Overview
Background
Leprosy is the most common treatable cause of neuropathy in the world. [1] In all patients with leprosy, the nerve tissue is involved. The dermal nerves are infected in all skin lesions, including those due to indeterminate leprosy of childhood. Clinical examination is often sufficient to reliably diagnose leprous neuropathy.
Leprosy is a particularly common cause of neuropathy in developing countries, although it is also seen in developed countries. In the United States, the prevalence of leprosy may increase with increasing immigration from regions in which the disease is endemic. [2]
Go to Leprosy for complete information on this topic.
Patient education
It is important to educate patients about the condition and the consequences of neuropathy. Periodic screening is recommended to detect signs and symptoms of neuropathic feet.
Any change in status may require a change in treatment protocol (possibly a different style of shoe or orthotic). If any new injury, redness, swelling, or temperature change is noted, it should be brought to the attention of a healthcare professional.
History
Signs and symptoms of nueropathy vary, depending on the type of leprosy.
Sensory neuropathy is far more common than motor neuropathy, but pure motor neuropathy can occur. Mononeuropathy and mononeuritis multiplex can occur, with the ulnar and common peroneal nerves most often involved. Symmetric peripheral neuropathy may also occur. Patients with skin lesions overlying peripheral nerve trunks are more prone to the development of sensory or motor impairment. [3]
Symptoms of leprous neuropathy usually include the following:
-
Anesthetic, painless, nonitchy skin patches
-
Deformities due to weakness and wasting of muscles innervated by the affected peripheral nerves (eg, claw hand or foot drop secondary to muscle weakness)
-
Sensory symptoms, such as diminished to complete loss of sensation, paresthesias in the distribution of affected nerves, and neuralgic pain when the nerve is struck or stretched
-
Spontaneous blisters and trophic ulcers consequent to sensory loss
Symptoms seen in leprosy reactions are as follows:
-
Type 1 reaction (reversal reaction) - Sudden onset of redness of the skin and appearance of new skin lesions
-
Type 2 reaction (erythema nodosum leprosum [ENL]) - Multiple skin nodules, fever, joint pains, muscle pains, and redness of eyes; severe neuritic pain and rapid evolution of peripheral nerve damage, resulting in claw hand or foot drop
Physical Examination
Peripheral nerve hypertrophy
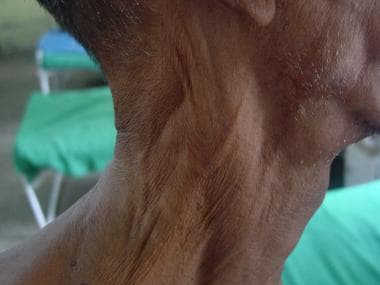
Nerve trunks are enlarged palpably in 40-55% of patients (see the image below); this sometimes predates sensory loss in the corresponding nerve territory. Nerve hypertrophy must be differentiated from a healthy nerve that may be palpable.
Sensory cutaneous nerves running to the proximal edge of a skin lesion may be thickened in tuberculoid (TT) and borderline tuberculoid (BT) leprosy. Nerves with a predilection for thickening include the following:
-
Great auricular nerves
-
Supraclavicular nerves as they cross the clavicle
-
Ulnar nerves just above the elbow (the ulnar nerve is the one most commonly thickened)
-
Dorsal cutaneous branches of the ulnar nerve at the wrist
-
Median and superficial radial nerves
-
Femoral cutaneous and lateral popliteal (common peroneal) nerves as they wind around the neck of the fibula
-
Superficial peroneal nerves in front of the ankles
-
Posterior tibial nerves immediately below the internal malleoli
-
Sural nerves
In TT disease, thickening is usually confined to 1 nerve. In lepromatous (LL) disease, the increase in nerve size is symmetrical; however, the degree of nerve thickening may differ between the 2 sides. The enlargement often may be segmental rather than diffuse and uniform.
Disparity may be noted between specific areas of thickened nerves and distribution of sensory/motor signs.
Sensory and motor abnormalities
Facial nerve palsy due to involvement of branches to the frontalis or orbicularis oculi leads to frontalis weakness or lagophthalmos. It may be unilateral or bilateral but spares other muscles innervated by the facial nerve. Sensory loss may occur in the malar region and cornea.
