Practice Essentials
Glioblastoma multiforme (GBM) is the most common and most aggressive of the primary brain tumors. The previous World Health Organization (WHO) classification of primary brain tumors lists GBM as a grade IV astrocytoma based entirely on the histopathological findings. [1] However, the 2021 WHO grading system considers only isocitrate dehydrogenase (IDH) wild-type diffuse gliomas in adults with histopathological features consistent with the previous definition as GBM. [2]
Signs and symptoms
Headache is one of the most common symptoms of GBM, especially if the tumor is arising in the posterior fossa. Incidence varies between 23% and 56%. [3, 4]
Seizures are the other common non-specific manifestation of any brain tumor, seen in ~20% cases of GBM at presentation.
Progressive focal neurological deficits can develop typically over weeks to months in GBM patients
Diagnosis
The preferred workup for GBM is diagnostic neuroimaging studies.
Brain MRI with and without gadolinium contrast is the most sensitive and specific study. GBM tumors characteristically have low-signal intensity on T1-weighted images and high-signal intensity on T2-weighted images. With contrast, the tumors usually enhance. The enhanced T1-weighted images typically have a central hypodensity surrounded by a thick enhancing rim of the tumor.
CT scan can be ordered with or without contrast when MRI is contraindicated or unavailable. On CT scan, GBMs have a variable, inhomogeneous hypodense or isodense appearance with surrounding edema. GBMs tend to infiltrate along the white matter tracts and frequently involve and cross the corpus callosum.
Management
Although the prognosis of GBM is uniformly poor, treating patients in an attempt to improve the quality of life is worthwhile. The current standard of care includes maximal safe surgical resection, followed by a combination of radiation and chemotherapy with temozolomide.
Background
Glioblastoma multiforme (GBM) is the most common and most aggressive of the primary brain tumors. The previous World Health Organization (WHO) classification of primary brain tumors lists GBM as a grade IV astrocytoma based entirely on the histopathological findings. [1] However, the 2021 WHO grading system considers only isocitrate dehydrogenase (IDH) wild-type diffuse gliomas in adults with histopathological features consistent with the previous definition as GBM. [2] See the image below.
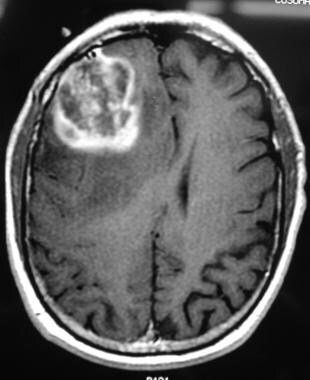 T1-weighted axial gadolinium-enhanced MRI demonstrates an enhancing tumor of the right frontal lobe. Image courtesy of George Jallo, MD.
T1-weighted axial gadolinium-enhanced MRI demonstrates an enhancing tumor of the right frontal lobe. Image courtesy of George Jallo, MD.
Pathophysiology
Glioblastoma multiforme (GBM) tumors are highly malignant, infiltrate the brain extensively, and grow rapidly; at times they may become enormous before turning symptomatic. They can arise de novo or by malignant transformation of a previously low-grade astrocytoma. Malignant transformation occurs by the sequential accumulation of genetic or molecular alterations and abnormal regulation of growth factor signaling pathways within the astrocytes [5] . Primary GBM usually presents with amplified and mutated epidermal growth factor receptors, whereas secondary GBM can have increased signaling through the PDGF-A receptor. There are many other molecular alterations defined in this cancer, including an amplification of the MDM2 gene, PTEN mutations, P53 mutations, IDH1 mutations, MET amplification, homozygous deletion of CDKN2A, and many more. The genomic and molecular landscape of GBM has evolved exponentially over the past few years. [6] The transition from a lower grade GBM to a higher grade is associated with inactivation of the retinoblastoma (RB1) gene and hyperactive MDM2. [7]
Many environmental risk factors are linked to the development of GBM such as exposure to therapeutic ionizing radiation or vinyl chloride or pesticides, smoking, and working in petroleum refining and synthetic rubber manufacturing industries. [6] Excessive use of mobile phones was also initially implicated in the pathogenesis. However, a meta-analysis published in 2007 did not show any association with the incidence of tumor development in people who used cell phones for at least 10 years. [8]
Certain hereditary syndromes are associated with GBM such as neurofibromatosis type 1 (NF1), neurofibromatosis type 2 (NF2), Li-Fraumeni syndrome, hereditary non-polyposis colorectal cancer (HNPCC/Lynch syndrome), Turcot syndrome/brain tumor-polyposis syndrome (BTPS), multiple endocrine neoplasia type 1 (MEN1), nevoid basal cell carcinoma syndrome (NBCCS), Gorlin-Goltz syndrome, and tuberous sclerosis complex (TSC). [6]
Epidemiology
Frequency
Among primary brain tumors, malignant astrocytomas are the most common in all age groups. (However, among all brain tumors, metastases are the most common.) Glioblastoma multiforme (GBM) tumors are the most common primary brain tumors in adults, accounting for 12–15% of intracranial tumors and 50–60% of primary brain tumors. Approximately three per 100,000 people develop the disease each year, although the regional frequency may be higher. [9, 10] GBM constitutes 45.2% of all malignant brain tumors, 54.4% of all high-grade gliomas, and 80% of all primary malignant brain tumors. [6] Several authors have reported a true increase in the incidence of brain tumors, especially among the elderly, and many have attributed the observed changes to developments in diagnostic imaging or changes in the classification system. [11]
Mortality/Morbidity
Morbidity is from the tumor location, progression, and pressure effects. The overall prognosis for GBM has changed little in the past 2 decades, despite major improvements in neuroimaging, neurosurgery, radiation treatment techniques, adjuvant chemotherapy, and supportive care. Few patients with GBM survive longer than 3 years and only a handful survive 5 years. Previously reported long-term survivors of GBM may be patients diagnosed with GBM who harbor low-grade glioma, pleomorphic xanthoastrocytoma, ganglioglioma, or other lesions.
