Overview
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system (CNS) characterized by widespread lesions due to infection of oligodendrocytes by JC virus, a ubiquitous human polyomavirus estimated to latently infect the kidneys of 50% of adults. [1] It is a small non-enveloped double-stranded DNA virus that was identified as the etiological agent in 1967 and was named JC virus in 1971 after John Cunningham, from whom it was first isolated. It occurs almost exclusively in immunosuppressed individuals, e.g., patients with AIDS, hematological and lymphoreticular malignancies, autoimmune rheumatological diseases, or those having undergone organ transplantation. PML has also been reported in patients receiving immune therapy with monoclonal antibodies (eg, natalizumab, rituximab) and various other immunosuppressants, including prednisone, cyclophosphamide, methotrexate, and cyclosporine. [2]
PML is associated with both HIV-1 and HIV-2. [3, 4] HIV infection accounts for almost 85% of the total cases, and its prevalence in this population is around 4–5%. [5, 6, 7] It is currently one of the AIDS-defining illnesses in HIV-infected patients.
HIV-associated PML also occurs during immune recovery following the initiation of highly active antiretroviral therapy (HAART). [8] Such cases are associated with an inflammatory reaction in brain lesions and contrast enhancement on neuroimaging studies. The outcome of inflammatory PML is more variable than that of PML in end-stage AIDS. [9, 10, 11, 12]
Most patients with HIV infection develop PML in the setting of a poor immunological status expressed by a low CD4 cell count (< 200/µL). Very few reports have described of PML in HIV-infected patients in the setting of better immunological function (ie, CD4 counts > 500/µL). [13, 14]
Recurrence of PML despite many years of immune recovery in HIV has been reported. [15]
Pathophysiology
Progressive multifocal leukoencephalopathy (PML) is caused by reactivation of the endemic JC polyomavirus. As many as 90% of healthy individuals have serum antibodies to this virus, but less than 10% show any evidence of ongoing viral replication. In cases associated with the initiation of highly active antiretroviral therapy (HAART), immune recovery may uncover pre-existing subclinical PML. [16]
The virus is thought to enter the body via the respiratory or oral route. After its entry, it becomes latent in the kidneys, lymphoreticular tissues, and brain. The primary infection is asymptomatic. Periods of viral replication without any clinical symptoms occur and can be detected when it is shed in the urine. When reactivation happens in the setting of immune suppression, viral replication ensues, causing dissemination to the brain. Alternatively, latent reactivation of the virus can occur in the brain itself in this setting. [17] Viral particles are shown to be carried to the CNS via B-lymphocytes in the setting of systemic dissemination.
According to a study of HIV-negative controls and HIV-positive patients with or without PML, a third of individuals from all subgroups had JC virus DNA in the urine. [18] In the same study, JC virus DNA was detected in 43% of lymphocyte samples and in 63% of plasma samples in HIV-positive patients with PML. In HIV-positive patients without PML, however, JC virus DNA was detected in only 13% of lymphocyte samples and in 22% of plasma samples. In HIV-negative controls, no lymphocyte or plasma samples harbored JC viral DNA.
HIV gene products, such as Tat, may be able to transactivate the JC viral promoter directly. This provides an additional pathogenic mechanism beyond general immunosuppression. The viral infection in oligodendrocytes is lytic. It undergoes DNA replication and synthesis of viral capsid proteins inside the cell. The virus infects other cells from a central nidus in a circumferential manner, leading to the expansion of the demyelinating lesion. Astrocytes that are infected by the virus enlarge and take bizarre appearance (distortion of the nuclei with enlargement or multiple nuclei) and resemble the tumor cells in giant cell astrocytomas.