Wasting and weakness usually progress pari passu (ie, at the same rate). In some patients, however, wasting is more prominent than weakness. These signs involve predominantly the ulnar nerve at the elbow, median nerve at the wrist, and common peroneal nerve at the fibular head.
With respect to sensory modalities, thermal sensation is affected first, followed by pain and touch. Proprioception and vibration modalities are often preserved. Topographical distribution of sensory loss is variable. Graded sensory testing with standardized nylon microfilaments or computer-assisted sensory examination (CASE) may be helpful to detect early sensory loss.
Deep tendon reflexes generally preserved because the muscle spindles and large-fiber nerves are not involved.
Extremities, deformities, and trophic changes
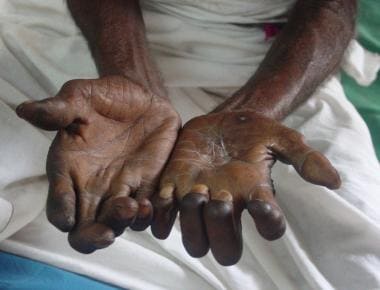
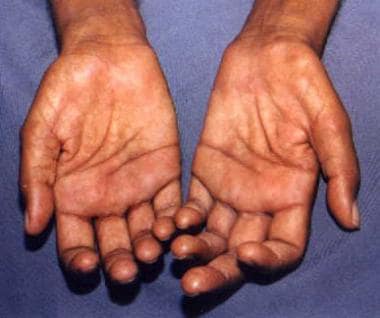
Claw-hand deformity (usually indicating ulnar nerve involvement) is most common, though it is a nonspecific manifestation of leprosy (see the images below).
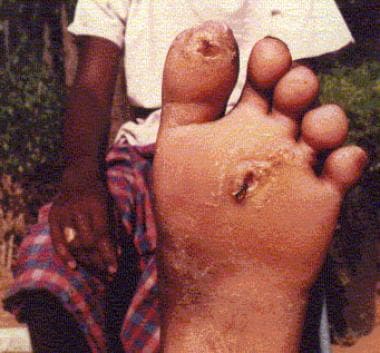
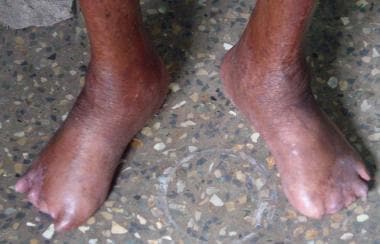
Trophic ulcers, a common, nonspecific complication of pain sensation loss, occur on the sole of the foot and on the hands and fingers (see the images below). Absorption of fingers and toes may be noted.
Abnormalities of autonomic nervous system
Autonomic nerve involvement is manifest clinically as varying degrees of impaired sweating and possible anhidrosis. [4] In general, visceral autonomic nerves are not involved, though conflicting experiences with cardiac dysautonomia have been reported. [5, 6]
Findings in type 1 and 2 reactions
Type 1 (reversal reactions) and type 2 reactions (ENL) exhibit distinct clinical features (see the table below).
Table 1. Comparison of Type I and Type 2 Reactions (Open Table in a new window)
Features |
Type I (Reversal) Reaction |
Type 2 (ENL) Reaction |
Leprosy type at risk |
BL, BB, BT |
LL, BL |
Onset |
Gradual, over a few weeks |
Sudden |
Clinical symptoms and signs |
Malaise |
Fever, malaise, arthritis, edema, hepatosplenomegaly, lymphadenopathy, orchitis, iridocyclitis |
Skin lesions |
Increased erythema, induration of preexisting lesions, and appearance of new lesions |
New erythematous lesions or tender nodules on face, extremities, and trunk |
Nerve involvement |
Frequent, often severe |
Frequent, often severe |
BB = midborderline; BL = borderline lepromatous; BT = borderline tuberculoid; LL = lepromatous. |
||
In type 1 (reversal) reactions, physical findings include erythema and edema in skin lesions. New skin lesions appear. Affected nerves increase in size and become tender with signs of damage of involved nerves. Patients with skin lesions overlying peripheral nerve trunks should be carefully monitored for development of sensory or motor impairment [7]
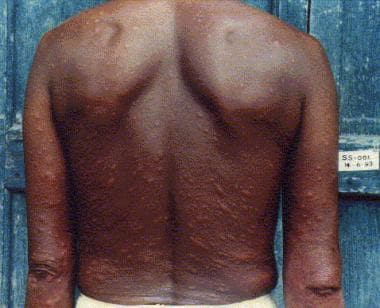
Type 2 reactions (ENL) are characterized by acute peripheral nerve damage leading to claw hand or foot drop. The involved nerve trunk—usually ulnar just above the elbow, median at the wrist, lateral popliteal at the fibular head—becomes tender and increases in size. Other features include multiple acute and tender skin nodules (see the image below), arthritis, edema, hepatosplenomegaly, lymphadenopathy, orchitis, and iridocyclitis.
