Overview
Background
Tardive dyskinesias (TDs) are involuntary movements of the tongue, lips, face, trunk, and extremities that occur in patients treated with long-term dopaminergic antagonist medications. Although they are associated with the use of neuroleptics, TDs apparently existed before the development of these agents. People with schizophrenia and other neuropsychiatric disorders are especially vulnerable to the development of TDs after exposure to conventional neuroleptics, anticholinergics, toxins, substances of abuse, and other agents.
TDs are most common in patients with schizophrenia, schizoaffective disorder, or bipolar disorder who have been treated with antipsychotic medication for long periods, but they occasionally occur in other patients as well. For example, people with fetal alcohol syndrome, other developmental disabilities, and other brain disorders are vulnerable to the development of TDs, even after receiving only 1 dose of the causative agent.
TD has been associated with polymorphisms of both the dopamine receptor D2 (DRD2) gene, [1] TaqI A and TaqI B and associated haplotypes, [2] and of the dopamine receptor D3 (DRD3) gene, [1, 3] the dopamine transporter (DAT) gene, and the manganese superoxide dismutase (MnSOD) gene.
Dysfunction of the dopamine transporter has been hypothesized to play a role in the development of TD. However, Lafuente et al did not find evidence of involvement of a polymorphism with a variable number of tandem repeats (VNTD) in the DAT gene (SLC6A3) in dyskinesias induced by antipsychotics. [4] Thus, further research is needed to investigate the role of the dopamine transporter in the development and maintenance of TD.
Galecki et al reported the association of a polymorphism of the manganese superoxide dismutase (MnSOD) gene and TD. [5]
TDs may be differentiated from acute movement disorders that commonly occur in the same patient groups. The acute movement disorders that occur as manifestations of effects of neuroleptics and other dopamine antagonists include akathisia, acute dystonia, and other hyperkinetic dyskinesias.
Acute effects of dopamine antagonists also include parkinsonian syndromes manifested by bradykinesia, rigidity, and pill rolling tremor. The acute movement disorders resulting from exposure to dopamine antagonists are commonly termed extrapyramidal syndromes (EPSs).
The occurrence of acute movement disorders on exposure to dopamine antagonists is increased in female patients and older patients. Use of potent dopamine antagonists, prolonged exposure to dopamine antagonists, and prior occurrence of acute movement disorders on exposure to dopamine antagonists are also associated with an increased risk for the occurrence of acute movement adverse effects.
Withdrawal dyskinesias may also occur as treatment with dopamine antagonists is decreased or withdrawn. They are often refractory to all therapeutic modalities. In addition to the prototypic orofacial dyskinesia, tardive syndromes also include a spectrum of hyperkinesias occurring during or after prolonged treatment with dopamine antagonists.
Pathophysiology
Extrapyramidal dysfunction
For most of the past century, movement disorders (ie, abnormal adventitious movements) have been categorized as EPSs caused by lesions of the extrapyramidal system of the central nervous system (CNS).
The pyramidal system, controlling voluntary movements, includes precise anatomic pathways from the cortex to muscle. Voluntary movements through the pyramidal systems are visible. An example of a classic disorder of the pyramidal system is a stroke, resulting in paralysis of an extremity.
Corticospinal lesions above the pyramidal decussation typically result in paralysis of volitional movements of the contralateral half of the body and a fixed posture with flexion of the upper extremity and extension of the lower extremity. Bilateral corticospinal lesions of the upper pons and midbrain typically cause extension of all 4 extremities and decerebrate rigidity with dorsiflexion of the cervical and thoracolumbar spine. Unilateral lesions of the upper pons and midbrain often result in extension of the ipsilateral arm and leg.
By contrast, extrapyramidal motor activities result in automatic movement and static, postural movement activities that are not noticeable (see Table 1 below). The extrapyramidal system includes theorized connections within the basal ganglia, the striatopallidonigral system, and other structures of the central nervous system that contribute to the regulation of movement, including related brainstem nuclei and the cerebellum.
Table 1. Classic Characterization of Pyramidal and Extrapyramidal Systems (Open Table in a new window)
Characteristic |
Pyramidal |
Extrapyramidal |
Anatomy |
Precisely demarcated pathways from cortex to muscle |
Hypothesized pathways among basal ganglia and other structures of the central nervous system |
Physiologic movements |
Voluntary |
Involuntary |
Pathologic movements |
Paralysis, paresis, hyperreflexia, and spasticity |
Akathisia, athetosis, ballismus, chorea, dystonia, myoclonus, stereotypy, tic, and tremor |
Classic disorders of the extrapyramidal system include a variety of involuntary movement disorders. Some of these movement disorders include dyskinesias such as akathisia, chorea, dystonia, myoclonus, stereotypy, tic, and tremor.
The pathophysiology of extrapyramidal disorders has been disputed on the grounds that some of these disorders may not involve lesions of the basal ganglia and, in addition, may not be involuntary. Because of the problems inherent in the concept of the extrapyramidal system, caution must be exercised in the classification of movement disorders as EPSs, and new approaches to the classification of movement disorders may be helpful.
Bradykinesia versus hyperkinesia
Dyskinesia is a type of movement disorder that is subdivided into bradykinesias and hyperkinesias. Bradykinesias are characterized by abnormal slowness (eg, rigidity), difficulty initiating and terminating actions, and the masked facial expression of patients with Parkinson disease. Hyperkinesias are purposeless movements, including akathisia, chorea, dystonia, myoclonus, stereotypy, tic, and tremor.
This binary classification of movement disorders is based on the observed phenomenology, etiology, and topography. Practitioners and researchers may be confounded by this approach to classification and may prefer instead to use clinical impressions. Methods of data analysis, including linear and logistic regression, linear discriminant function analysis, factor analysis, inverted factor analysis, tree approaches, dynamic clusters analysis, and principal component analysis, may facilitate the classification of movement disorders.
Dopamine system
Although the pathophysiology of TD is not well understood, it is hypothesized that central dopamine blockade plays a role in the pathogenesis of this condition. It is also hypothesized that acute movement disorders result, in part, from the blockade of dopamine receptors by dopamine antagonists.
Several mechanisms have been proposed by which TD may develop, including the following:
-
Striatal dopamine receptor supersensitivity may be responsible
-
Chronic dopamine blockade may result in upregulation of dopamine receptor responsiveness
-
Compensatory supersensitivity of dopamine receptors may develop after long-term blockade; long-term blockade of dopamine D2 receptors in the basal ganglia by dopamine D2 antagonists (eg, neuroleptics) may produce TD
-
When dopamine D2 -receptor blockade is reduced (even slightly), an exaggerated response of the postsynaptic dopamine D2 -receptor (even to low concentrations of dopamine) may result
-
Striatal disinhibition of the thalamocortical pathway from imbalance of D1 and D2 receptors may be involved
-
Neurodegeneration secondary to lipid peroxidation or excitotoxic mechanisms may be responsible
Although the dopamine D2 receptor has traditionally been implicated in the pathogenesis of TD, there is mounting evidence to indicate that in some individuals, the dopamine D3, D4, and D5 receptors are involved.
Most likely, genetic traits produce a vulnerability to TD when a susceptible individual is exposed to particular agents. For example, the MscI polymorphism of the dopamine D3 receptor gene has been associated with the development of TD. Support for the hypothesis that TD may result from blockade of postsynaptic dopamine receptors in the basal ganglia and other parts of the brain exists in the form of the beneficial effects of increasing doses of neuroleptics for some patients with TD. Thus, dopamine antagonists may mask TD.
