Overview
Single- or repetitive-pulse stimulation of the brain causes the spinal cord and peripheral muscles to produce neuroelectrical signals known as motor evoked potentials (MEPs). [1] Clinical uses of MEPs include as a tool for the diagnosis and evaluation of multiple sclerosis and as a prognostic indicator for stroke motor recovery,
The most famous pioneers in MEP research were Penfield and Boldrey, who made direct observations by stimulating the human brain with weak electrical shocks in conscious patients who were undergoing surgery. [2] From 1950-1970, several other studies of electrical stimulation of the exposed motor cortex (ie, during neurosurgical procedures) were performed in animals and humans to study the pyramidal pathway and other corticospinal connections.
Noninvasive elicitation of MEPs was made possible by Merton and Morton in 1980. [3, 4] They designed a high-voltage transcranial electrical stimulator that excited the motor cortex using cutaneous electrodes, which were placed over the scalp. Using their technique, a contraction of contralateral muscles is recorded in a conscious subject after transcranial electrical stimulation (TES). The clinical usefulness of this method has remained limited by the local discomfort of the electrical currents that are applied over the scalp. An exception to this limitation is its use for intraoperative monitoring.
The development of transcranial magnetic stimulation (TMS), in 1985, opened new possibilities for MEP studies. Barker et al created a new type of cortical magnetic stimulator, based on the principle of electromagnetic induction. [5] The device is composed of a main unit, which contains a bank of heavy-duty capacitors. The hand-held part is freely movable so that it can be placed over any part of the body. The investigator holds the stimulating coil tangentially over the motor cortex, and a technician holds a digitizing pen over the stimulating coil to record its 3-dimensional position; this allows stereotactic mapping of the motor cortex. MEPs are recorded with surface electrodes, which are placed over small hand muscles.
Although magnetic stimulation was first used to stimulate the peripheral nervous system (PNS) and muscles, cortical stimulation has become the focus of many studies.
Corticospinal Connections
Motor cortex
The main motor cortical area is located on the anterior wall of the central sulcus and the adjacent portion of the precentral gyrus. This area corresponds to area 4 of Brodmann. It is rich in pyramidal neurons, which provide the anatomical substrates for the motor output function of area 4.
Electrical stimuli over area 4 produce activation of contralateral muscles; the face, mouth, and hand muscles occupy about two thirds of the primary motor area. The size of the cortical representation of muscles is less a function of the muscle mass than of precision of the muscle movements. Secondary and tertiary areas of motor function can be mapped roughly around the primary motor cortex.
The primary motor cortex contributes more fibers to the corticospinal tract than any other region. Numerous observations support contributions from several other areas, including the frontal and parietal cortices. Ipsilateral projections are far less numerous than contralateral ones, being estimated to make up 1.8-5.9% of corticospinal connections.
Pyramidal tract
Fibers of the corticospinal tract and corticobulbar tract originate from the sensorimotor cortex around the central sulcus. The human pyramidal tract contains more than 1 million fibers. Most fibers are myelinated and have a small diameter (1-4mm); only a small portion (3-5%) are large-diameter fibers (10-22mm) that originate in Betz cells from area 4.
In humans, only 5% of the fibers of the corticospinal tract originate from Betz cells in area 4. The concept of pyramidal pathways with fibers originating only from Betz cells in the primary motor cortex has been invalidated. Indeed, a large portion of the corticospinal neurons have nonmotor functions, especially those originating in sensory or associative areas.
Subcortical projections of the pyramidal pathway
Pyramidal fibers converge in the corona radiata, toward the posterior arm of the internal capsule. In the pons, they divide into multiple longitudinal pathways, which merge in the medulla oblongata to form the pyramidal tract after branching out efferences to motor nuclei of cranial nerves.
