Overview
Background
Electroencephalography (EEG) remains the primary diagnostic test of brain function, especially in those with seizures or epilepsy, but is no longer used for identification and localization of gross structural brain lesions as neuroimaging with CT and MRI have taken that role. EEG assesses electrical brain function and is complementary to the neuroimaging techniques that assess anatomical brain changes. Unlike relatively new functional imaging procedures such as functional MRI (fMRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET), EEG provides a continuous measure of cortical function with excellent time resolution and is relatively inexpensive being nearly a century-old clinical tool. EEG is especially valuable in investigation of patients with known or suspected seizures or encephalopathy. [1]
Seizures are infrequent events in the majority of patients in an outpatient setting, making recording of ictal EEG time-consuming and labor intensive in the traditional EEG lab setting, but new portable devices allow for much longer sampling including in the home setting. The mainstay of diagnosis, therefore, remains detection of interictal (ie, between seizures, from the Latin icere, to strike) epileptiform discharges. Epileptiform discharges simply are transients with a characteristic "spiky" morphology discussed in greater detail in this chapter. Continuous video-EEG monitoring, developed over the last 50 years to facilitate recording of ictal events, also greatly increases the time available to detect interictal epileptiform discharges (IEDs). In the diagnosis of epilepsy and localization of seizure onset, both the intericital and ictal recordings are extremely informative. As EEG digital technology improves, there is growth in continuous bedside EEG monitoring to assist neurologic assessment in neurologically unstable patients, and more seizures are detected. Computerized spike and seizure programs are improving as well.
History
The first human EEG recording was done by Hans Berger in the 1920s. Interictal and ictal epileptiform EEG patterns were first identified in the 1930s, leading to distinction between partial/focal and primary generalized seizures. The basic concepts developed by such pioneers as Fred and Erna Gibbs, William Lennox, and Herbert Jasper underlie our current understanding of the clinical neurophysiology of epilepsy [2, 3, 4] ; subsequent work has led mainly to improvements in detection and interpretation of findings they first noted decades ago.
Definition and classification of interictal discharges
The International Federation of Societies for Electroencephalography and Clinical Neurophysiology (IFSECN) [5] describes interictal discharges as a subcategory of "epileptiform pattern," in turn defined as "distinctive waves or complexes, distinguished from background activity, and resembling those recorded in a proportion of human subjects suffering from epileptic disorders…." This somewhat circular definition makes clear that criteria must be verified empirically.
Interictal discharges may be divided morphologically into sharp waves, spikes, spike-wave complexes (also called spike-and-slow-wave complexes), and polyspike-wave complexes (also called multiple-spike-and-slow-wave-complexes). In practical terms, the morphological distinctions are less important than the certainty with which these entities can be distinguished from physiologic or nonspecific sharp transients. The other major issue is that some artifacts look like interictal discharges and require a well-trained electroencephalographer to interpret the waveforms accurately. If there is doubt, a repeat EEG may be of great benefit to sort things out, as misinterpretation may lead some patients to be placed on seizure medications needlessly. IEDs may occur in isolation or in brief bursts; bursts longer than a few seconds are likely to represent electrographic seizures rather than interictal discharges.
The following definitions are in use: [5]
-
Sharp wave - Transient, clearly distinguishable from background activity, with pointed peak at conventional paper speeds and a duration of 70-200 milliseconds (ms)
-
Spike - Same as sharp wave, but with duration of 20 to less than 70 ms
-
Sharp-and-slow-wave complex - Pattern consisting of a sharp followed by a slow wave (classically the slow wave being of higher amplitude than the sharp)
-
Spike-and-slow-wave complex - Pattern consisting of a spike followed by a slow wave (classically the slow wave being of higher amplitude than the spike)
-
Multiple spike-and-slow-wave complex - Same as spike-and-slow-wave complex, but with 2 or more spikes associated with one or more slow waves
Pathophysiology
The underlying cellular pathophysiology of focal spikes is believed to be the paroxysmal depolarization shift (PDS). Several decades of studies using cortical application of penicillin or other excitatory agents have revealed a stereotyped neuronal correlate of the interictal spike as recorded at the cortical surface. Sustained neuronal depolarization mediated by an influx of calcium ions underlies a train of action potentials associated with sodium influx. Repolarization and usually hyperpolarization, mediated mainly by potassium, follow this sustained depolarization. The corresponding extracellular field, forming the basis of the surface EEG, shows a negative peak during calcium and sodium influx, falls back to and then below baseline during hyperpolarization, and finally returns gradually to baseline.