Increased dopamine transport (DAT) uptake after treatment with quetiapine has been reported with the amelioration of TD in a 67-year-old woman. [6]
Nicotine may play a role in the pathophysiology of TS. Cigarette smokers appear to have increased metabolism of dopamine D2 antagonists. Nicotinic agonists appear to relieve dyskinesias in some people with Tourette syndrome, a condition characterized by the presence of motor and phonic tics. The relation between TD and the use of cigarettes and other nicotinic agonists remains to be clarified. The results of an animal study suggest the possibility that nicotine may be useful for improving the TD associated with antipsychotic use. [7]
Tan et al reported an inverse correlation of plasma levels of brain-derived neurotrophic factor and dyskinetic movements in people with schizophrenia who had TD. [8] Thus, brain-derived neurotrophic factor appears to have a protective effect in the nervous system against TD with people with schizophrenia.
Modestin et al observed that a fluctuating course of the illness characterizes people with TD. They also reported that length of illness is highly correlated with the development of TD. [9]
Bishoi et al noted that curcumin, an antioxidant, may prevent the development of dyskinesias induced in animals by drugs that block dopamine receptors. [10]
Adenosinergic receptor system
Bishnoi et al provided evidence of the involvement of the adenosinergic receptor system in the development of TD in rodents. [11] Haloperidol induced vacuous chewing movements, orofacial movements, and facial stereotypies in rats. These changes were reversed after treatment with adenosine or caffeine. These findings provide evidence that adenosine, a major inhibitory neurotransmitter in the CNS, plays a role in TD. Additionally, these results suggest potential therapeutic agents for clinical trials.
Etiology
Drugs
TD can be caused by long-term treatment with dopamine antagonists. It can also be caused by both high-potency and low-potency traditional neuroleptics, including long-acting depot formulations (eg, decanoate and enanthate). Greater D2 dopamine receptor blockade at the trough levels of the neuroleptics may be associated with a greater degree of TD. [12] Amisulpride has been associated with TD, [13, 14] but in general, newer atypical antipsychotic agents, including olanzapine and risperidone (and its metabolite paliperidone [15] ), appear to carry less risk of TD. [16]
The antiemetic metoclopramide, a potent D2 dopamine receptor antagonist, may cause TD, particularly in elderly patients. TDs have also been reported with the use of antihistamines, fluoxetine, amoxapine (a tricyclic antidepressant), and other agents (see Table 2 below).
Table 2. Medications Causing Tardive Dyskinesia (Open Table in a new window)
Category |
Agents |
Antipsychotic agents (ie, neuroleptics) |
Butyrophenones: droperidol, haloperidol, dibenzodiazepines, loxapine Diphenylbutylpiperidines: pimozide Indolones: molindone Phenothiazines: chlorpromazine, fluphenazine, mesoridazine, perphenazine, thioridazine, trifluoperazine Thioxanthenes: thiothixene |
Newer atypical antipsychotic agents (sporadically linked to TDs) |
Olanzapine Quetiapine Risperidone Paliperidone Amisulpride |
TD = tardive dyskinesia. |
|
A number of nonneuroleptic compounds are associated with dyskinesias that usually resolve with dose reduction or discontinuance (see Table 3 below).
Table 3. Nonneuroleptic Medications Linked to Dyskinesias (Open Table in a new window)
Category |
Agents |
Anticholinergics |
Benzhexol Biperiden Ethopropazine Orphenadrine Procyclidine |
Antidepressants |
MAOIs: phenelzine SSRIs: fluoxetine, sertraline Trazodone TCAs: amitriptyline, amitriptyline-perphenazine, amoxapine, doxepin, imipramine |
Antiemetics |
Metoclopramide Prochlorperazine |
Antiepileptic drugs |
Carbamazepine Ethosuximide Phenobarbital Phenytoin |
Antihistamines |
Various |
Antihistaminic decongestants |
Combinations of antihistamines and sympathomimetics |
Antimalarials |
Chloroquine |
Antiparkinson agents |
Bromocriptine Carbidopa-levodopa Levodopa |
Anxiolytics |
Alprazolam |
Biogenic amines |
Dopamine |
Mood stabilizers |
Lithium |
Oral contraceptives |
Estrogens |
Stimulants |
Amphetamine Methylphenidate Caffeine |
MAOI = monoamine oxidase inhibitor; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant. |
|
Tardive dyskinesia and psychogenic movement disorders
Psychogenic movement disorders are often florid and bizarre (see Catatonia). [17, 18] The motions of these disorders typically defy the boundaries that circumscribe neurologic disorders. They usually do not resemble classic TD. Psychogenic movement disorders generally represent conversion disorders, neurologic symptoms expressed by a patient who believes that the symptoms are present. [19] The patient is apparently physically healthy. Conversion disorders are not well understood. [19]
Often a stress, positive or negative, has occurred in the individual’s life. People with psychogenic movement disorders often have experienced a major life event (eg, failure to attain an expected promotion, death of a loved one). [19]
Malingering disorders may occur when an individual seeks disability and other compensation. Specifically, malingering occurs when the patient seeks a tangible reward for being sick. A person who malingers may seek to be excused from work or school because of the feigned illness. A malingerer may seek compensation in the form of disability payments for the alleged illness.
Factitious disorder occurs when an individual feigns illness in order to assume the sick role. People with factitious disorders appear to seek the attention accorded to a patient. Like conversion disorders, factitious disorders are not well understood.
An extremely severe form of factitious disorder is manifested as Munchausen syndrome. Munchausen syndrome is characterized by the apparently deliberate feigning of symptoms and signs. People with Munchausen syndrome may fabricate elaborate and bizarre stories. They may agree to multiple operations in an attempt to diagnose and treat the fabricated illnesses.
Munchausen syndrome by proxy occurs when a parent seeks treatment for a child, typically an infant who cannot speak. The parent fabricates a history of false symptoms for the healthy child, which leads to unnecessary diagnostic and therapeutic interventions. Munchausen syndrome by proxy is a form of child abuse. Suspected cases of Munchausen syndrome by proxy merit report to child protective services.
Although people with psychogenic movement disorders may seek and demand medication and surgery, they are likely to experience severe adverse effects. Therefore, pharmacologic and surgical interventions should be avoided in patients with psychogenic movement disorders. These disorders must be differentiated from voluntary expressions of emotion characteristic of cultural and ethnic groups, such as zaghrouta. [20, 21] Psychiatric consultation is indicated.
Genetic factors
A genetic basis for TD has not been identified. In particular, a functional polymorphism of the gene coding for human glutathione S-transferase P1 (GSTP1) does not appear to be associated with TD. [22] Additionally, CYP3A4 and CYP2D6 gene polymorphisms are apparently unassociated with TD. [23] TD has been associated with polymorphisms of the dopamine D3 receptor Ser9Gly [24] and of the serotonin 2A [1] and 2C receptor genes. [24, 1]
Reports of associations between TD and polymorphisms of nicotinamide adenine dinucleotide phosphate (NADPH) quinine oxidoreductase 1 (NQO1) and superoxide dismutase 2 (SOD2, MnSOD) genes have not consistently been confirmed by subsequent studies. [25]
Brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms may be associated with the development of TD and the severity of TD in whites. [26] Thus, the enhancement of BDNF may be protective against the development of TD, particularly in whites. [26]
Epidemiology
Frequency
In 1997, Goetz estimated that tardive dyskinesia (TD) occurs in approximately 15-30% of persons who receive long-term treatment with neuroleptics. [27] TD is more likely to occur in individuals who have manifested acute adverse effects of exposure to dopamine antagonists. The prevalence of TD is higher in cigarette smokers. [28]
The various subtypes of TD vary markedly in frequency. For example, orofacial, buccolingual, and masticatory dyskinesias are common, but only 1–2% of people treated with dopamine antagonists develop tardive dystonia.
Orofacial TDs differ from peripheral TDs with regard to the occurrence of comorbid acute movement disorders. Acute tremor, acute akathisia, and acute Parkinsonism are more common in people with peripheral TD. Distinguishing acute and TDs in an individual patient can represent a serious diagnostic challenge.