In patients with PML who develop immune reconstitution, the entry of JCV-specific T cells, B cells, and monocytes into the CNS at the site of JCV infection helps eliminate the virus. However, a subgroup of PML patients shows no or limited response despite immune reconstitution. A recent study of 4 patients with multiple sclerosis who developed natalizumab-associated PML and granule cell neuronopathy demonstrated that mutations in the VP1 protein of the JCV may generate altered peptides that escape efficient CD4 T-cell recognition, resulting in impaired support for JCV-specific CD8 T cells that are crucial for eliminating JCV infection of the CNS. [19]
Epidemiology
A United States study of 9,675 cases of progressive multifocal leukoencephalopathy (PML) from 1998 to 2005 found that 82% of cases were associated with HIV infection. [20]
The incidence of PML is low in India and Africa, possibly due to diagnostic challenges and differences in JC virus isolates. [21, 22]
Before the advent of highly active antiretroviral therapy (HAART), PML occurred in as many as 4% of AIDS cases. Like other HIV/AIDS-related opportunistic tumors and infections, the incidence of PML has decreased in the HAART era. [23, 24]
According to the Italian NeuroAIDS study 2000-2002 (IRINA), PML is the third most common cause of encephalopathy in HIV-infected patients, after Toxoplasma encephalitis and HIV encephalopathy. [25]
In the United States, the mortality associated with PML has decreased significantly since 1996, when HAART became the standard of care. The PML-associated death rate decreased from 2.76 deaths per 1 million population in 1992-1995 to 0.66 in 2002-2005, in large part because of fewer deaths among HIV-infected patients. [26]
Prognosis
In the pre-HAART era, the prognosis in patients with progressive multifocal leukoencephalopathy (PML) was dismal, with death occurring in approximately 95% of patients within 4–6 months after diagnosis in most cases. Approximately 8% of patients experienced spontaneous recovery.
With the widespread adoption of highly active antiretroviral therapy (HAART), the incidence of PML decreased substantially. In addition, several case series have shown prolonged survival for patients receiving HAART. [27] In a series of 118 consecutive patients from Spain, 63.6% survived for a median of 114 weeks (2.2 y) after diagnosis of PML. [28]
CD4+ T-cell counts less than 100/μL at baseline are associated with a higher mortality rate. Death may result not from the neurologic condition but from end-stage immune deficiency.
In a clinical outcome study of 60 patients with PML (73% HIV+) who were prospectively evaluated for the presence of JC virus (JCV)-specific CD8+ cytotoxic T-lymphocytes (CTL) in blood, estimated probability of survival at 1 year was 52% for HIV+/PML and 58% for HIV-negative patients with PML. The presence of JCV-specific CTLs was associated with a trend toward longer survival in patients with PML. [29]
A study by Lima et al of 24 PML patients whose survival exceeded 5 years found that by the end of the follow-up period, 33% of patients had no significant disability despite persistent symptoms; 25% had slight disability and were living independently; 21% were moderately disabled, requiring some help during activities of daily living; and 21% had moderately severe disability, requiring constant help or institutionalization. [30]
Clinical Presentation
Patients with progressive multifocal leukoencephalopathy (PML) typically experience insidious onset and steady progression of focal symptoms that include behavioral, speech, cognitive, motor (eg, head tremor [31] ), and visual impairment. Though the neuropathological evaluation reveals the multifocal nature of the disease, its presentation is typically unifocal; however, MRI may demonstrate multifocal pathology. Unlike other major opportunistic disorders that produce focal brain lesions (eg, cerebral toxoplasmosis, primary CNS lymphoma), which characteristically progress over the course of hours or a few days, PML evolves over several weeks. [32] However, PML demonstrates more rapid progression than AIDS dementia complex (ADC). Involvement of the brainstem is more commonly seen in PML associated with AIDS than with other entities. As individual lesions expand, either concentrically or along white matter tracts, manifestations may worsen and involve a larger territory. For example, initial weakness of one leg may progress to hemiparesis. [32] Patients with more preserved immune status may show a slower progression of the disease than those with a immunocompromised state.