The PDS is a model based on intracellular and extracellular single-cell recording. [6] In vivo, however, neuronal networks in hippocampus and neocortex are critical to production of both interictal and ictal discharges. [7] A neuronal network involving thalamus as well as cortex is responsible for producing the generalized spike-wave complex that is the hallmark of idiopathic generalized epilepsies; this network is similar to that thought to be responsible for generating sleep spindles. A complex interaction of excitatory and inhibitory firing of thalamic reticular, thalamic relay, and neocortical pyramidal neurons generates the rhythmic burst firing underlying spike-wave complexes. [8] The slow wave of the complex is thought to represent an inhibitory event, consistent with the major clinical manifestation of arrest of activity in generalized absence seizures (see Absence Seizures).
Simultaneous scalp and intracranial EEG recording has revealed that standard electrodes record only a relatively small proportion of spikes detectable at the cortical surface. Involvement of a relatively large cortical area, 6-10 cm2, is required for spikes to be recorded at the scalp. [9, 10]
Distinction From Normal or Nonspecific Sharp Transients
Challenge for the Novice Electroencephalographer
Perhaps the greatest challenge that faces the novice electroencephalographer is to distinguish true epileptiform discharges from normal or nonspecific sharply contoured waveforms. [11, 12] The latter can be divided into physiologic transients, stereotyped normal EEG variants (see Normal EEG Variants), or nonspecific waveforms. There are also many artifacts that can be mistaken for IEDs. The advent of digital EEG has helped to interpret these waveforms in different montages and modifying recording parameters to accurately determine the true nature of the waveforms. The increasing sophistication of computer EEG analysis, capablities, and machine learning may aid in EEG interpretation that was previously unthinkable and may replicate the computerized EKG read, which was a much easier task. The Responsive Neuro Stimulation (RNS) device already uses a small computer implanted into the skull to read and identify spikes and seizures using a combination of line length and frequency algorithm in real time.
Sharply Contoured Physiologic Phenomena
These are recognized readily because of their state dependence, characteristic localization, and reactivity. [2]
Vertex waves and K-complexes
Vertex waves appear in late stage 1 sleep, persist into deeper stages, and are maximal over the vertex, with variable but usually symmetric spread to parasagittal head regions. K-complexes correspond to partial arousals and first appear in stage 2. All the sleep-related vertex phenomena can be sharply contoured, especially in children.
Positive occipital sharp transients of sleep
Positive occipital sharp transients of sleep (POSTS) appear in drowsiness and light sleep. They can occur in isolation or in 4- to 6-Hz trains. Analysis of polarity at the occipital leads shows a surface positivity that is relatively uncharacteristic of epileptiform discharges.
Mu rhythm
This central sensorimotor phenomenon is typically a rhythmic discharge at 8-11 Hz, having a comblike, sharp morphology, which transiently suppresses with active or passive movement, or with thinking about movement, of the contralateral limb. It is somewhat like alpha rhythym as it is of alpha frequency but central, and is reactive/suppressed (to motor movement as compared to eye closure). Fragments of the mu rhythm can appear at times; the characteristic morphology and frontocentral, central, or centroparietal topography aid in identification.
Normal Variants and Patterns of Uncertain Significance
Wicket spikes
These waves have an arciform appearance with a negative sharp component similar to that of mu, and usually occur in brief bursts at frequencies of 6-11 Hz. They are most common in older adults during drowsiness and light sleep. When isolated, especially in combination with nonspecific temporal slowing common in elderly patients, they can be confused easily with epileptiform discharges. However, they have symmetric upslope and downslope, do not cross the baseline, and do not significantly interrupt the background (see following image, lower tracing).
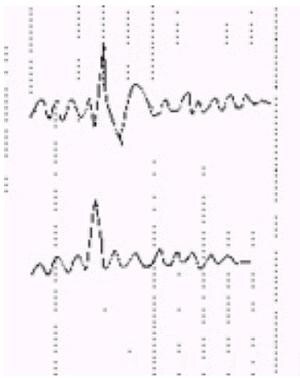 Schematic of an interictal epileptiform discharge (IED), upper tracing, vs nonspecific sharp transient, lower tracing. Note interruption of the background, asymmetric contour with descending limb falling below the baseline, and aftercoming slow wave associated with the IED; sharpness of the peak does not distinguish the 2 waveforms.