Age-, sex-, and race-related demographics
TD occurs in all ages. Connor et al found that 5.9% of 95 young people aged 7–21 years who received dopamine antagonist treatment for 3 months had TD. [29] Advanced age is a major risk factor for TD. [30] The prevalence of TD is 29% in elderly patients receiving dopamine antagonist treatment for 3 months and 26-67% in patients undergoing long-term treatment.
Elderly female patients appear to be particularly susceptible to the development of TD. [30] Young men are prone to develop tardive blepharospasm and tardive dystonia.
TD occurs in persons of every race. [30] Studies in different populations have identified overall prevalences ranging from 1% to 65%. Africans and African Americans appear to be especially vulnerable to TD after exposure to low doses of neuroleptics for short durations.
However, it is difficult to draw any firm conclusions from these findings, because the investigators conducted their studies in different settings. A number of other variables, such as therapeutic approaches, methodologic inconsistencies, diet, weather, and varied assessments, may also contribute to the differences reported in various racial groups.
Patient Education
Fully inform the patient (or the legal surrogate if the patient is incompetent) of the possible courses of action. Discuss with the patient the advantages and disadvantages of dopamine antagonist treatment. A written treatment plan that documents agreement with the treatment course between the clinician and patient is helpful. Regularly review and revise the treatment plan as needed.
If a patient exhibits a movement disorder when taking a drug, then gradual discontinuance of the causative drug generally is a wise course if the patient can tolerate a reduction in dose. [31] Advise the patient to avoid receiving dopamine-receptor blocking drugs. Advise the patient to obtain a medical alert bracelet to warn against the administration of dopamine-receptor blocking drugs.
For patient education resources, see the Sleep Disorders Center, as well as Restless Legs Syndrome.
Presentation
History
Patients often have movement disorders that may actually represent a mixture or overlap of several dyskinesia disorders. Individuals treated with neuroleptics may demonstrate both acute and chronic effects, manifested by acute dyskinesias and tardive dyskinesia (TD). Akathisia and tics may be manifested simultaneously after long-term treatment with neuroleptics.
When a patient is seen for the first time, diagnosis of acute and chronic dyskinesias may be difficult without a careful history. Precise documentation of a patient’s complete movement history and medication history may facilitate accurate delineation of movement disorders. Therefore, a full neurologic and pharmacologic history may provide the basis for distinguishing idiopathic Tourette disorder from acute medication-induced tardive tics.
Patients and families often cannot provide accurate histories; thus, firm diagnoses may be impossible. Because acute and tardive medication effects may occur simultaneously, the distinction may be challenging in a clinical setting. Observing patients carefully on a regular basis and precisely documenting (through the use of structured rating instruments) the phenomenology and topography of movements and the pharmacologic treatments at each visit will help provide a basis for accurate future diagnosis of acute dyskinesia and TD.
Although TD has been observed after exposure to various substances (eg, levodopa, amphetamine, and metoclopramide), the prototypical TD is the orofacial (ie, buccolingual, masticatory) hyperkinesia induced by neuroleptics (antipsychotic dopamine D2-receptor blockers). People with other movement disorders and those with diabetes mellitus are at increased risk of TD.
Neuroleptic-induced TD is characterized by choreiform, athetoid, and rhythmic movements of the tongue, jaw, trunk, and extremities that have persisted for at least 4 weeks and that began during treatment with neuroleptics or within 4 weeks of discontinuing neuroleptics. Diagnosis of neuroleptic-induced TD generally requires exposure to neuroleptics for at least 3 months. At least 1 month of exposure is typically required if the patient is aged 60 years or older. Oral and genital pain can be prominent manifestations of TD.
Neuroleptic-induced TD is excluded if symptoms and signs result from another neurologic or medical disorder, ill-fitted dentures, or other medications. For example, hyperthyroidism may manifest with choreiform movements of the limbs. Furthermore, neuroleptic-induced TD cannot be diagnosed if symptoms and signs result from an acute neuroleptic-induced movement disorder.
Antiparkinsonism agents usually do not improve neuroleptic-induced dyskinesias. Decreasing the dose of the neuroleptic may increase the movements temporarily. In some patients, increasing the dose of the neuroleptic diminishes movements, thereby masking the TD.
Although the neuroleptic dose may temporarily diminish the disorder, regular increments are needed to achieve apparent beneficial effects. Over the long term, maintaining and increasing doses of neuroleptics to mask TD may be ineffective. However, situations may exist in which masking of TD by continuing and escalating neuroleptic doses may be justified. Benefits and risks must be weighed. Neuroleptic-induced TDs are absent during sleep.
On initial examination, obtain a history of neurologic disorders that may involve the basal ganglia (eg, cerebrovascular disease, encephalitis, head trauma, and neoplasms). Obtain a family history for hereditary dyskinesias associated with Huntington disease, Wilson disease, and torsion dystonia.
Inquire about medications, including amphetamines, levodopa, and substances that may result in dyskinesias (see Etiology). Specifically note whether antiemetic medications (especially metoclopramide, prochlorperazine, and related compounds) are being administered.
Distinction from similar conditions
Unlike TD, Sydenham chorea is a disorder associated with a history of group A streptococcal infection and rheumatic fever in children. It typically affects children and adolescents 6 months or more after an infection with group A streptococci. Prompt administration of antibiotic therapy for infections with group A streptococci dramatically reduces the incidence of Sydenham chorea. The female-to-male ratio is approximately 2:1.
Sydenham chorea is characterized by the rapid onset of chorea, muscular weakness, hypotonia, dysarthria, obsessions, compulsions, and other behavioral and emotional disturbances. After an abrupt or insidious onset, Sydenham chorea worsens over 2-4 weeks and then resolves over 3–6 months. Chorea may persist after the episode has ended. One fifth of patients with Sydenham chorea experience a recurrence, typically within 2 years of the initial episode.
Pantothenate kinase-associated neurodegeneration (PKAN) (Hallervorden-Spatz disease) occurs in patients aged 10–15 years, a different age group than that of persons with TD. PKAN is an extremely rare, progressive neurogenetic disorder with autosomal recessive inheritance associated with dementia and death (approximately 20 y after onset). It is characterized by rigidity, dystonia, choreoathetosis, spasticity, foot deformity, and intellectual deterioration. It is associated with excessive iron deposition in the basal ganglia that can be observed on MRI.
Assessment tools
Differentiate neuroleptic-induced TD from spontaneous dyskinesias and mannerisms of psychosis and other mental disorders. [32] Several rating scales have been developed to identify the presence and severity of TD. [33, 34, 35, 36, 37, 38]
The most widely used instrument is the Abnormal Involuntary Movement Scale (AIMS) developed by the Psychopharmacology Research Branch of the National Institute of Mental Health (see the image below). [39] Because the AIMS can be readily administered in a few minutes, it is recommended in patients receiving treatment with substances that may cause TD. Administer the AIMS at baseline before the institution of pharmacotherapy to document any movements present, then at least every 3 months thereafter during the course of treatment.
Part of the AIMS assessment includes observation of the patient when he or she is distracted by other activities. Patients may suppress movements while concentrating intensely. Therefore, administration of the AIMS can be supplemented by requesting that the patient perform additional tasks during the assessment. Movements may then be demonstrated when the patient is concentrating on the additional tasks.
For example, during the administration of item 5 of the AIMS assessment, have the patient sit in a chair with hands on knees, legs slightly apart, and feet flat on the floor. Examine the entire body for movements while the patient is in this position, then ask the patient to count backwards from 30.
In addition, during item 5 of the AIMS, ask the patient to sit with hands hanging unsupported (between the legs if male, or hanging over the knees if female and wearing a dress). Observe the hands and other body areas, then request that the patient describe in detail the path traveled that day. Ask the patient where the trip started, what streets were traveled, where turns were made, where the trip terminated, and what floor and room were entered. These procedures stimulate the patient and may provoke the appearance of movement disorders.