At the junction between the medulla oblongata and the spinal cord, 75-90% of the fibers cross through the midline to constitute the crossed (ie, indirect) pyramidal pathway. The remaining fibers make up the uncrossed (ie, direct) pyramidal pathway. A large part of direct pyramidal tract fibers actually cross the midline at the spinal cord level (ie, through the white anterior commissura), making the projections bilateral.
Magnetic and Electrical Stimulators
Magnetic stimulation
Magnetic stimulation of the nervous system can occur only in the setting of a rapidly changing magnetic field. Subjects exposed to a constant field strength (eg, during magnetic resonance imaging [MRI]) do not experience stimulation of nervous tissue. The intensity of the secondarily produced electrical field in nervous tissue (and of the stimulation) is related to the speed of change in magnetic field strength.
Formation of the magnetic pulse starts in the main unit of a magnetic stimulator, which contains a large bank of electrically charged heavy-duty capacitors. When triggered, these capacitors rapidly discharge through a cable into the hand-held coil, producing a brief burst of high current (up to 4000V or several thousand amperes [A]). The current that moves through the hand-held coil produces a large magnetic field (1-3T) that lasts only 50-200 milliseconds.
The stimulating coil consists of tightly wound and well-insulated copper coil. As a result of the brief magnetic field induced from the coil, a secondary electrical field that circulates in the opposite direction to the magnetic field is produced. The strength of the electrical field is related in part to the first derivative of the magnetic flux over time: the more rapid the change in the magnetic field, the stronger the intensity of the secondary electrical field and of nervous stimulation.
Most commercially available stimulators can produce stimulations at a rate as high as 5Hz, although some can produce repetitive stimulations as high as 50Hz. A big advantage of magnetic stimulation over electrical stimulation is magnetic stimulation's ability to penetrate tissues regardless of electrical resistance. The drop-off is essentially the same for air, bone, fat, muscle, and saline.
The magnitude, waveform, and rise time of the magnetic field are important parameters of the stimulation. The diameter, shape, and thickness of the coil are also important. Because of these multiple variables, the measurement of intensity of stimulation usually is expressed as a percentage of the maximal output of the stimulator.
In choosing coils, the tradeoff is between strength and focality of stimulation. Coil diameter may vary between 5cm and 15cm. Large-diameter coils stimulate over a wider area but are less focal than small-diameter coils. With the round coils, the highest intensity electric field is measured at the edges of the coil, with lower intensities in the center.
To obtain more focality, the use of a butterfly coil (also called "figure of 8" coil) is recommended. The focality of these coils makes them particularly suitable for use in mapping out the upper limb and hand musculature.
Electrical stimulation
Electrical stimulators have a simpler design than magnetic stimulators. The stimulation is transmitted through cutaneous electrodes. The main advantage is a better depth of penetration, allowing direct spinal cord stimulation. The main limitation is the local discomfort that is created by the stimulation.
Electrical stimulators contain a capacitor that produces constant current, high-voltage pulses of brief duration for percutaneous stimulation. The output current range is 0-1000 milliamperes, from a source voltage as high as 400V. The pulse-width range can be varied from 50 milliseconds to 2 milliseconds. The voltage is kept constant during the stimulation, but the intensity of stimulation depends on the skin impedance.
Some electrical stimulators can deliver repetitive (2-9) pulses, which have been shown to facilitate induction of motor responses. These stimulators can be particularly useful for monitoring the spinal cord during surgical procedures.
Electrophysiology of MEPs
Generation of motor evoked potentials
Electrical stimulation of the exposed cortex has been studied in animal models for several decades. An initial D (ie, direct) wave is followed by several I (ie, indirect) waves, which come at periodic intervals (usually about 1 millisecond). D waves represent the direct excitation of corticospinal tract neurons, while I waves reflect indirect depolarization of the same axons via corticocortical connections. This propagation pattern of descending impulses has been confirmed and demonstrated in humans during surgical procedures, using epidural recordings made after cortical magnetic stimulation.