Schematic of an interictal epileptiform discharge (IED), upper tracing, vs nonspecific sharp transient, lower tracing. Note interruption of the background, asymmetric contour with descending limb falling below the baseline, and aftercoming slow wave associated with the IED; sharpness of the peak does not distinguish the 2 waveforms.
14- and 6-Hz positive spikes
These are characteristically maximal in posterior temporal areas and have a typical combination of 5- to 7-Hz and 13- to 17-Hz frequencies. The spiky portion of the waveform has a surface positive polarity. This pattern is common in adolescents and young adults and appears in drowsiness and light sleep.
Rhythmic temporal theta bursts of drowsiness (psychomotor variant)
These notched or sharply contoured 5- to 6.5-Hz waves occur in bursts or runs, appear in early drowsiness, and usually disappear in stage 2 sleep. They often shift sides during a given recording. Since they may last more than 10 seconds, they can be confused with ictal as well as interictal discharges. The older term psychomotor was used in the past as a synonym for complex partial seziures (which are now called focal impaired aware), but the psychomotor variant had no clear association with seizures, thus is a pattern of uncertain clinical significance.
Subclinical rhythmic epileptiform discharge of adults
Subclinical rhythmic epileptiform discharge of adults (SREDA) is an unusual pattern somewhat similar to rhythmic temporal theta bursts but is maximal over parietal regions and mimics an ictal discharge; it may be preceded by what appear to be isolated sharp waves in the same region. SREDA has no clinical correlation and typically is recorded in older patients without seizures.
"Phantom" spike-waves
The classic form has very small, often surface-positive, spike components linked to 6-Hz theta waves, and a posterior or ill-defined field, thus the name "phantom" thought to be benign variant.
Benign epileptiform transients of sleep (small sharp sleep)
The name benign epileptiform transients of sleep (BETS) belies its clinical signifance. Seen in temporal regions during drowsiness or sleep as small spikes/discharges without slow wave; similar discharges can be associated with epilepsy when they are frequent, consistently unilateral, or associated with focal slowing. [3] They are also called small sharp sleep (SSS), so the hallmark is that they are small, do not have an aftercoming slow wave, and are seen only in sleep.
Nonspecific Sharp Transients
The EEG consists of a spectrum of frequencies whose relative power is varying constantly. Sharply contoured waves can occur as a result of random superimposition of different frequency waves. [12] As noted above in relation to wickets, nonspecific sharp transients lack the following characteristics of IEDs:
-
Interruption of background true epileptiform discharges (see following image) - Rhythms
 Schematic of an interictal epileptiform discharge (IED), upper tracing, vs nonspecific sharp transient, lower tracing. Note interruption of the background, asymmetric contour with descending limb falling below the baseline, and aftercoming slow wave associated with the IED; sharpness of the peak does not distinguish the 2 waveforms.
Schematic of an interictal epileptiform discharge (IED), upper tracing, vs nonspecific sharp transient, lower tracing. Note interruption of the background, asymmetric contour with descending limb falling below the baseline, and aftercoming slow wave associated with the IED; sharpness of the peak does not distinguish the 2 waveforms.
-
Asymmetric upstroke and downstroke, with downstroke usually being less steep and descending below the baseline
-
Aftercoming slow wave
In general, interpretation of any single waveform should be conservative; if truly pathological, it generally will recur during the recording, especially if sleep is included. As with all EEG interpretation, it should incorporate the patient's history, the all important clinical correlation.
Localization and Clinical Significance of IEDs
Partial Versus Generalized Seizures
The fundamental distinction between partial (now called focal) and generalized seizures is reflected in the dichotomy of focal and generalized ictal and interictal discharges. Since recording seizures is rare in routine clinical practice, interictal discharges, along with the history, form the basis of distinguishing seizure types and their corresponding epileptic syndromes. If needed, prolonged recordings such as video EEG, which could span several days, can provide both interictal and ictal data. Specifically, the distinction between complex partial (focal impaired aware) and absence seizures, or between primarily and secondarily generalized tonic-clonic seizures, often depends on determining whether interictal discharges are focal or generalized. Some patients have mixed seizure disorder, such as Lennox-Gastaut syndrome, and have both partial and primary generalized seizures.