The AIMS may be modified to allow repeated brief assessments during the course of a single patient examination to check for possible effects of interventions. In other words, by administering the modified AIMS regularly after the administration of a treatment, the time course of abnormal movements may be regularly recorded and plotted. [40]
To assess the appropriateness of treatment with antipsychotic medication, [33, 34, 41] the Psychoactive Medication Quality Assurance Rating Survey (PQRS) (see the first image below) can be used at initial assessment and then repeated annually, [42] and the PQRS Screening Criteria (see the second image below) can be repeated monthly [43] .
Specific tardive dyskinesias
Specific TDs resulting from long-term use of dopamine antagonists (eg, orofacial dyskinesia, tardive akathisia, tardive blepharospasm, tardive dystonia, tardive myoclonus, and tardive tics) may occur concurrently.
Tardive akathisia is manifested by repetitive tapping, squirming, and marching movements. It occurs as the dose of the dopamine antagonist is decreased after long-term treatment. People with akathisia complain of inner restlessness and the inability to remain still. Unlike other movement disorders, akathisia can be diagnosed solely on the basis of the patient’s subjective symptoms in the absence of any objective signs. The Hillside Akathisia Scale is often helpful (see Physical Examination).
Blepharospasm (repetitive, forceful, sustained contraction of the orbicularis oculi) may occur as an isolated finding. When it occurs in conjunction with dystonia of the lower face, jaw, and neck, it is referred to as Meige syndrome. Although blepharospasm has been reported with dopaminergic and sympathomimetic agents and antihistamines, it has also been associated with long-term treatment with dopamine antagonists.
Tardive blepharospasm is defined as the presence of repetitive sustained contractions of the orbicularis oculi that have lasted for at least 1 month and that developed during or within 3 months of the discontinuance of treatment with dopamine antagonists (in the absence of other disease or familial causes). Symptoms of tardive blepharospasm fluctuate. Fatigue, anxiety, work, and light exacerbate tardive blepharospasm, whereas rest and sleep relieve it.
Tardive dystonia occurs in 1-2% of individuals during long-term treatment with dopamine antagonists. It must be differentiated from acute dystonia, which occurs in the first days of neuroleptic treatment or after an increase in the dose. Tardive dystonia presents as fixed posturing of the face and neck (eg, anterocollis, retrocollis, torticollis), extremities, and trunk. It may be either localized, involving 1 or more body parts, or generalized. Unlike TD, tardive dystonia may improve with anticholinergic medication.
Unlike TD, torsion dystonia is an inherited disorder with childhood onset that typically occurs in people of Ashkenazi Jewish descent.
Tardive myoclonus, a rare disorder, presents as brief jerks of muscles in the face, neck, trunk, and extremities.
Tardive tourettism resembles Tourette syndrome [44] and presents during or after treatment with dopamine antagonists. Typically, it begins in individuals older than 21 years, whereas Tourette syndrome commonly presents by the age of 7 years. Tardive tourettism is characterized by frequent, multiple motor and vocal tics, echolalia, echopraxia, coprolalia, and copropraxia. Tics may be suppressed temporarily while inner tension builds; this inner tension is relieved by an explosion of tics. Tardive tourettism may occur with other TDs.
Tardive tremor is a hyperkinetic movement disorder associated with long-term treatment with dopamine antagonists. Kuo and Jankovic have reported the occurrence of tardive gait as a tardive dyskinesia. [45]
Physical Examination
As noted, neuroleptic-induced orofacial hyperkinesia is the prototypical form of TD. It is characterized by irregular movements of variable amplitude and low frequency. TD is expressed in the tongue, cheeks, mandible, perioral area, and other regions of the face, fingers, and toes. Various facial movements are observed. TD may be observed in the upper face with excessive blinking and brow wrinkling.
Orofacial dyskinesias appear as involuntary, repetitive, and stereotyped facial grimacing with twisting or protrusion of the tongue. The individual may initially be unaware of these movements until family and friends draw attention to them. Puckering, smacking, opening, and closing of the lips may occur constantly. The person may appear to be chewing or sucking on items. The movements resemble those of people with ill-fitting dentures.
Inquire about the use of dentures. Inquire if the person is aware of movements in the mouth, face, hands, and feet. Ask if dentures or teeth bother the patient. The tongue may protrude briefly out of the lips. If asked to hold the tongue in a protruded position, the person may be unable to maintain protrusion for longer than 1 second. Although the individual may attempt to disguise the movements by placing the hand to the mouth, in time, the movements become constant during waking hours and cannot be suppressed by the patient.
TD is commonly associated with involuntary athetoid movements (slow, snakelike writhing) of the extremities, including wiggling, twisting, and tapping the fingers and toes. To perform a full assessment, ask the individual to remove shoes and socks so that the movements of the toes and feet can be observed fully. Movements typically become constant during waking hours. Often, the individual cannot suppress them for longer than 1 second.
Guitar- and piano-playing movements and other flexion and extension movements of the fingers or wrists can be observed. Flexion and extension movements of the ankles and toes are characteristic. Dyskinetic movements of the neck, trunk, and pelvis are occasionally seen. Jerking movements of the abdomen and diaphragm, resulting in respiratory irregularity, may occur.
Neuroleptic-induced TD is present at rest and diminishes or subsides when the affected body part is activated. For example, squeezing the hand of another person often eliminates finger dyskinesias, tongue protrusion commonly reduces tongue dyskinesias, and mouth opening diminishes orofacial dyskinesias. Simply pointing out these movements and asking the patient to stop can reduce the movements. For example, orofacial movements may be stopped by placing the patient’s fingers on his or her lips.
Neuroleptic-induced TD is increased when the patient’s attention is drawn away from the movements, as when the examiner asks the patient to move a different body part. For example, finger dyskinesias may be increased by asking the patient to walk with arms resting comfortably at the sides. Asking the patient to repeatedly touch the thumb to each finger sequentially in both hands may amplify TD in the tongue and the face. Provocative distracting movements may be necessary to induce movement in mild TD. Distraction is a key component of the AIMS. [46]
Tardive akathisia includes the presence of subjective symptoms of restlessness and the urge to move. It refers to the inability to sit down or remain still. People with tardive akathisia exhibit constant pacing and moving of the hands and feet. They typically shift their weight from one foot to the other when standing and swing their legs when sitting.
Akathisia can be objectively and readily assessed in clinical settings by using the Hillside Akathisia Scale (see the image below). [47, 48, 49] For this evaluation, examine the patient with his or her feet bare and hands exposed so that movements of the extremities can be observed. Ask the patient to sit, stand, and lie still for 2 minutes in each position.
In each patient position, inquire about the presence of a sensation of inner restlessness and an urge to move. After the patient has maintained the designated position for a full minute, ask, “Do you feel restless inside? Do you have the urge to move? Are you able to keep your feet still?” If the patient responds that these sensations are present, ask him or her to quantify the magnitude of the urge to move as mild, moderate, or severe. [47, 48, 49]
In addition, ask if the urge to move is distressing. If the patient is experiencing distress, ask him or her to quantify the feeling as mild, moderate, or severe. For clinical assessments, the patient may be assessed in the sitting and standing positions only; assessment in the lying position may be omitted. Score the evaluation at the conclusion of the assessment session. [47, 49] Perform this assessment at the initial evaluation and then regularly throughout the course of treatment to determine beneficial and adverse effects. [47, 49]
Tardive blepharospasm manifests with findings similar to those of other forms of blepharospasm. Repetitive, forceful, and sustained contractions of the orbicularis oculi are observed. Dyskinetic blinking may occur.