As these multiple volleys descend the corticospinal tracts, they summate at the anterior horn cells in the spinal cord. Although the first D wave may not bring the alpha motoneuron to fire, summation of subsequent I waves may reach the threshold and trigger neuronal firing. While this summation may result in a single discharge, the spinal motoneuron also may fire repeatedly after a sufficiently intense, single cortical stimulation. Consequently, the amplitude of an evoked potential after cortical stimulation may be larger than the response produced by supramaximal stimulation of the corresponding peripheral nerve.
Facilitation of motor evoked potentials
The excitability threshold for eliciting motor evoked potentials (MEPs) can be decreased by performing a voluntary contraction of the target muscle. In parallel to such threshold changes, a decrease in latency of the response to stimulation (2-6 milliseconds) can be observed with respect to the MEPs that are obtained with no contraction of the target muscle. The origin of this facilitation remains controversial. Some authors ascribe it, at least partially, to modifications of cortical excitability. Others support a segmental spinal mechanism, which seems to play a major role.
The conduction speed as measured in several studies (60-70 milliseconds) is compatible with propagation through fast corticospinal tract axons.
Safety issues
Heating
Magnetic stimulation creates intense electrical discharges. Repetitive stimuli make the coil hot, and this can be a problem in clinical practice. Commercially available devices have auto-shutdown systems and temperature monitors that indicate coil overheating. These features protect patients but may prolong the duration of the procedure by 100-200%, an inconvenience that may be reduced by using several interchangeable coils or water-cooled coils.
Noise
The magnetic stimulator generates a high-intensity noise artifact that lasts about 1-2 milliseconds. Although no acoustic damage has been documented in humans, ear protectors attenuate the sound pressure level and have been recommended.
Seizures
Seizures are reported rarely in seizure-prone individuals. Stimulation at a high rate clearly can induce seizures, even in healthy subjects, but the effect of low-rate stimulation is controversial. Some studies suggest a beneficial role for TMS in decreasing the frequency of epileptic seizures. Patients with epilepsy still are excluded routinely from magnetic stimulation studies.
Kindling (ie, induction of permanent seizure focus) has never been induced in animals with stimulation at less than 10Hz, despite prolonged stimulation.
TMS Methodology
Stimulation
For circular coils, the direction of the current in the coil (clockwise or counterclockwise) must be selected first. The right hemisphere is stimulated preferentially with clockwise currents; the left hemisphere, by counterclockwise currents. The stimulating coil must be held firmly and tangentially against the stimulated structure.
Response recording
Motor evoked potentials (MEPs) usually are recorded with surface electrodes in target muscles. Coaxial needle electrodes can be used for more selective recording, mostly in research studies. Superimposition of 2 or more tracings of reproducible morphology helps to identify the parameters of interest correctly.
MEP parameters
Threshold of stimulation
This is the level of stimulation that is needed to obtain reliable MEPs over 50 microvolts in about 50% of 5-10 stimulations. Lower thresholds are found for the hand and forearm, lower still for the truncal lower limb and pelvic musculature.
Response latency
Two techniques, the central method and the peripheral method, have been developed to subtract peripheral conduction time from the total scalp-to-muscle latency.
The central method uses a magnetic or electrical impulse over the cervical or lumbar spine. The stimulation point has been demonstrated to be at the intervertebral foramen, so the central conduction time includes the proximal root. For lumbosacral stimulation and the best response in the tibialis anterior, the inner edge should be moved to the L5 vertebral level. As the stimulation site for the nerve root is also at the intervertebral foramen, the central motor conduction time (CMCT) includes conduction through the cauda equina. The peripheral method uses F-wave latency.
Amplitude
Amplitude (peak to peak) is best expressed in terms of the percentage of the muscle response amplitude evoked by supramaximal peripheral nerve stimulation of the target muscle.