Differential Diagnosis
Treatment progress and decreasing prevalence make differential diagnosis difficult, and neurologic expertise is required. Silent nerve lesions may be present long before symptomatic neuropathy appears. Detection of asymptomatic leprous neuropathy by means of careful clinical examination and electrophysiologic studies in persons at risk can aid early detection and treatment. [8]
Epidemiologic studies reveal that many cases occur within families. This pattern may lead to misdiagnosis of a hereditary neuropathy. Differentiating leprous neuropathy from hereditary sensory neuropathy may be difficult. Both may involve family members, the clinical pictures may be similar, and the diagnosis may be missed unless skin smears or skin or nerve biopsy is performed.
Neuritic leprosy produces no obvious skin lesions, and skin smears are negative for acid-fast bacilli (AFB). Suspect this diagnosis when persons from areas of endemic disease present with nerve thickening and associated nerve deficit. In some studies, neuritic leprosy was reported in 5-15% of patients with leprosy. Some cases may have been treated partially; others may subsequently evolve to classic leprosy. Nerve biopsy is essential for diagnosis.
Even in areas of endemic disease, not all persons with thickened nerves have leprosy; other neuropathies must be excluded. Various neuropathies, especially those involving small fibers of the peripheral nerve, may closely mimic that of leprosy.
The involvement of the median nerve at the wrist, the ulnar nerve at the elbow, and the common peroneal nerve at the fibular head are common in leprosy and may lead to difficulty in distinguishing this condition from idiopathic entrapment neuropathy involving these nerves. A history of previous residence or travel to an area of endemic disease, a careful search for skin lesions, and a careful search for nerve enlargement, combined with an appropriate diagnostic workup for leprosy, may confirm the diagnosis.
In leprosy, the nerve is often thickened and involves areas proximal to the entrapment site. Motor weakness and wasting are often more severe in leprosy than in a carpal tunnel syndrome. Idiopathic entrapment neuropathy must not be misdiagnosed as neuritic leprosy (ie, leprosy without skin lesions).
Nerve Conduction Studies
Abnormalities on nerve conduction studies [9] include the following:
-
Segmental slowing of conduction at common sites of entrapment (eg, elbow segment of the ulnar nerve)
-
Prolonged distal latencies
-
Reduced (sensory or motor) nerve conduction velocities
-
Reduced amplitude of compound muscle action potentials
-
Absent or low-amplitude sensory nerve action potentials
-
Pattern of abnormalities suggesting mononeuropathy, mononeuropathy multiplex, entrapment neuropathy, or generalized polyneuropathy
The ulnar, common peroneal, median, and tibial nerves are most commonly involved. Changes in nerve conduction are more severe if the nerves are clinically affected than if they are not. Nerve-conduction velocity may be decreased before any sensory deficit appears, and this finding can be used to detect asymptomatic nerve involvement. [10]
Conduction velocity in the index branch of the radial cutaneous nerve can be reduced in early leprosy and even in the contacts of patients with leprosy. Similar studies in the dorsal cutaneous branch of the ulnar and the great auricular nerves can also be useful.
In lepromatous (LL) disease, nerve thickening is not correlated with impaired nerve conduction. Palpably enlarged nerves may be functional, though they may eventually fail.
