptional age with episodes of arm and leg extension without EEG correlates. This segment of normal, active sleep shows irregular respirations; frequent eye movements on EOG; and a mixed pattern of delta, theta, and alpha frequency activity.
-
Tracé discontinu (TD). An infant of 24 weeks' gestational age at age 4 weeks with an intraventricular hemorrhage and left shoulder twitching. Periods of alternating high-voltage mixed frequencies and periods of voltage suppression are normal findings before 28-30 weeks' postconceptional age.
-
Tracé alternant (TA). An infant of 42 weeks' postconceptional age delivered via cesarean birth, with Apgar scores of 4/4/4 and left arm and leg jerking movements. TA occurs with regular respirations, low-voltage tonic EMG, and minimal eye movements. Periods of relative voltage attenuation may occur periodically during quiet sleep.
-
Burst suppression. An infant of 42 weeks' postconceptional age with asphyxia. An alternating pattern of high-voltage mixed frequency activity and voltage attenuation in a term infant indicates severe diffuse cerebral dysfunction. Compare this to tracé discontinue (TD), in which similar activity may be considered normal prior to 30 weeks' gestational age.
-
Voltage attenuation, focal. An infant of 40 weeks' postconceptional age with a left middle cerebral artery infarction and intermittent posturing and hyperextension of the neck. Background activity demonstrates attenuation of delta and fast activity on the left indicative of a structural lesion on the left.
-
Positive temporal sharps. An infant of 41 weeks' postconceptional age with a fever (temperature, 102°F) and 3 episodes of right arm and leg jerking with eye deviation that last 5-10 seconds each. Left positive temporal sharps are seen in the 4th and 5th seconds at T3 and independently at T4 during the 8th second.
-
Excessive sharp transients. An infant of 36 weeks' postconceptional age with hypoxic ischemic encephalopathy and seizures on days 2 and 3 of life. Sharp transients are seen in the 1st and 3rd seconds at T4.
-
Alternating seizure 1. An infant of 32 weeks' gestational age at 40 weeks' conception with serratia meningoencephalitis. Surgery for drainage and dural repair was performed. This seizure begins at 00:36:12 in the right posterior quadrant with sharply contoured rhythmic delta at 1.5-2 Hz. Note the compressed nature of the time base, with each gradation representing 1 second. By 00:36:50, the spread to the left posterior quadrant is evident. The seizure continues in the next figure.
-
Alternating seizure 2. In the first 10 seconds of the page, the electrographic seizure pattern in the right posterior quadrant attenuates, leaving behind the ongoing left posterior quadrant discharge. By 00:37:32, spread to T3 is evident. Seizures in neonates often alternate or "ping pong" from one side to the other.
-
Electrographic seizure, surface positive. An infant of 40 weeks' postconceptional age with partial seizures and hypocalcemia. An asymptomatic surface-positive electrographic seizure occurs at C4 with rhythmic spikes at 4 Hz.
-
Electrographic seizure, focal 1. An infant of 40 weeks' postconceptional age with meningitis. This page contains 2 minutes of compressed EEG to demonstrate the entire extent of the seizure. At 00:37:32, repetitive T4 spikes begin, and, over the next 10 seconds, they become rhythmic spikes. This spreads centrally, and, by 00:39:05, the discharge begins to end with slow spike discharges at T4 and then completely attenuates.
-
Electrographic seizure focal 2. Expanded time base of seizure onset from the previous image. In the 2nd second, a clear rhythmic spike onset is seen in the right temporal region.
-
Pseudo theta. An infant of 35 weeks' gestational age at age 3 days. Patient is having alternating jerking movements of all 4 extremities. Focal monorhythmic theta at 7 Hz is seen in the right temporal and left central regions, independently.