Latency and amplitude of MEPs vary among healthy subjects and no reference normal values for MEPs in healthy older adults are available. Clinically useful reference values for MEPs by TMS can be provided in healthy adults older than 70 years. As in the younger population, standing height is important in defining normal MEPs. The difference between sexes might be due to the lower height of women. [6]
Contraindications
Contraindications to TMS include the following:
-
Pacemaker
-
Spinal or bladder stimulator
-
Previous skull opening or trauma
-
History of epilepsy (relative)
-
Presence of metallic foreign body
TES Methodology
After skin preparation with acetone, stimulation is performed using cutaneous electrodes. Specific montages allow preferential stimulation of specific muscle groups. For example, motor evoked potentials (MEPs) are induced preferentially in hand muscles if the anode is placed 7cm away from the vertex, on the line joining the vertex to the tragus, and the cathode is placed over the vertex.
TES cannot stimulate individual muscles selectively. Conversely, TMS can stimulate discrete muscles or muscle groups preferentially. Responses in TES are recorded in target muscles with surface or coaxial electrodes, as in TMS.
Clinical Applications of MEPs
Multiple sclerosis
The diagnosis of multiple sclerosis (MS) is based on the detection of multiple inflammatory, demyelinating white matter lesions, which are disseminated in time and space. In many patients, clinical assessment is insufficient and paraclinical studies must be performed. These tests, such as MRI, cerebrospinal fluid (CSF) studies, visual evoked potentials (VEPs), and somatosensory evoked potentials (SEPs), may be used in conjunction with motor evoked potential (MEP) studies to establish the diagnosis of MS. [7]
The duration of MEPs is increased in patients with definite (MS), a phenomenon that is compatible with increased temporal dispersion of the impulses that arrive at the motor neuron pool. MEP studies may have 2 applications in MS: (1) as a diagnostic tool and (2) as an index of corticospinal pathway dysfunction. [8]
In MS, a prolongation of the CMCT may be explained by reduced stimulus conduction in the large-diameter corticospinal fibers; this phenomenon is caused by demyelination or incomplete remyelination. The temporal stimulus summation that is necessary for large motor anterior cells to discharge may be reduced. Dispersion of the conduction velocity of individual axons may be increased.
In patients with definite MS, a correlation between CMCT and manual dexterity (but not muscle strength) has been reported. The lack of correlation between CMCT and isometric muscle strength, hyperreflexia, or spasticity is attributed to the role of the fast-conducting pyramidal tract in generating rapid phasic muscle action important for fine motor skills but not for strength of muscle contraction (which can be carried by different descending tracts).
MEP studies can be useful for monitoring the electrophysiologic correlates of clinical response to treatment. In patients who show clinical improvement after steroid treatment, CMCT decreases toward normal values and can remain stable if disease activity is controlled.
MEPs are more sensitive than SEPs in MS, with an overall incidence of abnormality greater than 70%. In patients with definite MS, prolonged CMCT to muscles in the lower extremities is observed in 77-89% of patients, while abnormal VEP findings are observed in 74.4% of these patients.
Recording MEPs in upper and lower extremities increases the sensitivity of the study, because lesions that are caudal to C8 can be detected. Nociti and colleagues have shown that SEPs reflect the upper limb motor performance in MS. [9, 10]
Cervical myelopathy
MEP recordings from thenar and tibialis anterior muscles appear to be especially sensitive (84-100%) in the detection of cervical myelopathy. MEP studies can indicate whether lesions identified on anatomic computed tomography (CT) scanning or MRI studies have neurophysiologic significance.