Prolongation of the refractory period is considered a more sensitive parameter than conventional motor or sensory conduction in detecting early nerve damage in clinically asymptomatic nerves. [11]
Abnormalities in visual and brainstem auditory evoked potentials have been reported in LL disease, suggesting central nervous system (CNS) involvement. [12]
Neurophysiological studies of ulnar nerves in patients with type 1 and type 2 reactions indicate axonal and demyelinating processes across the elbow. In type 2 reactions, changes of demyelination (ie, conduction block) is a primary event, occurring as an acute phenomenon, while in type 1 reactions, temporal dispersion, a subacute phenomenon, is seen. [13]
Nerve Biopsy
Nerve biopsy occasionally reveals abnormalities even in contacts of patients with leprosy. The results may rule out other diseases such as polyarteritis nodosa, hereditary neuropathies, or chronic inflammatory demyelinating polyradiculoneuropathy. Recognizing that not all people with thickened nerves, even those of in regions of endemic disease, have leprosy is important.
Nerve biopsy is probably more sensitive than skin biopsy, though false-negative histologic results may be seen when clinically uninvolved nerves are sampled. Skin and nerve histologic results are often incongruous; results in patients with multibacillary (MB) leprosy in nerve may show paucibacillary (PB) leprosy in skin. The best results of nerve biopsy are obtained when the findings are interpreted in laboratories with special expertise in such diseases. In purely neuropathic leprosy, nerve biopsy is the only way to confirm the diagnosis.
Biopsy of a clinically involved cutaneous nerve may be more informative than routine biopsy of the sural nerve, radial cutaneous nerve, or dorsal branch of the ulnar nerve. Sural nerve biopsy is usually performed at the level of the lateral malleolus, where it passes between the calcaneum and the lateral malleolus. Biopsy of the radial cutaneous nerve or dorsal branch of the ulnar nerve is at the level of the dorsum of the wrist. Fascicular nerve biopsy causes less sensory deficit than full-thickness biopsy.
The nerve-biopsy specimen is divided into 5 pieces, each treated with 1 of the following fixatives:
-
Formalin for hematoxylin and eosin and silver stains for axons
-
Flemming solution for myelin stains (Weigert-Pal technique)
-
Glutaraldehyde for electron microscopy
-
Formol-calcium solution for teased-fiber preparations
-
Frozen specimen for enzyme histochemical technique
The following staining techniques are useful to examine histopathological changes in leprosy-affected nerves: [14]
-
Job-Chacko modification of Fite-Faraco stain for AFB: M. leprae is weakly-acid fast and may not be demonstrated by Ziehl-Neelsen staining
-
Gomori’s-Grocott methanamine silver stain to demonstrate remnants of M. leprae
-
Luxol fast blue stain for myelin
-
Van Gieson’s stain for collagen fibers
-
Bodian's silver stain for axons
-
Immunohistochemical stains for nerves
-
Immunohistochemistry and immunofluorescent microscopy to demonstrate mycobacterial antigens and PCR studies
Histologic Findings
Lepromatous disease
Lepromatous (LL) leprosy is associated with characteristic features on nerve biopsy, as follows.
On light microscopy, overall nerve structure is preserved. Involvement is asymmetric between and within individual fascicles. Nerve cross-sections show an inflammatory reaction affecting the epineurium and perineurium, causing increased nerve volume.
Macrophages and Schwann cells filled with organisms and debris (foamy cells) appear in the epineurium, endoneurium, and perineurium. In the perineurium, foamy macrophages infiltrate and separate individual layers, fibroblasts and perineurial cells proliferate, and collagen is deposited. This produces onion-skinning of the nerve fascicles. [15] Proliferation of connective tissue (peri- and endoneurial fibrosis) is not as prominent as in tuberculoid (TT) disease.
Lymphocytic vasculitis affects nerve blood vessels in all nerve compartments. The vessels remain permeable to blood. This feature is seen in persons who have received treatment for leprosy before the biopsy.
Mycobacterium leprae organisms are extremely numerous and are often seen in globoid clumps on Ziehl-stained paraffin-embedded specimens. They are found in all nerve compartments and affect a large variety of cells, including perineurial cells, fibroblasts, cells of the macrophage histiocyte lineage, Schwann cells, and endothelial cells. Fite, auramine rhodamine, or toluidine blue (in plastic-embedded sections) staining also demonstrates M leprae. A few viable M leprae organisms in Schwann cells persist even after treatment completion.
On myelin and silver (axon) stains, small myelinated and unmyelinated fibers are lost in early stages; large myelinated fibers are lost in later stages. Symptomatic neuropathy is associated with severe axonal loss. Nerve fiber density decreases to 5% of control values, compared to 25-30% in silent hypertrophy of the radial cutaneous nerve.