Unlike tardive dystonia, torsion dystonia is characterized by twisting and sustained contractions of muscles resulting in rapid, repetitive, distressing movements. Torsion dystonia usually begins with inversion of the foot and spasm of the proximal limb muscles, resulting in gait abnormalities. Scoliosis, torticollis, and tortipelvis may occur in torsion dystonia. Patients may experience considerable impairment in performing activities of daily living. Spasmodic torticollis presents in adults and is characterized by torticollis, anterocollis, or retrocollis.
Tardive tics may be observed in affected patients. Because the patient may suppress the tics temporarily, they may not be observed during the initial encounter.
Tardive tremor manifests as involuntary rhythmic sinusoidal movements of limbs, head, neck, or voice. Tardive tremors are persistent. Unlike cerebellar tremors, which are present on voluntary motion and not at rest, and psychogenic tremors, which diminish during the course of long examinations, tardive tremors are usually present at rest and with voluntary movement.
The presence of dementia in a patient in whom TD is suspected merits consideration of Huntington disease, Wilson disease, or a central nervous system (CNS) neoplasm. The presence of hemiparesis, asymmetric reflexes, and other focal deficits indicates the need for further assessment to exclude structural brain lesions.
The presence of jaundice, hepatomegaly, abdominal pain, or Kayser-Fleischer rings in the cornea requires further assessment to exclude Wilson disease. Kayser-Fleischer rings may be observed best with a slit-lamp examination. Ophthalmologic consultation is mandatory for patients in whom Wilson disease is suspected.
Wilson disease is characterized by the presence of choreiform movements, tremors, diminished dexterity, marked rigidity, dystonia, dysarthria, and neuropsychiatric manifestations. The presenting manifestation may be psychosis. Check serum ceruloplasmin and the copper transporter gene in every patient in whom Wilson disease is suspected. The onset of movement disorders in patients younger than 50 years merits ruling out Wilson disease, a treatable disorder.
Tachycardia, sweating, and a goiter suggest hyperthyroidism. TD may coexist with other neuroleptic-induced movement disorders, including parkinsonism (manifested by tremor, rigidity, and bradykinesia). Distinguish TD from acute dystonic reactions induced by medications and from neuroleptic withdrawal dyskinesias. Unlike TDs, withdrawal dyskinesias remit within a month after discontinuance of neuroleptics.
Postural instability is common in Huntington disease but uncommon in neuroleptic-induced TD. Unlike TD, Huntington disease appears with chorea in the face and the proximal extremities. The term chorea implies a dancelike distinctive gait.
The development of TD in people with schizophrenia is associated with the development of cognitive impairment. [50]
Assessment tools
Characterization and classification of TDs and other movement disorders are facilitated by the administration of the Movement Disorders Checklist by trained raters to score the presence or absence of traits of movements (see the images below). Each discrete movement is rated separately on its own page. This checklist can be readily used by practitioners in clinical settings.
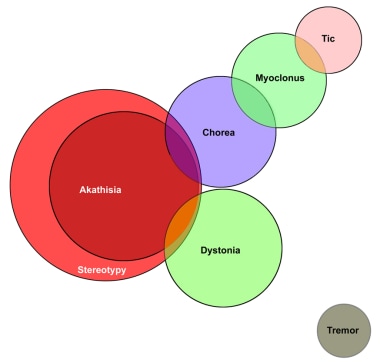
With dichotomous random variables (ie, indicator functions), algorithms in the form of linear regression equations express the relationships among dyskinesias and other movement disorders. The formulation can be expressed as a Venn diagram (see the image below).
As the diagram indicates, every case of akathisia is also a case of stereotypy, and therefore, the presence of akathisia implies the presence of stereotypy. Some cases of chorea can be classified as akathisia and stereotypy, whereas other cases of chorea can be classified as myoclonus. In addition, some cases of tics can also be classified as myoclonus. Some cases of dystonia can be classified as akathisia.
The Venn diagram also indicates that tic is entirely separate and distinct from akathisia, chorea, dystonia, stereotypy, and tremor. Furthermore, the diagram demonstrates that tremor is distinct from the other movement disorders.
The presence of stereotypies can be readily assessed by using the Timed Stereotypies Rating Scale. [51]
Observe the patient with his or her feet bare and hands exposed. Ask him or her to sit still in a chair for 10 minutes. Place a check mark on the score sheet the first time each movement occurs during each 30-second interval of the observation period. The timing can be established by playing a recording containing a series of 30-second intervals adding up to 10 minutes (listen to the audio file below). The recording may be played live in the patient’s presence. To avoid distracting the patient, the examiner may listen through headphones.
Audio segment (MP3)
Another option is to record the patient on video for at least 10 minutes and then to rate the video later, using an audio recording of 30-second segments adding up to 10 minutes. Optimally, the patient is rated both live and on video. The live rater notes the seconds on the image of the video screen of the videocamera to determine each 30-second segment of the 10-minute observation period. The video recording is rated by examiners who are blind to the status of the patient. Test-retest reliability can thus be determined by assessing the ratings of the live and video sessions.
Video recordings often miss crucial events, such as a tear or a jerk. The score sheet contains blank spaces for other head/neck stereotypies in items 21 and 22 and for other stereotypies in items 49-60 at the end of the form, where additional patient movements (eg, extending arms at the elbow or extending legs at the knee) can be added. Additional patient utterances (eg, grunts, snorts, throat clearing, vowels, syllables, words, sentences) can also be added in the blank spaces for other stereotypies in items 49–60.
If the sessions are recorded on video, the recordings may be played back frame by frame to facilitate the observation of each occurrence of every stereotypy.
Because some of the terms of the Timed Stereotypies Rating Scale may not be well known, some further definitions are provided. Item 9, the bonbon sign, is present when the patient presses the tip of the tongue against the cheek as if tasting a piece of candy in the mouth. Item 48, the Bronx cheer, is a colloquial euphemism for a sound occurring when air is forcefully expressed through tightly closed lips resembling the noise of a flatulent retort.
Diagnostic Considerations
Tardive dyskinesia (TD) is common in individuals with psychotic disorders (eg, schizophrenias, schizoaffective disorders, or bipolar disorders) who are treated with antipsychotic medications, especially dopamine antagonists, for many years. Generally, TD is diagnosed if 1 of the following circumstances is present:
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 2 movements of at least mild intensity while taking a neuroleptic
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 1 movement of at least moderate intensity while taking a neuroleptic
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 2 movements of at least mild intensity within 4 weeks of the discontinuance of the neuroleptic
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 1 movement of at least moderate intensity within 4 weeks of the discontinuance of the neuroleptic
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 2 movements of at least mild intensity within 8 weeks of the discontinuance of a depot neuroleptic
-
A person who has taken neuroleptics for at least 3 months (1 month if older than 60 years) develops at least 1 movement of at least moderate intensity within 8 weeks of the discontinuance of a depot neuroleptic
In addition to the conditions listed in the differential diagnosis (see below), other problems to be considered include the following:
-
Conversion disorder [19]
-
Compulsions
-
Dyskinesias secondary to caffeine
-
Dyskinesias secondary to chloroquine
-
Dyskinesias secondary to estrogen
-
Dyskinesias secondary to lithium
-
Dyskinesias secondary to phenytoin
-
Dyskinesias secondary to schizophrenia
-
Factitious disorder
-
Fahr syndrome
-
Hyperthyroidism
-
Hypoparathyroidism
-
Malingering
-
Meige syndrome
-
Munchausen syndrome
-
Munchausen syndrome by proxy
-
Polycythemia rubra vera
-
Poorly fitting dentures
-
Somatization disorder
-
Spontaneous dyskinesias
-
Sydenham chorea
-
Syphilis
-
Systemic lupus erythematosus
-
Wilson disease
-
Dementia in Parkinson disease
-
Dementia in progressive supranuclear palsy
Differential Diagnosis
See the list below:
Workup
Approach Considerations
Optimal management of tardive dyskinesia (TD) involves diagnosis and treatment of conditions that resemble TD (eg, seizure disorders, syphilis, thyroid disease, and Wilson disease). Workup may include selected laboratory studies, as well as imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT).