MEP studies may be more sensitive than SEPs in the detection of cervical myelopathy, possibly because cervical spondylosis (often with prominent bony spurs projecting from the vertebral body) predominantly involves the anterolateral quadrant of the spinal cord. This potentially could affect descending motor tracts in the corticospinal tract, leaving dorsal column pathways relatively unaffected. [11]
Spinal cord injury
Monitoring MEPs is a significantly reliable technique to assess spinal cord ischemia during thoracoabdominal aortic aneurysm repair. [12, 13]
The prognostic value of MEP studies in spinal cord injury (SCI) is limited, probably because of the spinal shock in the acute phase. In one study, among 10 patients who presented within 2 weeks after injury, MEPs were absent below the level of the lesion in 7 patients with complete paraplegia; MEPs remained absent 6 months later. In 3 patients with incomplete quadriplegia and subsequent recovery, MEPs were recordable in 2 subjects and absent in 1 subject.
Among 25 patients studied within 6 hours after onset and monitored over 6 weeks, MEPs were not obtained in patients without preceding clinical evidence of voluntary contraction.
MEPs recorded from the lower extremities can fail to provide rapid detection of spinal cord ischemia in the upper thoracic level after cross clamping of the descending thoracic aorta.
Currently utilized MEP and SSEPs are unable to differentiate between central and peripheral paraplegia in patients with acute spinal cord ischemia during thoracoabdominal aortic replacement. Researchers have proposed combining MEP and peripheral compound muscle action potential induced by posterior tibialis nerve stimulation, enabling the surgeon to quickly distinguish between central and peripheral neurologic injury. [14]
Motor neuron disease
Reported MEP abnormalities in motor neuron disease include absence of response, prolongation of latency, enhanced cortical threshold and, most commonly, low-amplitude polyphasic responses. The degeneration of spinal motor neurons explains the tendency of MEPs to be of short duration.
The average survival duration of patients with a normal CMCT is not significantly different from those with an abnormal CMCT. MEP studies have limited use in the diagnosis of amyotrophic lateral sclerosis; they do not provide significant prognostic information.
Stroke motor recovery prognosis
The recovery of motor function after a stroke varies. In the first days, the motor prognosis is difficult to establish from clinical and even head CT scan data.
The clinical application of MEPs in stroke is done mainly to evaluate the prognosis for recovery. (MEPs have a better predictive value for functional prognosis than do SEPs.) Most studies suggest that the early presence of MEPs after a stroke indicates a good recovery of daily functions. Absence of MEPs is associated with variable outcomes, usually poor. For patients who do not respond to cortical stimulation at stroke onset, the risk of poor functional recovery at 12 months is high and the probability of stroke-related death during this period is greater than for patients who do respond.
Takarada et al tested the hypothesis that transient vascular occlusion increases the excitability of the primary motor cortex during force exertion. The MEP amplitudes were enhanced with occlusion under all conditions, with the exception of 60% contraction. In contrast, no significant difference was observed between the MEP amplitudes obtained from the occluded or nonoccluded, relaxed flexor carpi ulnaris muscle. These results suggest that transient vascular occlusion increases the excitability of the motor cortex only during force exertion. [15]
Prolonged CMCT is found mainly in subcortical lesions. Severe cortical strokes are more likely to result in absent MEPs.
Reliability of intrasession MEP size is excellent in the lower limb of patients with stroke using as few as 6 MEPs, but intersession reliability is poor. Comparing MEP size measures across 2 or more sessions is questionable in the lower limb of patients with stroke. [16]
Transcranial magnetic stimulation-evoked neurophysiological parameters are useful measures for monitoring post-stroke patients. [17, 18]
Parkinson disease
Basic research findings in Parkinson disease support a decrease of the excitatory drive from the thalamus, which projects to the cortex in this disorder, and an enhanced cortical excitability.
Rapid (5Hz) repetitive TMS (rTMS) over the dominant hemisphere has been reported to shorten reaction and movement times in Parkinson disease. Some have reported improvement that lasted 20 minutes after rTMS. Other groups have reported negative results. The use of rTMS probably will be limited to electrophysiologic studies of cortical excitability in Parkinson disease; its therapeutic use remains anecdotal.
Writer's cramp
Handwriting was reportedly improved after 1Hz rTMS over the left hemisphere. The decreased intracortical inhibition in individuals with writer's cramp was corrected after 1Hz rTMS.