On electron microscopy, organisms are seen as membrane-bound, round- or rod-shaped, electron-dense structures. They often are surrounded by a clear halo, which is composed of bacterial metabolites and/or denatured host cytoplasmic components. Bacteria are found easily in macrophages and Schwann cells of unmyelinated fibers but are less frequently seen in Schwann cells associated with myelinated fibers.
In early leprous neuropathy, electron microscopy demonstrates naked or thinly myelinated axons, suggesting primary demyelination. Axonal pathology appears at a late stage.
Endothelial cells may appear swollen, with loss of cell junctions and other signs of damage to the blood-nerve barrier. Multilayering and thickening of the basement membrane around vessels occurs in all types of leprosy but is a nonspecific change of many chronic neuropathies.
In patients with silent hypertrophy of the superficial radial cutaneous nerve, teased-fiber preparation reveals segmental abnormalities of the myelin sheath, including segmental demyelination, a wide nodal gap between 2 internodes, and short remyelinated internodes. [10]
Demyelinated fibers are often clustered and linked closely with debris-laden macrophages. Axonal degeneration of nerve fibers predominates in some. In experimental animals, segmental demyelination was predominant during early infection. Axonal degeneration was evident in advanced infections.
Paucibacillary (tuberculoid) disease
In TT disease, the nerve may be completely destroyed, and normal nerve structures may not be identifiable. Involvement may also be multifocal, with damaged fascicles found adjacent to entirely normal ones.
Perineurium is thickened markedly, often fused with the epineurium into a thick fibrotic mass and infiltrated by inflammatory cells and small vascular channels. [15] The entire endoneurium may be replaced by a single granuloma. Caseation necrosis may occur and may even progress to a nerve abscess. Granulomas consist of epithelioid histiocytes, multinucleated giant cells, and variable numbers of lymphocytes and plasma cells.
Bacilli are absent from the lesions, but M leprae antigens may be demonstrated in nerves by immunohistochemical methods.
When no evidence of infection with M leprae can be found, differentiating sarcoid neuropathy from TT leprous neuropathy may be impossible. Immunologic and molecular techniques may be needed to confirm the etiology.
Medical Treatment
Significant nerve destruction occurs during the reactive phase of both type 1 reactions (reversal reactions) and type 2 reactions (erythema nodosum leprosum [ENL]).
Acute granulomatous inflammation can destroy nerves to the extent of causing caseation necrosis of nerve tissue and irreversible paralysis. Swelling of nerves due to sudden increase in inflammatory cells and edema within an unyielding perineurium produces ischemia and paralysis.
Inflammatory reaction in the nerve is suppressed by corticosteroid treatment with antileprosy treatment. [16, 17] Corticosteroids are used for patients with severe nerve involvement, nerve abscess, impending paralysis, or extensive and acutely inflamed skin lesions. High-risk patients may require corticosteroids for 6 months with MDT as a preventive measure. [18] Nerve damage can occur even after clinical cure and release from treatment.
Local treatment measures include the following:
-
Appropriate splints (eg, for wrist drop or foot drop)
-
Exercises
-
Prevention of corneal exposure when the facial nerve is involved
-
Prevention of injuries to anesthetic areas
-
Prevention and protection of plantar ulcers through the wearing of special controlled-rigidity footwear for redistributing pressure
-
Rest, splints, and physiotherapeutic measures may be appropriate. Thalidomide is not useful for reversal reactions.
Management of decreased sensation
Particular attention should be paid to managing decreased sensation. Most damage to insensitive tissues is preventable. Wounds in insensitive tissues heal as promptly as those in normal tissues.
Every wound must be splinted and protected from recurrent injury. Patients should be instructed to inspect eyes, hands, and feet daily for evidence of injury and should be encouraged to seek early medical attention if injury is present.
Suitable, safe occupations should be found for patients. Tools and everyday items (eg, door keys) should be modified to remove sources of high pressure and shear. Gloves should be worn for potentially damaging tasks. Footwear must be soft, have total contact, and avoid shearing and direct forces. Goggles must be worn to prevent drying of cornea and introduction of foreign bodies.
When sympathetic denervation results in anhydrosis, patients should keep their hands and feet soft by soaking them in water morning and night. This should be followed by occlusive cream or ointment application to lock in moisture.