Tardive blepharospasm should be evaluated with electroencephalography (EEG) and a complete ophthalmologic evaluation, including slit-lamp examination to rule out the Kayser-Fleischer rings of Wilson disease.
Laboratory Studies
Deficiency of serum ceruloplasmin due to an abnormal copper transporter gene characterizes Wilson disease. Urine copper collection may be abnormal. In addition, liver function tests and liver transaminases may be abnormal. Also, check the copper transporter gene in patients in whom Wilson disease is suspected.
In addition, the following tests may be appropriate:
-
Thyroid function tests to exclude thyroid dysfunction
-
Serum biochemistry, serum copper, serum ceruloplasmin, thyroid function tests, and syphilis serology to evaluate tardive blepharospasm
-
Connective tissue disease screening tests to exclude systemic lupus erythematosus and other vasculitides
-
Red blood cell (RBC) counts to exclude polycythemia rubra vera
-
Serum calcium level
CT, MRI, SPECT, and PET
Findings from brain CT and MRI are typically normal in patients with TD. Nevertheless, these imaging studies may assist in the differential diagnosis. In Huntington disease, atrophy of the caudate nucleus is commonly seen on CT and MRI of the brain. In Fahr syndrome, calcification is often seen in the brain, particularly in the basal ganglia. Imaging results can also exclude neoplasm and cerebral infarction.
Physiologic imaging studies (eg, PET and SPECT) of patients with TD may demonstrate increased glucose metabolism in the globus pallidus and the precentral gyrus. PET may help differentiate TD and other disorders with minimal gross cerebral structural changes [18] from disorders with characteristic findings on neuronuclear imaging. [52, 53] Amelioration of TD resulting from the administration of quetiapine can be confirmed by the demonstration of increased uptake of the dopamine transporter (DAT) verified by SPECT. [6, 54, 55]
Proton magnetic resonance spectroscopy has demonstrated neural damages in the left lenticular nucleus in a group of patients with TD.
Treatment & Management
Approach Considerations
Before administering any treatment that may block dopamine receptors, obtain informed written consent. In addition, all patients currently treated with dopamine antagonists, even those with schizophrenia treated with traditional neuroleptics for many years, merit reevaluation for possible change of medication. Atypical antipsychotics, such as risperidone and clozapine, appear to have a lower risk of tardive dyskinesia (TD).
Obtain written informed consent to acknowledge the risk of possible TD from patients treated with dopamine antagonists for any diagnosis including migraine, hiccups, and gastroesophageal reflux. Movement disorders may be aggravated by the administration of dopamine-receptor blocking drugs. Throughout the course of therapy, reevaluate the need for continuation of neuroleptics. [42, 43, 41, 35, 37, 38] Patients with TD who require continued treatment with a neuroleptic may benefit from atypical neuroleptics.
Oxidative stress may contribute to the development of TD. Dehydroepiandrosterone (DHEA), an endogenous antioxidant, is hypothesized to function as a neuroprotective agent against TD. A genetic basis to protect against the development of TD through the endogenous production of DHEA has not been confirmed.
Before and during treatment, assess the need for treatment with psychoactive medication. Administration of any medication to pregnant women, including dopamine antagonists, may be dangerous to the fetus.
If uncertain about the possible existence of a psychological component to a movement disorder, request psychiatric consultation. People with psychogenic movement disorders may request and demand surgery and other treatments associated with morbidity and mortality. A prudent clinician withholds inappropriate treatments from people with psychogenic movement disorders.
If infection is considered, lumbar puncture is indicated to obtain samples of cerebrospinal fluid for laboratory analysis.
A few single case reports suggested that lesioning surgery, such as pallidotomy or thalamotomy, may have some efficacy in this context. [56] A greater number of series have evaluated the application of deep brain stimulation to the internal globus pallidus, and all reported notable improvements of motor symptoms without major psychiatric side effects.
Pharmacologic Therapy
The evidenced-based guidelines of the American Academy of Neurology recommend the use of clonazepam and ginkgo biloba for TD. [57, 58, 31]
Since these guidelines were last issued, the FDA has approved valbenazine (Ingrezza), the first drug to treat tardive dyskinesia. Valbenazine is a selective vesicular monoamine transporter 2 (VMAT2) inhibitor. These drugs modulate the presynaptic packaging and release of dopamine into the synapse, and may offset the movement-related effects of antipsychotics and other dopaminergic blockers. Approval was based on the KINECT 3 trial that included patients with schizophrenia, schizoaffective disorder, or a mood disorder who had moderate or severe tardive dyskinesia. Of the 225 participants, 205 completed the study. Based on the AIMS dyskinesia score, results showed valbenazine significantly improved tardive dyskinesia compared with placebo. [59]
Deutetrabenazine (Austedo) is a novel, highly selective vesicular monoamine transporter type 2 (VMAT2) inhibitor. FDA has approved deutetrabenazine tablets for the treatment of adults with tardive dyskinesia (TD). The approval was based in part on the results of two 12-week, randomized, double-blind, placebo-controlled, multicenter trials, which compared changes in involuntary movements in 335 patients with TD who took deutetrabenazine or placebo.
For the first trial, 222 patients were randomly assigned to deutetrabenazine (12 mg/day, 24 mg/day, 36 mg/day) or placebo. Two dosage levels—24 mg/day and 36 mg/day—were associated with statistically significant improvement in patients’ Abnormal Involuntary Movement Scale (AIMS) score, from baseline to week 12, compared with placebo. [60]
For the second trial, 113 patients received daily doses of placebo or deutetrabenazine, starting at 12 mg/day, with titration up to 48 mg/day over a six-week period. This was followed by a six-week maintenance period at an average dose of 38.3 mg/day. From baseline to week 12, the AIMS total score decreased significantly in patients receiving deutetrabenazine compared with those receiving placebo. [61]
Fukasawa et al reported that clonazepam successfully alleviated the symptoms of TD and spontaneous oral dyskinesia. [62] Clonazepam (1-4.5 mg/day) showed a decrease of TD symptoms by 35% compared with placebo in a 12-week trial (n=19). [63]
Primary prevention of TD by using the lowest effective dose of neuroleptic for the shortest period is recommended. When TD is diagnosed, reduce or discontinue the causative agent if possible. [33, 34, 42, 43, 41, 49, 35, 36, 37, 38] The risk of a permanent movement disorder must be weighed against the risks of exacerbating psychosis. In addition, TD may worsen initially after neuroleptics are discontinued.
Atypical neuroleptics may control psychosis while reducing the risk of TD. Whereas traditional neuroleptics primarily block dopamine D2 receptors, atypical neuroleptics bind variably to dopaminergic, serotonergic, alpha-adrenergic, histaminic, and muscarinic receptors.
In particular, clozapine has been recommended as treatment for patients with TD who require antipsychotics. Clozapine is one of the most effective atypical neuroleptics for treatment-refractory schizophrenia. Although clozapine has been associated with TD, [64] the incidence of TD with this and other atypical agents appears markedly less than that of traditional neuroleptics.
The benefits of clozapine may result from its affinity for the D4 receptor. However, risperidone and clozapine may be ineffective in treating negative and positive symptoms in some patients. Treatment with clozapine requires regular hematologic evaluation to avoid fatal agranulocytosis.
In one study, increased uptake of the dopamine transporter (DAT), verified by positron emission tomography (PET), followed the administration of quetiapine to a 67-year-old woman with amelioration of TD. [6]
In a group of adult men with TD, administration of branched-chain amino acids markedly reduced the movements of TD. [65] Such intervention is contraindicated in pregnant women. This promising treatment requires investigation in other locations to confirm its safety and efficacy.