Epilepsy
Some believe that TMS induces seizures when used to evaluate patients with partial epilepsy. TMS has actually appeared to be poorly epileptogenic at low frequencies of stimulation (induction rate in epileptic patients: 0-92%). To what extent low-rate TMS may induce seizures, even in susceptible individuals, remains questionable. Although rTMS can provoke seizures, the effect depends on the individual (epileptic vs nonepileptic), antiepileptic treatment, and stimulus type (intensity-frequency).
Therapeutic benefit of rTMS
Intracortical inhibition is reduced in epileptic patients. Because low-rate rTMS can increase intracortical inhibition, this paradigm was used as a treatment in patients with intractable epilepsy. A substantial reduction of seizure frequency was reported in an open study.
Determination of language dominance before surgical procedures
Induction of speech arrest after stimulation of the dominant hemisphere is possible, but the reported success rate ranges from between 50-100% of subjects. This seems to depend at least partly on the stimulation technique (ie, coil type) used, but it remains to be clarified fully in further studies.
Surgical monitoring
The standard method of surgical spinal monitoring has been the use of SEP studies. Special stimulators that can provide 3-4 short-interval, successive pulses can overcome the reduction in evoked potentials caused by anesthetic agents. Special anesthetic methods using ketamine, etomidate, or propofol are required. However, because myogenic MEPs can be affected by most anesthetic agents and muscle relaxants, anesthesiologists are required to properly understand MEPs and to manage anesthesia carefully. [19]
Tamkus et al in their study revealed that the use of inhalation anesthesia during adult spinal surgery is associated with significantly higher rates of false-positive changes compared with total intravenous anesthesia during transcranial electrical motor evoked potentials monitoring. This relationship appears independent of preoperative motor status. Further study and multivariate analysis of anesthetic agents, diagnosis, and symptoms is necessary to elucidate the impact of these variables. [20]
Macdonald et al have made some recent recommendations on the basis of current evidence and expert opinion. [21] They have emphasized that intravenous anesthesia, usually consisting of propofol and an opioid, is optimal for muscle MEPs. Muscle MEP warning criteria are tailored to the type of surgery and based on deterioration clearly exceeding variability with no confounding factor explanation.
Intraoperative neurological assessment during aneurysmal clipping under awake craniotomy is feasible and safe, and it should be valuable for the assessment of ischemia, especially in the anterior choroidal artery. From a neurophysiologic viewpoint, MEP may be insufficiently sensitive for evaluating voluntary movement under ischemia. [22]
Eleraky et al showed that temporary nerve root clipping combined with MEP and SEP monitoring compliments the impact of neuromonitoring in the intraoperative management of patients with thoracic spine tumors and significantly improves the neurologic outcome. [23] Yeon et al showed that the use of transcranial MEP monitoring can be used to reduce ischemic complications by allowing prompt corrective measures to be taken during aneurysm surgery. [24]
Kobayashi et al recently did the first prospective multicenter study to investigate the alarm point of transcranial electrical stimulation MEP. The authors recommend the designation of an alarm point of a 70% decrease in amplitude for routine spinal cord monitoring, particularly during surgery for spinal deformity, ossification of the posterior longitudinal ligament, and extramedullary spinal cord tumor. [25, 26]
Lateral decubitus positioning
Monitoring transcranial MEPs recorded from the tibialis anterior muscle has been useful in identifying emerging peroneal nerve compression secondary to lateral decubitus positioning. [27]
Research Applications of MEPs
Double-pulse studies
Intracortical inhibition (ICI) or facilitation (ICF) defines the inhibitory or facilitatory action between areas of the motor strip itself or between cortical areas of the same hemisphere.