Although seizures have been considered a rare manifestation of PML, Lima et al found that seizures occurred in 18% of PML patients. [33] Many of the PML patients presenting with seizures had demyelinating lesions immediately adjacent to the cortex. Seizures usually responded well to treatment and did not affect survival.
In PML related to immune reconstitution, onset may occur weeks to months after the initiation of antiretroviral therapy.
Visual symptoms in PML result from visual pathway involvement, not from optic neuritis as in other inflammatory demyelinating diseases. [34]
Physical Examination
Focal neurologic signs include aphasia, hemiparesis, ataxia, cortical blindness, limb apraxia, brainstem symptoms and, less frequently, head tremor. Focal signs tend to be related to posterior brain (eg, occipital lobes). Gait abnormalities occur in up to 65% patients, and cognitive dysfunction is seen at the time of presentation in up to 30% people.
Conjugate gaze abnormalities are common. This is the initial presentation in more than 30% of patients. Abnormalities may progress to quadriparesis and coma. Occasionally, neurologic signs are diffuse rather than focal.
Workup
In a patient with steadily progressive focal neurologic deficits consistent with progressive multifocal leukoencephalopathy (PML), neuroimaging is indicated. The combination of a characteristic clinical picture and typical imaging findings supports a confident presumptive diagnosis of PML. [32]
Computed tomography or magnetic resonance imaging
With CT scan or MRI of the brain, single or multiple confluent lesions without mass effects are seen, most frequently in the parieto-occipital white matter. Occasional infratentorial lesions are usually asymmetrical. The demyelinating plaques involve subcortical U fibers but tend to spare the cortical ribbons and deep gray matter structures; however, cases have been described that have involvement of deep gray matter. Subcortical gray matter or the spinal cord may be involved, but rarely. Gray matter involvement has a scalloped appearance.
PML sometimes can resemble lymphoma, toxoplasmosis, or HIV encephalitis. However, the absence of a mass effect or displacement of normal structures is more consistent with PML than these other disorders. [32] Rarely, PML can also present as a mass lesion with enhancement on postcontrast MRI scans. Magnetization transfer ratio (MTR) is typically low in PML cases compared with normal white matter and that of HIV-infected white matter without PML.
CT scan may show hypodense lesions. MRI scan is far more sensitive than CT scan. [35] On MRI, PML lesions characteristically are hypointense on T1-weighted images; this finding may be subtle but can help distinguish PML lesions from those of other diseases (eg, white matter lesions of HIV encephalitis). On T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences, PML lesions are characteristically hyperintense (see the image below). [32]
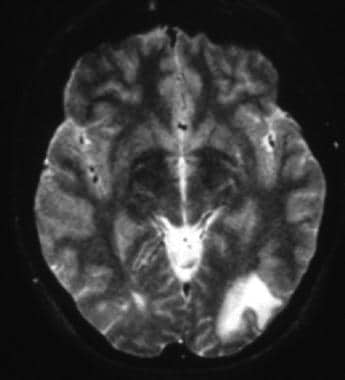 T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
Neuroimaging in patients with inflammatory PML may demonstrate atypical features, including a mass effect of the PML lesions with surrounding edema. Contrast enhancement, which is uncommon in classic PML and tends to be sparse when it does occur, may be striking in patients with inflammatory PML. [32] Any area of the white matter can be affected but is usually in the cerebellum.
Lumbar puncture
Cerebrospinal fluid (CSF) is usually normal, but protein levels may be elevated slightly. Normal CSF findings serve to rule out other etiologies. CSF pleocytosis can sometimes occur, but the cell count is usually less than 20/µL. JC virus culture in the CSF is usually unrevealing.