Other therapeutic agents for which there is some anecdotal support include vitamin E, levodopa (see carbidopa/levodopa), benzodiazepines, botulinum toxin, reserpine, tetrabenazine, propranolol, [66] and dopamine-depleting agents. Ondansetron, a selective 5-hydroxytryptamine-3 antagonist, has helped some individuals with TD. Discontinuance of anticholinergic therapy may relieve TD. A controversial strategy for treating TD is to continue or increase the dose of the dopamine antagonist.
Tardive blepharospasm can respond favorably to reduction or cessation of dopamine antagonists. Individuals who must be treated with neuroleptics often respond favorably to atypical neuroleptics. Additional treatments to consider include anticholinergics, dopamine-depleting agents, benzodiazepines, clozapine, and botulinum toxin.
For tardive tics, remove the causative neuroleptic if possible. If the patient cannot tolerate the absence of the neuroleptic, substitute an atypical neuroleptic. Pimozide, clonidine, and haloperidol can be helpful in some patients with tardive tics.
Clozapine has treated tardive tremor successfully. Propranolol is useful for tardive akathisia. Ertugrul and Demir reported the occurrence of TD in a man treated with clozapine for 1 year who had previously received other dopamine antagonists. [64] The previous exposure to dopamine antagonists may have contributed to the development of TD in this patient.
Lin et al reported beneficial effects of zotepine in patients with clozapine-induced TD. [67]
In an open-label trial that included 22 people with schizophrenia, Miyaoka et al reported that yi-gan san, a traditional Japanese herbal medicine, alleviated psychosis and TD. [68] These promising findings await confirmation by controlled clinical trials.
Aripiprazole was reported to relieve the dyskinetic movements of TD in a 65-year-old man with schizophrenia. [39] However, aripiprazole itself can lead to tardive movement disorders. [69] In a case report from Japan, switching from aripiprazole to quetiapine yielded improvements in dyskinesia, dystonia and psychotic symptoms. [70]
Slotema et al reported nonsignificant improvement in orofacial TD after the administration of botulinum toxin. [71]
A study by van Harten et al suggested that lithium may have a protective effect in some patients, preventing the development of TD. [72]
Zopiclone, an agonist for the A receptor complex of gamma aminobutyric acid (GABA), was reported to alleviate TD in 2 adults. [73]
Bishnoi et al demonstrated a beneficial effect of progesterone on an animal model of TD. [74] They hypothesized that the favorable effect of progesterone was modulated by means of the GABA-ergic and neuroprotective actions of alloprogesterone, a metabolite of progesterone.
Iwata et al, in a preliminary open-label trial designed to examine the efficacy, tolerability, and safety of zonisamide against TD in psychiatric patients with TD associated with antipsychotic treatment, found that zonisamide appeared to be safe and effective in this setting; larger well-designed trials will be required to assess these findings further. [75]
Special concerns with discontinuance of dopamine antagonists
Abrupt cessation of dopamine antagonists may lead to an acute exacerbation of symptoms (which presumably were controlled by medication). Accordingly, caution must be exercised in reducing and discontinuing treatment. Life-threatening conditions, such as malignant neuroleptic syndrome, are exceptional situations in which immediate discontinuance may be justified.
Abrupt cessation of treatment with dopamine antagonists may precipitate a florid psychosis with delusions, hallucinations, and suicidal or homicidal behavior. Whenever possible, it is preferable to taper the dose slowly (by 10% increments of the original dose) while closely observing the patient for exacerbation of psychotic symptoms.
Total discontinuance is often difficult or impossible for people whose TD has been treated pharmacologically. Some patients need a small dose of dopamine antagonists on a long-term basis. They may require hospitalization if the dopamine antagonist is discontinued completely.
Because Wilson disease is a treatable, preventable psychosis, evaluate patients with abdominal pain and mental dysfunction for Wilson disease. Obtain ophthalmologic consultation for patients in whom Wilson disease is suspected. Check serum ceruloplasmin and the copper transporter gene in patients who may have Wilson disease.
Approach to Psychogenic Movement Disorders
Patients with psychogenic movement disorders, somatoform disorder, somatization disorder, hypochondriasis, hysteria, conversion disorder, [19] malingering, Munchausen syndrome, and factitious disorders usually have manifestations that rule out TD. Nevertheless, clinicians may be tempted to consider treatment for possible TD in people who have psychiatric and psychological problems. The desire of the clinician to offer a therapeutic intervention may be intensified by the requests and demands of patients for surgery and other help.
Prudent clinicians must exercise extreme caution to avoid providing pharmacologic and surgical treatments to patients with psychogenic movement disorders. These patients are likely to experience extreme adverse effects and no beneficial effects from such treatments. In particular, surgery, including psychosurgery, is contraindicated for psychogenic movement disorders.
A tactful suggestion that stress may be contributing to the symptoms is appropriate. Typically, people who manifest psychogenic movement disorders have recently had life experiences that are stressful. The life stresses can be both positive, such as a promotion, and negative, such as the death of a loved one. [19] Psychotherapy may then provide a more effective means of expressing the psychological distress often associated with psychogenic movement disorders. Referral to mental health professionals should always be considered.
Occasionally, psychogenic movement disorders may be alleviated by the administration of a small intravenous injection of lorazepam or sodium amobarbital. Although this procedure is not pathognomonic of psychogenic movement disorders, it may be helpful for confirming a diagnosis of a movement disorder due to hysteria, somatization disorder, somatoform disorder, or conversion disorders.
Prevention
Movement disorders may be aggravated by the administration of dopamine-receptor blocking drugs. In vulnerable patients, the administration of even a single dose of a dopamine-receptor blocking drug may lead to incapacitating movement disorders.
Patients with developmental disabilities, fetal alcohol syndrome, schizophrenia, and other neuropsychiatric disorders may be exquisitely vulnerable to TD upon exposure to dopamine-receptor blocking drugs. If a patient exhibits a movement disorder when given a drug, discontinuance of the causative drug is generally wise. Advise the patient to avoid receiving dopamine-receptor blocking drugs. Make a note in the patient’s record, including the electronic patient record if possible, to warn against the administration of these drugs.
In addition, advise all of the clinicians treating the patient to refrain from administering dopamine-receptor blocking drugs. Advise the patient to obtain a medical alert bracelet warning against the administration of dopamine-receptor blocking drugs.
Consultations
Consultation with an ophthalmologist is indicated for evaluating tardive blepharospasm, excluding Wilson disease by means of slit-lamp examination, or both.
Consultation with a neurologist is advisable. Information about neurologists in various areas of the United States is available from the American Academy of Neurology, 1080 Montreal Ave, St Paul, MN 55116; (651) 695-2717; fax (651) 695-2791.
Consultation with a movement disorders specialist may help clarify the diagnosis and determine optimal treatment. Information about movement disorder experts in various areas of the United States is available from The Movement Disorder Society, 611 East Wells St, Milwaukee, WI 53202; (414) 276-2145; fax (414) 276-3349.
Psychiatric consultation is indicated for people with possible psychogenic movement disorders. These conditions include some of the most bizarre and florid movement disorders known. Typically, psychogenic movement disorders do not demonstrate the usual phenomenology and topology of TD, though it is possible for a patient to have both a psychogenic movement disorder and TD.
If a psychological component is suspected, the primary clinician must tactfully and diplomatically suggest to the patient that a consultation to investigate psychological aspects may be helpful. The patient should agree to psychiatric consultation before the specialist is asked to visit. A course of psychotherapy likely carries less morbidity than pharmacologic interventions.
Consultation with a movement disorders specialist may be helpful to confirm a psychogenic movement disorder and to recommend the appropriate psychological treatments by experienced clinicians.