ICI and ICF can be elicited by using a double-pulse (DP) technique with focal stimulations (see the image below). With intervals of between 1 and 5 milliseconds, DP studies test the influence of short-range inhibitory GABA (gamma aminobutyric acid)-ergic interneurons. With intervals of between 7 and 30 milliseconds, DP studies measure the influence of short-range excitatory interneurons.
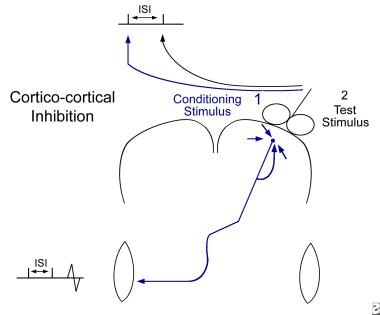 Double-pulse study. Using a common single coil, a conditioning stimulus (1) is given over the target hemisphere a short delay prior to a test stimulus (2) over the same location. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of intracortical inhibition and facilitation.
Double-pulse study. Using a common single coil, a conditioning stimulus (1) is given over the target hemisphere a short delay prior to a test stimulus (2) over the same location. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of intracortical inhibition and facilitation.
DP studies have been useful in evaluating the effect of rTMS on cortical excitability in several neurologic diseases.
Interhemispheric conditioning studies
Interhemispheric inhibition can be demonstrated in humans by studying the transcallosal effects of magnetic stimulation on motor cortical excitability (see the image below). Two stimulating coils are positioned at the optimal positions ("hot spots") on both hemispheres. A conditioning stimulus is performed to activate one hemisphere with a preset delay before a test stimulation is administered over the other hemisphere.
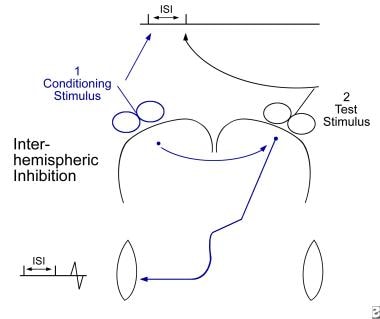 Interhemispheric conditioning study. A conditioning stimulus (1) is given over the contralateral hemisphere a short delay prior to a test stimulus (2) over the target hemisphere. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of transcallosal inhibition or excitation.
Interhemispheric conditioning study. A conditioning stimulus (1) is given over the contralateral hemisphere a short delay prior to a test stimulus (2) over the target hemisphere. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of transcallosal inhibition or excitation.
Previous studies have suggested that in right-handed individuals, the inhibition after stimulation of the "dominant" hemisphere was more marked than after stimulation of the "nondominant hemisphere."
Interhemispheric inhibition appears to be decreased significantly in patients with an abnormality of the anterior part of the corpus callosum.
Mapping studies
Magnetic stimulation studies can address specifically the issue of cortical reorganization by using mapping procedures that employ focal stimulations with "figure-of-8" coils.
Single pulses are used to sequentially stimulate successive positions over the scalp, usually 1cm apart. The intensities or latencies of responses then can be plotted on a 2-dimensional (2-D) map, which is obtained in reference to the vertex or other anatomical landmarks. A refined technique, which uses frameless stereotactic localization of the stimulating coil and of the subject's head, allows the researcher to project the TMS maps directly onto 3-D brain reconstruction images. (See the image below.)
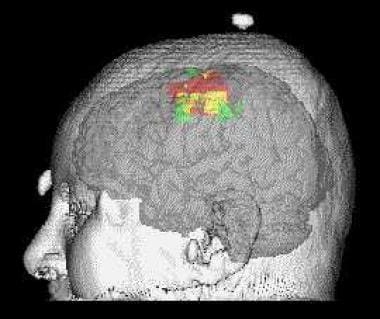 Example of a motor map obtained by transcranial magnetic stimulation (TMS), using a stereotactic technique. The TMS map is represented in red. It is compared with a map obtained with functional magnetic resonance imaging (MRI), in green. The overlap of the TMS and functional MRI maps is represented in yellow. The TMS and functional MRI mapping techniques can provide complementary information on motor control.