Race-, sex-, and age-related demographics
High-grade astrocytomas (HGAs) are slightly more common in whites than in blacks, Latinos, and Asians. GBM is slightly more common in men than in women; the male-to-female ratio is 3:2. While GBM occurs in all age groups, its incidence is increasing in elderly patients. A true increase in the incidence of primary brain tumors exists, which cannot be explained by the aging population, better imaging techniques, or earlier detection at surgery. [6, 9]
Prognosis
With optimal treatment, the median survival of patients with glioblastoma multiforme (GBM) is about 12 to 15 months. [12] However, only 3–7% of patients survive for more than 5 years. [13] In the United States between 2012 and 2016, five-year survival was 6.8%. [13] The overall prognosis for GBM has changed little since the 1980s, despite major improvements in neuroimaging, neurosurgery, radiotherapy, and chemotherapy techniques. [14] Despite all the advancements in the treatment, a prospective trial demonstrated a median survival of only 16.6 months with 34% of patients surviving at 2 years. [15]
Various prognostic factors implicated in survival include age, performance status, histological grade of the tumor, specific molecular markers (MGMT methylation, mutation of IDH1, IDH2 or TERT, 1p19q codeletion, overexpression of EGFR, etc.), and the extent of resection. [16]
Various studies demonstrated that patients with GBM who are younger than 40 years have an 18-month survival rate of 50%, while those aged 40–60 years have an 18-month survival rate of 20%, and those older than 60 years have a rate of only 10%. In some series, age appears to be an even more important prognostic factor than histology.
The survival of patients with GBM decreases as KPS decreases. Patients with a KPS of more than 70 have an 18-month survival rate of 34%, while those with a Karnofsky Performance Scale (KPS) score of less than 70 have an 18-month survival rate of 13%. Additional factors such as the extent of surgical resection, seizures as the initial presentation, and tumor location with superficial tumors have been variably associated with outcome. [14]
An animal study in rats investigated the use of monoclonal antibodies 8H9 as interstitial infusion showed a significant volumetric response and prolonged survival (54 d for untreated rats vs 120 d for treated rats) as a potential target therapy for high-grade gliomas. [17]
A study by Wang et al demonstrated that overexpression of EphA7 was predictive of adverse outcomes in patients with primary and recurrent GBM, independent of microvascular density (MVD) expression. Moreover, the high density of both MVD and EphA7 expression predicted the disease outcome more accurately than EphA7 alone. [18]
A study by Liang et al demonstrated that nuclear FABP7 was preferentially expressed in infiltrative gliomas only and associated with poor prognosis in EGFR-overexpressing glioblastoma. The study suggested that FABP7 immunoreactivity could be used to monitor the EGFR-overexpressed GBM progression. [19]
Studies are focusing attention on identifying molecular markers like anaplastic oligodendroglioma to predict response or resistance to specific treatments. One such interest is the expression of the MGMT (O6 -methylguanine–DNA methyltransferase) gene. The protein product of this gene, 06 alkyl guanine DNA-alkyl-transferase (AGAT), is shown to be a major mechanism for tumor resistance to alkylating agents. [6, 20] Recent clinical trials for malignant gliomas now often include the determination of MGMT expression status. Several other molecular markers, such as epidermal growth factor receptor, platelet-derived growth factor receptor, vascular endothelial growth factor receptor, loss of chromosome 10, mutation or loss of the p53 gene, expression of the YKL-40 gene, and loss or mutation of PTEN gene, are being investigated. [6]
Studies are also focusing on new targets such as receptor blockade. Glutamatergic system alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor-blocker talampanel, may be beneficial in this disease. In one study, talampanel was added to standard radiation and temozolomide in adults with newly diagnosed glioblastoma to estimate the overall survival as well as talampanel toxicity as a secondary measure. The study concluded that talampanel was well tolerated, and compared with European Organization for Research and Treatment of Cancer (EORTC) data, median survival seemed superior (20.3 vs 14.6 mo, respectively). Therefore, talampanel can be added to radiation therapy and temozolomide without significant additional toxicity. [21]
Patient Education
During the course of diagnosis, treatment, and follow-up care, educate the patient and family about the course and prognosis of the tumor to help them cope with the physical and emotional burden. Set this goal during discussion with the patient in the presence of family members, nurses, physicians, social services, and spiritual services. In addition, frequent contacts, regular follow-up care, and involvement of support groups are necessary.
For patient education resources, see the Cancer Center and Brain Cancer.
-
T1-weighted axial gadolinium-enhanced MRI demonstrates an enhancing tumor of the right frontal lobe. Image courtesy of George Jallo, MD.
-
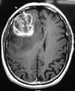
T2-weighted image demonstrates notable edema and midline shift. This finding is consistent with a high grade or malignant tumor. Image courtesy of George Jallo, MD.
-
Histopathologic slide demonstrating a glioblastoma multiforme.
-
Magnetic resonance spectroscopy is representative of a glioblastoma multiforme.