Background
Neural tube defects (NTD) are significant birth deformities of the central nervous system that occur due to a defect in the neurulation process of embryogenesis. They are among the most common type of birth defects and are thought to have multifactorial etiology, including multigenetic and environmental influences. Because the neural tube is ultimately formed from the migration and fusion of the neural plate, the type and severity of malformation varies based on the location of the defect. This includes both cranial and spinal cord malformations. Since rostral and caudal neuropore closure is the last phase of neurulation, they are particularly vulnerable to defects. Consequently, a majority of NTDs arise in these areas. [1, 2]
NTDs can be classified as “open” or “closed” types, based on embryological considerations and the presence or absence of exposed neural tissue (i.e., failure of incomplete fusion of the neural plate).
-
Open NTDs frequently involve multiple aspects of the CNS (e.g., associated hydrocephalus, Chiari II malformation) and are due to failure of primary neurulation, thus the neural tube fails to appropriately close along the dorsal midline. Neural tissue is completely exposed, or covered by a membrane, with associated cerebrospinal fluid (CSF) leakage. [3] Open NTD’s represent roughly 80% of all NTD’s, with the most common being meningocele (spina bifida), myelomeningocele, encephalocele, and anencephaly.
-
Closed NTDs are localized and confined to the spine (the brain is rarely affected) and result from a defect in secondary neurulation. Neural tissue is not exposed and the defect is fully covered by epithelium, although the skin covering the defect may be dysplastic (i.e., tuft of hair, dimple, birthmark, or other superficial abnormality). [4]
Cranial manifestations include the following: [5]
-
Anencephaly
-
Encephalocele (meningocele or meningomyelocele)
-
Craniorachischisis totalis
-
Congenital dermal sinus
Spinal presentations include the following: [5]
-
Spina bifida occulta
-
Spina bifida in relation to a dermoid cyst
-
Spina bifida aperta (meningocele, myelomeningocele (see images below), meningomyelocele, myeloschisis)
-
Split-cord malformations
-
Diastematomyelia
-
Diplomyelia
-
Caudal agenesis
-
Lipomatous malformations (lipomyelomeningocele)
For more information on the classification of neural tube defects, see Medscape Reference article Imaging in Spinal Dysraphism and Myelomeningocele.
Pathophysiology
Two distinct and critical processes are involved in the formation of the neural tube: primary neurulation and secondary neurulation (i.e., canalization). [6] The neural plate and the notochord are formed during early embryonic development. The neural groove develops by the third gestational week and the neural folds subsequently form bilaterally.
Primary neurulation (weeks three and four during embryogenesis, forming the early brain and spinal cord): [7]
-
The neural folds elevate, approximate each other, and start fusing along the dorsal midline, thus forming the neural tube. This begins during the third week after conception.
-
The cranial neuropore closes during the fourth gestational week. The last area to close is the commissural plate.
-
The point of initial closure occurs at the caudal rhombencephalon, or cranial neuropore, on day 25 of embryogenesis.
-
The cutaneous ectoderm fuses first, followed by the neuroectoderm.
-
The caudal neuropore closes between T11 and S2, two days after closure of the cranial neuropore (roughly day 27)
-
Occurring in parallel to this process, the cutaneous ectoderm separates from the neuroectoderm to form the overlying skin, while the lateral mesoderm migrates between the 2 ectodermal layers to form the posterior vertebral arches.
Secondary neurulation (canalization: weeks five and six, forming the early sacral and coccygeal cord)
-
This characterizes further neural development caudal to the caudal neuropore after the termination of primary neurulation, and the caudal neuropore closes
-
This process includes formation of the filum terminale and conus medullaris from a poorly differentiated cell mass of the medial eminence.
-
Because of differential growth between the vertebral column and the spinal cord, the conus becomes more rostral during later development.
-
By 8 weeks after conception, spinal cord tissue runs the entire length of the spinal cord. [8]
Open NTDs have been suggested to result from defective primary neurulation while defective secondary neurulation gives rise to closed NTDs. However, this issue is not settled. Another possible explanation is that open NTDs (spina bifida in particular) result from defects in either primary or secondary neurulation, depending on their site being cranial or caudal to the posterior neuropore (ie, upper and lower spina bifida, respectively).
Epidemiology
Epidemiological studies have demonstrated multifactorial influences underlying NTDs. Variations in incidence have been seen across geographical variation, ethnic groups, socioeconomic status, and genetic predisposition.
Frequency
United States
The incidence of NTDs declined 50% between 1970 and 1989 (0.6–1.3 cases per 1000 pregnancies) in the United States, thought to be due to improved prenatal care and nutrient fortification (i.e., folate) of common food products. [9] During this period, the proportion of spina bifida cases increased relative to anencephaly.
The race ratio of whites to other races for isolated NTDs decreased and the risk of isolated NTDs in female infants also decreased.
The highest incidence is in Appalachia (1 case per 1,000 live births).
Incidence is higher in the Eastern United States than on the West Coast.
International
NTDs are among the most common birth defects globally. [9] They exhibit a marked geographical variation, with the incidence higher in Great Britain and lower in Japan.
In white populations, the lowest birth incidence was noted in mainland Europe and the highest in Great Britain (especially Ireland).
A study of long-term trends in prevalence of NTDs in Europe found that, overall, the pooled total prevalence of NTD during the study period was 9.1 per 10, 000 births. Prevalence of NTD fluctuated slightly but without an obvious downward trend, with the final estimate of the pooled total prevalence of NTD in 2011 similar to that in 1991. [10]
Currently, the highest reported incidence is in Northern China (3.7 cases per 1000 live births).