Example of a motor map obtained by transcranial magnetic stimulation (TMS), using a stereotactic technique. The TMS map is represented in red. It is compared with a map obtained with functional magnetic resonance imaging (MRI), in green. The overlap of the TMS and functional MRI maps is represented in yellow. The TMS and functional MRI mapping techniques can provide complementary information on motor control.
TMS mapping has been used successfully in studying the changes of cortical sensorimotor maps in response to experimental injury to the PNS or central nervous system (CNS).
TMS mapping provided evidence that changes in cortical motor maps may, in some situations, occur rapidly (ie, during motor learning in healthy volunteers) or much more slowly (eg, after peripheral nerve transposition).
Stereotactic TMS mapping can be coregistered to functional MRI scan, positron emission tomography (PET) scan, or electroencephalogram (EEG) for various assessments of brain function.
Questions & Answers
Overview
What are motor evoked potentials (MEPs)?
What is the role of the motor cortex in motor evoked potentials (MEPs)?
What is the role of the pyramidal tract in motor evoked potentials (MEPs)?
What is the role of magnetic stimulation in motor evoked potentials (MEPs)?
What is the role of electrical stimulation in motor evoked potentials (MEPs)?
What is the role of electrophysiology in the generation of motor evoked potentials (MEPs)?
How is coil overheating prevented during the generation of motor evoked potentials (MEPs)?
Why are ear protectors recommended during the generation of motor evoked potentials (MEPs)?
What is the risk of motor evoked potentials (MEPs)-related seizures?
How does the current direction affect motor evoked potentials (MEPs)?
How are motor evoked potentials (MEPs) recorded?
What are the threshold of stimulation parameters for motor evoked potentials (MEPs)?
What are response latency parameters for motor evoked potentials (MEPs)?
What are the amplitude parameters for motor evoked potentials (MEPs)?
What is the role of transcranial electrical stimulation (TES) in motor evoked potentials (MEPs)?
What is the role of motor evoked potentials (MEPs) in the diagnosis of cervical myelopathy?
What is the role of motor evoked potentials (MEPs) in the assessment of spinal cord ischemia?
What is the role of motor evoked potentials (MEPs) in the diagnosis of motor neuron disease?
What is the role of motor evoked potentials (MEPs) for stroke motor recovery prognosis?
What is the role of motor evoked potentials (MEPs) in the treatment of Parkinson's disease?
What is the role of motor evoked potentials (MEPs) in the treatment of writer's cramp?
What is the role of motor evoked potentials (MEPs) in the treatment of epilepsy?
What is the role of motor evoked potentials (MEPs) in surgical monitoring?
What is the role of motor evoked potentials (MEPs) in identifying peroneal nerve compression?
What are double-pulse motor evoked potentials (MEPs) studies?
What are interhemispheric conditioning motor evoked potentials (MEPs) studies?
What is the role of motor evoked potentials (MEPs) in mapping studies?
-
Double-pulse study. Using a common single coil, a conditioning stimulus (1) is given over the target hemisphere a short delay prior to a test stimulus (2) over the same location. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of intracortical inhibition and facilitation.
-
Interhemispheric conditioning study. A conditioning stimulus (1) is given over the contralateral hemisphere a short delay prior to a test stimulus (2) over the target hemisphere. The conditioning effect is evaluated by comparing conditioned responses to baseline responses that are obtained without a conditioning stimulus. This allows measurement of transcallosal inhibition or excitation.
-
Example of a motor map obtained by transcranial magnetic stimulation (TMS), using a stereotactic technique. The TMS map is represented in red. It is compared with a map obtained with functional magnetic resonance imaging (MRI), in green. The overlap of the TMS and functional MRI maps is represented in yellow. The TMS and functional MRI mapping techniques can provide complementary information on motor control.