Polymerase chain reaction (PCR) of the CSF has been shown to be highly specific (92-99%) and sensitive (74-93%) for the detection of JC virus in patients with PML. [36] False negatives may be due to the low viral DNA in the CSF, storage of the specimen, low volume of the specimen, and loss of DNA during concentration. The false-positive rate has been reported to be around 2%. Measuring CSF JC virus DNA load is a reliable marker of disease activity in patients receiving HAART and has a potential use in drug trials. Conceivably, this test could eliminate the need for brain biopsy. However, the detection of JC virus in CSF may be less likely in patients with inflammatory PML. [32] If PML is suspected, even though the initial JC virus PCR is negative, the recommendation is to repeat the spinal fluid analysis.
Brain Biopsy
Brain biopsy has a sensitivity of 74–92% and a specificity of 92–100% in progressive multifocal leukoencephalopathy (PML). Mild cortical atrophy may be seen on biopsy specimens. Immunohistochemistry or in situ hybridization is the best method to confirm JC virus in the biopsy specimen.
Multiple demyelinative foci may be seen in the cerebral, cerebellar, and brainstem white matter and at the gray-white matter junction; in severe cases, such foci may be seen in the cortical gray matter. Foci may become confluent (see the images below). Oligodendrocytes at the gray-white junction are the most common sites of infection. JC virus infects not just the oligodendrocytes and astrocytes but also the granule cells of the cerebellum.
 This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
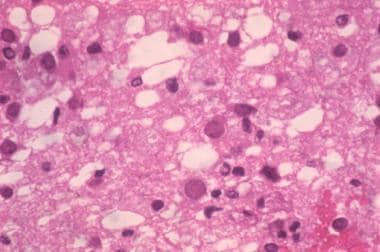 Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Perivascular inflammatory infiltrates are observed. Necrotic and cystic lesions may be present but are rare.
Nuclear inclusions may be seen in large ballooned oligodendrocytes and rarely in astrocytes, both of which show bizarre-looking nuclei. The inclusions contain viruses as identified by electron microscopy and immunohistochemistry. Because of the cellular atypia, PML can be occasionally misdiagnosed as glioma. [37]
Diagnosis
In April 2013, the Neuroinfectious Disease Section of the American Academy of Neurology released a consensus statement on the diagnostic criteria of progressive multifocal leukoencephalopathy (PML) that stated that PML can be diagnosed either histopathologically or with clinical, radiologic, and virologic evidence. [38]
The classic histopathologic features of PML (triad of demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei) with evidence of the presence of JC virus by electron microscopy, immunohistochemistry, or PCR establish the diagnosis. Alternatively, the presence of compatible clinical features, classic radiologic findings, and a positive CSF JC virus PCR is considered sufficient for the unequivocal diagnosis of PML. [38]
Lack of clinical or radiologic features despite CSF JC virus PCR positivity when diagnosis is based on clinical criteria, or failure to demonstrate JC virus despite classic histopathologic features when histopathologic criteria are used, reduces the diagnostic certainty to probable PML. Patients with probable PML should be managed as having PML. [38]
The diagnosis of PML remains less certain in the possible PML category.
The following tables demonstrate these criteria:
Table 1. Establishing the diagnosis with clinical, radiographic, and laboratory data. (Open Table in a new window)
Certainty of PML diagnosis |
Compatible Clinical Features |
Compatible Image Findings |
CSF JCV PCR |
Definite |
+ |
+ |
+ |
Probable |
+ |
- |
+ |
- |
+ |
+ |
|
Possible |
+ |
+ |
- /or not done |
- |
- |
+ |
|
Not PML |
- |
- |
- |
+ |
- |
- |
|
- |
+ |
- |
Table 2. Establishing the diagnosis with histopathology. (Open Table in a new window)
Certainty of PML diagnosis |
Classic Histopathologic Triad* |
Immunohistochemistry or Electron Microscopy |
Tissue JCV PCR |
Definite |
+ |
+ |
+ |
+ |
-/or not done |
+ |
|
+ |
+ |
-/or not done |
|
Probable** |
+ |
- |
-/or not done |
Possible |
- |
+ |
-/or not done |
Not PML |
- |
- |
-/or not done |
*Histopathologic triad: demyelination, bizarre astrocytes and enlarged oligodendroglial nuclei.