Neurosurgical consultation for consideration of deep-brain stimulation may be appropriate for severe cases refractory to other interventions. [66]
Questions & Answers
Overview
What are tardive dyskinesias (TDs)?
Which patients are at highest risk for tardive dyskinesia (TD)?
Which polymorphisms are associated with tardive dyskinesia (TD)?
How are tardive dyskinesias (TDs) differentiated from extrapyramidal syndromes?
What are withdrawal dyskinesias?
How is tardive dyskinesia (TD) characterized?
What are the subsets of dyskinesia?
What is the basis of the binary classification system for dyskinesias?
What is the pathophysiology of tardive dyskinesia (TD)?
What are proposed mechanisms by which tardive dyskinesia (TD) may develop?
Which dopamine receptors are involved in the pathogenesis of tardive dyskinesia (TD)?
What is the role of genetics in the pathogenesis of tardive dyskinesia (TD)?
What is the effect of increasing dopamine transport (DAT) uptake on tardive dyskinesia (TD)?
What is the role of nicotine in the pathophysiology of tardive dyskinesia (TDs)?
What is the association between length of illness and tardive dyskinesia (TD)?
What is the role of curcumin in the prevention of tardive dyskinesia (TD)?
What is the role of the adenosinergic receptor system in the development of tardive dyskinesia (TD)?
Which medications may cause tardive dyskinesia (TD)?
Which nonneuroleptic medications may cause tardive dyskinesia (TD)?
What are psychogenic movement disorders?
How are malingering disorders differentiated from tardive dyskinesia (TD)?
How are factitious disorders differentiated from tardive dyskinesia (TD)?
What is Munchausen syndrome by proxy?
How are psychogenic movement disorders treated?
What is the role of genetics in the etiology of tardive dyskinesia (TD)?
What is the prevalence of different forms of tardive dyskinesia (TD)?
How does the prevalence of tardive dyskinesia (TD) vary by age?
How does the prevalence of tardive dyskinesia (TD) vary by sex?
How does the prevalence of tardive dyskinesia (TD) vary among racial and ethnic groups?
What information about tardive dyskinesia (TD) should patients receive?
What are possible presentations of tardive dyskinesia (TD)?
What is the role of medical history in the evaluation of tardive dyskinesia (TD)?
What is the prototypical tardive dyskinesia (TD)?
What are the signs and symptoms of neuroleptic-induced tardive dyskinesia (TD)?
What is the focus of the medical history on initial exam in a patient with tardive dyskinesia (TD)?
How is Sydenham chorea differentiated from tardive dyskinesia (TD)?
How is tardive dyskinesia (TD) differentiated from other dyskinesias?
How is appropriateness of treatment of tardive dyskinesia (TD) assessed?
What is tardive akathisia diagnosed?
What is tardive blepharospasm?
What are the signs and symptoms of orofacial hyperkinesia in tardive dyskinesia (TD)?
How are the involuntary athetoid movements of tardive dyskinesia (TD) assessed?
How is neuroleptic-induced tardive dyskinesia (TD) assessed?
Which physical findings are characteristics of akathisia in tardive dyskinesia (TD)?
What questions should be asked during the exam for tardive dyskinesia (TD)?
Which physical findings are characteristic of blepharospasm in tardive dyskinesia (TD)?
Which physical findings help differentiate torsion dystonia from tardive dystonia?
When are tardive tics present in tardive dyskinesia (TD)?
Which physical findings are characteristic of tremor in tardive dyskinesia (TD)?
How is Wilson disease differentiated from tardive dyskinesia (TD)?
Which conditions should be included in the differential diagnoses of tardive dyskinesia (TD)?
How is Huntington disease differentiated from tardive dyskinesia (TD)?
What is the development of tardive dyskinesia (TD) in people with schizophrenia associated with?
Which assessment tools are used for the classification of tardive dyskinesia (TD)?
How are stereotypies in patients with tardive dyskinesia (TD) assessed?
What are the diagnostic criteria for tardive dyskinesia (TD)?
Which conditions should be included in the differential diagnoses of tardive dyskinesia (TD)?
What are should be considered in the differential diagnosis of tardive dyskinesia (TD)?
What should be included in the workup of tardive dyskinesia (TD)?
Which tests should be performed in the workup of blepharospasm in tardive dyskinesia (TD)?
Which lab tests are used to differentiate Wilson disease from tardive dyskinesia (TD)?
What is the role of lab testing in the diagnosis of tardive dyskinesia (TD)?
What is the role of imaging studies in the diagnosis of tardive dyskinesia (TD)?
What is the role of PET and SPECT imaging in the diagnosis of tardive dyskinesia (TD)?
What findings on proton magnetic resonance spectroscopy suggest tardive dyskinesia (TD)?
What must be done prior to treatment of tardive dyskinesia (TD)?
What are treatment options to offset oxidative stress in tardive dyskinesia (TD)?
When is psychoactive medication indicated during treatment for tardive dyskinesia (TD)?
How should psychogenic movement disorders be managed?
When is lumbar puncture indicated in the treatment of tardive dyskinesia (TD)?
What is the role of lesioning surgery in the treatment of tardive dyskinesia (TD)?
What does the AAN recommend for treatment of tardive dyskinesia (TD)?
Which drugs are FDA approved for treatment of tardive dyskinesia (TD)?
What is the role of deutetrabenazine (Austedo) as a treatment for tardive dyskinesia (TD)?
What is the role of clonazepam as a treatment for tardive dyskinesia (TD)?
How is tardive dyskinesia (TD) prevented?
What is the role of clozapine in the treatment of tardive dyskinesia (TD)?
What is the efficacy of quetiapine in the treatment of tardive dyskinesia (TD)?
What is the role of branched-chain amino acids in the treatment of tardive dyskinesia (TD)?
Which therapeutic agents are used for the treatment of tardive dyskinesia (TD)?
Which medications are used for the treatment of tardive blepharospasm?
How are tardive tics treated in patients with tardive dyskinesia (TD)?
Which medication is used to treat tardive tremor in tardive dyskinesia (TD)?
Which medication is used to treat tardive akathisia?
Which medication is used to treat clozapine-induced tardive dyskinesia (TD)?
What is the efficacy of Japanese herbal medicine as a treatment for tardive dyskinesia (TD)?
What is the role of aripiprazole in the treatment of tardive dyskinesia (TD)?
Which medication is used to treat orofacial tardive dyskinesia (TD)?
What is the role of lithium in the treatment of tardive dyskinesia (TD)?
What is the role of zopiclone in the treatment of tardive dyskinesia (TD)?
What is the role of progesterone in the treatment of tardive dyskinesia (TD)?
What is the role of zonisamide in the treatment of tardive dyskinesia (TD)?
When is hospitalization indicated in the treatment of tardive dyskinesia (TD)?
How is Wilson disease treated?
What are the treatment approaches for psychogenic movement disorders?
Which treatments are contraindicated for psychogenic movement disorders?
What is the role of psychotherapy in the treatment of psychogenic movement disorders?
What is the role of medication in the treatment of psychogenic movement disorders?
What is the role of dopamine-receptor blocking drugs in the management of tardive dyskinesia (TD)?
Which patients are at highest risk for tardive dyskinesia (TD)?
When is a consultation with an ophthalmologist indicated for patients with tardive dyskinesia (TD)?
Which specialist consultations are needed for patients with tardive dyskinesia (TD)?
Where can information about movement disorders specialist for tardive dyskinesia (TD) be found?
When is a psychiatric consultation indicated for patients with tardive dyskinesia (TD)?
When is consultation with a movement disorders specialist indicated for tardive dyskinesia (TD)?
When is neurosurgical consultation indicated for patients with tardive dyskinesia (TD)?
-
Diagnostic criteria for neuroleptic-induced tardive dyskinesia.
-
Tardive dyskinesia. Venn diagram of the classification of movement disorders.