Indian and Eastern Mediterranean populations (with the exception of Israeli Jews) also have relatively high incidences of NTDs [11] However, unlike the Western white populations, anencephaly is more common than spina bifida.
Brazil has experienced a decrease in infant and perinatal mortality, but no change in its under-five mortality due to congenital disorders, which are the second leading cause of infant death. Recommended changes include a revision of the policy of flour folic acid fortification. [12]
Mortality/Morbidity
Anencephaly is incompatible with life. [13] No differentiated supratentorial neural tissue is present, and the brain stem consists of nests of poorly differentiated neural elements.
The brain stem is believed by some to be not sufficiently developed to be responsible for the temporary brainstem reflexes that are observed. Some have implicated the upper cervical cord as the seat of these functions.
The survival of these newborns is limited to a few hours (rarely >2 days).
In an earlier policy statement, the American Medical Association recommended that organs could be harvested from anencephalic infants even before the traditional criteria of death are met. However, the statement has since been revoked.
Other NTDs may give rise to progressive neurological deterioration, which may present early after birth or later in life. The neurological deficits may be due to accompanying hydrocephalus, a Chiari II malformation, tethering of the cord, cystic mass, or fibrous band compressing the neural elements. Another possible complication is meningitis (infectious or chemical), especially in open NTDs.
The average recurrence risk of NTDs for parents with one affected child has been estimated to be about 5%, and that for monozygotic twins about 20%. Recurrence risks are higher in populations with a higher birth incidence.
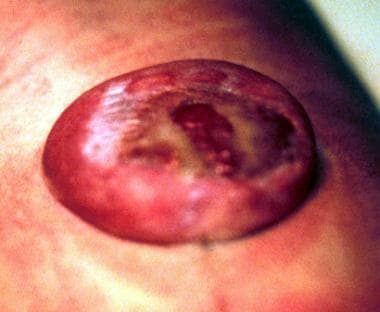
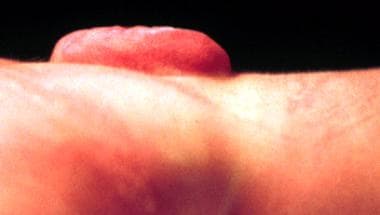
The most common NTD compatible with life and a positive prognosis is myelomeningocele (see the images below).
The incidence of myelomeningocele is 1 case in 1200–1400 live births. It is a disease affecting 6000–11000 newborns in the United States annually.
Paralysis, bladder and bowel incontinence, and hydrocephalus are the most common clinical complications. Severe intellectual disability is present in 10–15% of these patients.
Neurologic deficits are overall difficult to predict based on the level of the lesion, as some segments of the spinal cord may retain central connections and maintain partial function, allowing voluntary control or sensation in affected limbs. Most commonly, distal cord may retain function, but afferent pathways are interrupted. This may preserve reflexes and pain withdrawal, but voluntary movement and pain sensation are affected.
Prospects for independent ambulation are correlated with the level of the spinal lesion. Independent mobility is preserved for nearly all cases with low lumbar and sacral lesions; with lesions above L2, loss of quadriceps and iliopsoas muscle function often occurs, and independent mobility is unlikely. [14]
Bowel and bladder function are affected in roughly 90–95% of patients with myelomeningocele, manifesting as neurogenic bladder and/or fecal incontinence. [15]
Presence or severity of urinary dysfunction cannot be predicted by location of the spinal lesion or by neurologic exam. The level of spinal cord responsible for mediating bladder function is below the level that controls lower extremity function. [16]
Despite aggressive medical care, 10–15% of these children die prior to reaching the first grade. However, most children with isolated myelomeningocele (without major anomalies of other organs) survive to adulthood, and life expectancy is nearly normal. [17] Sixty percent have normal intelligence, although of these, 60% have some learning disability (math and problem solving being particularly difficult). Attention deficit disorder without hyperactivity also has been described in these children. Hydrocephalus is present in 85% but bears little relationship to intelligence. About 80% are socially continent (although many require clean intermittent catheterization).
Race
In studies done before the availability of prenatal screening and prophylactic vitamin supplementation, birth incidence of both spina bifida and anencephaly was reported as higher in the European white population than in the black population. [9]
In North America, the risk of NTDs was found to be highest in the Hispanic population (more than 3-fold higher than that for non-Hispanic whites).
Migration studies in the white migrant population showed a prevalence of NTDs that corresponded more closely to the risk of the place to which they had migrated, as opposed to the place of their origin. In contrast, similar studies in descendants of the Black and Asian migrant populations in Europe and North America showed a prevalence not substantially higher than those of their parent countries. These variations are consistent with the theory that NTDs are a phenotypically heterogeneous group of malformations with environmental factors, multifactorial inheritance in some cases, and single gene defects in others.
Sex
Anencephaly has a female preponderance, especially among premature births, with a female-to-male ratio of 3:1.
Other NTDs above the thoracolumbar junction show a mild female preponderance.
No such gender difference has been noted in more distal forms of spina bifida.
Age
Open NTDs are readily visible at birth, with the majority being discovered during pregnancy.
Closed NTDs may remain undetected for years, even decades, especially in the absence of cutaneous markers; it has been estimated that roughly 70% of asymptomatic patients with a closed NTD present with one or more cutaneous lesion. [18, 19]
-
Myelomeningocele in a newborn.
-
Myelomeningocele in a newborn - Lateral view.
-
Child with Chiari malformation, in whom the tonsils have descended to the level of C2.
-
MRI of a cervical syrinx in the sagittal plane.
-
MRI of a cervical syrinx in the axial plane.