**Presence of clinical and radiologic evidence not resulting from other disease processes increases the certainty of this category to definite.
Treatment and Management
Pembrolizumab, a humanized monoclonal antibody targeting programmed cell death protein-1 (PD-1) on lymphocytes, an immune checkpoint inhibitor developed to treat cancer, is the most encouraging treatment yet for the management of progressive multifocal leukoencephalopathy (PML). PD-1 is upregulated on chronically activated immune cells and its expression is thought to lead to decreased viral clearance. PD-1 is upregulated in CD4+ and CD8+ T cells in patients with PML, and is particularly enriched on JC virus-specific CD8 cells. As part of a pilot trial, pembrolizumab (2 mg/kg) was given every 4–6 weeks for up to 3 doses to eight patients with PML and evidence of progression of disease. Clinical improvement or stabilization was seen in five of the eight patients. [1]
Other than this new, experimental treatment for PML, the principal approach is antiretroviral therapy. Treatment guidelines for PML recommend (1) starting antiretroviral therapy immediately in patients with PML who are not on therapy and (2) optimizing the antiretroviral regimen for virologic suppression in patients who are receiving antiretroviral therapy but who remain HIV-viremic because of antiretroviral resistance. [32]
Intensive treatment with 4 classes of antiretroviral drugs, including enfuvirtide, has been reported as providing a possible survival benefit in PML patients with undetectable plasma HIV. Currently, evidence is insufficient to support a recommendation for or against this approach. [32]
The use of drugs that block the serotonergic 5HT2a receptor (eg, olanzapine, ziprasidone, mirtazapine, cyproheptadine, risperidone) has been suggested as treatment for PML, on the basis of a report indicating that this receptor can serve as the cellular receptor for JCV. Only anecdotal reports of this approach are available, however, and its routine use is not considered justified. [32]
Mefloquine has been suggested as one of the options based on its in vitro activity against the JC virus, but a recent trial showed the lack of efficacy. [39] The current consensus is that interferon-alfa does not help in the treatment of PML, though some reports initially suggested its use.
Inhbition of retrograde transport of the JCV to the endoplasmic reticulum was investigated by two compounds: retro-2cycl and brefeldin A. Both retro-2cycl and brefeldin A were shown to inhibit JCV infection in vitro, and retro-2cycl was also shown to inhibit infectious spread in cultured cells. [40] However, these compounds remain investigational and so far no studies show their effecacy or safety in humans.
In some individuals, especially those with very low CD4+ counts, worsening of PML or new-onset PML can be observed after the initiation of highly active antiretroviral therapy (HAART). This is thought to be secondary to immune reconstitution inflammatory syndrome (IRIS). IRIS is considered as a paradoxical deterioration of a preexisting infection that is related to the recovery of the immune system. It is suggested to occur due to an imbalance of CD8+/CD4+ T cells.
Anecdotal case reports of use of pulsed methylprednisolone have shown rapid improvement in IRIS-associated PML. [41] Treatment guidelines consider the use of corticosteroids justified in this setting (B rating). [32]
Treatment trials have been conducted with cidofovir (used in AIDS patients with CMV) [42] , topotecan (topoisomerase inhibitor), and interferon-alfa. [43] Results have been inconclusive, however, and treatment guidelines do not recommend the use of any of those agents. [32]
Cytosine arabinoside at 2 mg/kg/day for 5 days showed 30% response rate in one study with patients (non-AIDS–related PML ) in whom 85% mortality rate was expected in 1 year. [44] Cytosine arabinoside, however, failed in AIDS patients with PML.
Passive and active immunization against JCV infection and PML is a potential approach that is being considered for future evaluation. [40]
-
T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
-
This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
-
Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.