Once wounds have occurred, treatment is often prolonged and difficult. If a wound is infected acutely, antibiotics, bed rest, and elevation of the limb may be required. After the acute infection settles, the leg and foot may require a plaster cast to allow healing. Severely diseased tissue may require surgical amputation. Tarsorrhaphy may be beneficial in lagophthalmos.
Surgical Nerve Decompression and Reconstruction
Surgery improves sensation in selected patients with sensory impairment and most often prevents further deterioration. Optimal timing for nerve decompression must be established. A multidisciplinary team comprising a leprologist, a neurologist, physical and occupational therapists, and a surgeon with experience in peripheral nerve surgery is needed. [19, 20, 21]
Surgical treatment of acute nerve abscess consists of careful incision of the nerve sheath and drainage of the abscess. Surgical neurolysis or even fascicular dissection has been advocated to relieve intraneural pressure. Sensory loss, though generally irreversible, can often be ameliorated by simple longitudinal epineurotomy.
Surgical treatments for eliminating anatomic constrictions include medial epicondylectomy, anterior transposition of ulnar nerve, deroofing of the carpal tunnel, and decompression of the posterior tibial nerve at the flexor retinaculum.
A Cochrane review assessing the effects of decompressive surgery on nerve damage in leprosy found that after 2 years, there was no significant difference in nerve function improvement between people treated with surgery plus prednisolone and those treated with prednisolone alone. [22] Accordingly, nerve decompression is undertaken when signs of entrapment have not cleared after 3-4 weeks of steroid therapy, when neurologic status deteriorates despite steroid therapy, and when signs of nerve abscess or chronic entrapment are noted.
Posterior tibial neurovascular decompression by release of the flexor retinaculum with systemic administration of steroids may be beneficial in early acute and silent neuritis. Distal compression of the plantar branches should be relieved by slitting the calcaneal bands and ensuring free passage of the plantar branches to the sole of the foot. Nerve function, particularly autonomic and sensory modalities, recovers to a considerable extent.
Some authors believe that even in long-standing cases, vascular decompression may help in healing chronic plantar ulcers and prevent their recurrence. [23]
Peripheral nerve reconstruction using denatured muscle autografts may help to restore protective sensation in hands and feet. Nerve grafts may be helpful for patients with localized nerve lesions. [24]
In patients with Hansen disease, with foot drop not recovering with steroids and conservative treatment, tibialis posterior tendon transfer surgery with early rehabilitation may be useful. [25]
Imaging
Ultrasound of the median, ulnar and common peroneal nerves may help in the diagnosis of neuropathy due to Hansen's disease, including the pure neuritic type of Hansen's disease wherein skin lesions are absent. Ultrasound may also help to differentiate leprosy from other neuropathies in which nerve enlargement can occur. The ulnar nerve enlargement (increase in crosssectional area [CSA]) starts at the ulnar sulcus and is a maximum of four centimeters above the medial epicondyle and starts reducing further along the tract. [26] Nerve abscess may also be detectable with ultrasound. Emerging ultrasound techniques such as imaging of soft tissue strain, elasticity and intraneural doppler may also be useful. [27] The nerve abnormalities on ultrasound may also worsen after treatment.
-
Infiltrated, scaly skin patch, with thickening of the cutaneous nerve close to the patch, in a patient with tuberculoid leprosy.
-
Patient with lepromatous leprosy showing multiple skin nodules with diffuse infiltration.
-
Infiltration of the ear lobes in a patient with lepromatous leprosy.
-
Advanced lepromatous leprosy with amputation of toes and trophic ulceration.
-
Bilateral total claw hand.
-
Anteroposterior (AP) radiograph of the foot shows absence of the second to fifth toes and distal tapering of the metatarsals secondary to neuropathy; this is the licked–candy stick appearance. The phalanges of the great toe show destruction secondary to osteomyelitis
-
Sagittal fat-saturated T2-weighted image shows amputation of the great toe with increased signal intensity in the head of the first metatarsal indicating osteomyelitis. A small abscess is noted beneath the head of the first metatarsal.
-
Infiltration and thickening of skin in the face in lepromatous leprosy (leonine facies).
-
Claw-hand deformities of both hands in a patient with neural leprosy.
-
Plantar trophic ulcers in a patient with leprous neuropathy.
-
Peripheral nerve thickening in leprosy.