Focal Discharges
Focal discharges manifest as sharp waves or spikes (see following image); aftercoming slow waves help to distinguish these from nonspecific transients, but are not as prominent as in spike-wave complexes. Localization is usually straightforward from recordings made using the 10-20 system, but supplementary electrodes are often useful, especially T1 and T2 electrodes. Computerized mapping techniques continue to advance and may be helpful in specific cases. [13, 14] Descriptions of discharges in typical locations follow. [3]
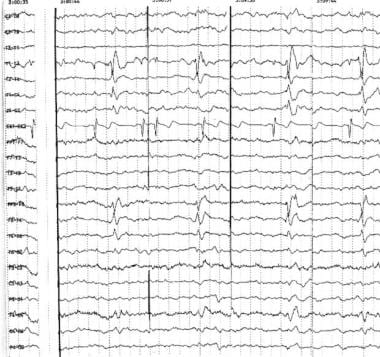 Right anterior temporal spikes. Note the typical contour and well-defined field of the interictal epileptiform discharges (IEDs) in each segment, detected at different times during long-term monitoring. Phase reversals are noted at T2 in the coronal montage (top 7 channels) and at F8 in the longitudinal bipolar derivations (below EKG channel 8).
Right anterior temporal spikes. Note the typical contour and well-defined field of the interictal epileptiform discharges (IEDs) in each segment, detected at different times during long-term monitoring. Phase reversals are noted at T2 in the coronal montage (top 7 channels) and at F8 in the longitudinal bipolar derivations (below EKG channel 8).
Temporal
The temporal lobe is the most common location of partial seizure generation and of interictal discharges. An electrical field's maximum at F7/F8 usually indicates anterior temporal location, especially if T3/T4 (T7/T8 in the new terminology) also is involved significantly, while maxima at T3/T4 indicate mid-temporal, and T5/T6 (P7/P8), posterior temporal, locations. Supplementary T1/T2 (ie, true anterior temporal) (see above image), zygomatic surface electrodes, or sphenoidal leads, can be more sensitive in recording anterior or mesial temporal discharges. T1 and T2 are very useful in prolonged video EEG recordings. Sphenoidal leads are slightly invasive but are useful in long-term video EEG monitoring of temporal lobe epilepsy where surgical decisions are in play. Nasopharyngeal electrodes are uncomfortable and prone to artifact, and they are not used routinely. Foramen Ovale electrodes require fluorscopy. However, they are invasive as well and their use is not very common.
Frontal
These may show maxima at frontopolar (Fp1/Fp2), dorsolateral frontal (F3/F4, possibly with spread to C3/C4), orbitofrontal (F7/F8, usually with frontopolar spread), or mesial (Fz-Cz) locations. Seizures arising at these different locations have different clinical characteristics (see Frontal Lobe Epilepsy). So-called secondary bilateral synchrony may occur, in which case distinction from generalized discharges is difficult, although a persistent asymmetry of amplitude and side of onset, as well as ipsilateral focal slowing, can be helpful in identifying the epileptogenic frontal lobe.
Parietal
P3/P4, possibly with central spread, is a less common location for seizure generation or interictal spikes.
Occipital
Discharges maximal at O1/O2 are tied less strongly to clinical epilepsy than discharges at other locations, especially in children. The special circumstance of "needle-like occipital spikes of the blind," occurring with congenital blindness in early childhood, has no relation to seizures.
Lateralized
Sometimes discharges are widespread over one hemisphere; if they are large, there is some volume conduction of the waveform to the other side.
Multifocal
Typically, this term refers to independent discharges occurring in each hemisphere, arising from at least 3 distinct locations that are separated by more than one interelectrode distance.
Generalized Discharges
Generalized discharges usually take the form of spike-wave or polyspike-wave complexes, and involve both hemispheres more or less symmetrically, most often with a midfrontal maximum at Fz, F3, and F4 (see following image). [3] Exceptions exist, with frontopolar or occasionally posterior maxima. Although these discharges are usually bilaterally synchronous and symmetric, amplitude maxima may shift from side to side within the same record or in serial recordings in the same patient.
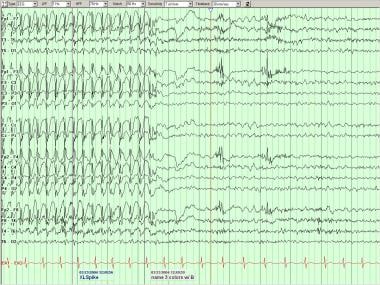 Generalized spike-wave complexes in a patient with idiopathic generalized epilepsy. Note phase reversals at F3 and F4 of both the spike and slow wave components.
Generalized spike-wave complexes in a patient with idiopathic generalized epilepsy. Note phase reversals at F3 and F4 of both the spike and slow wave components.
Frequency of the spike-wave bursts can be approximately 3 Hz (classical or typical), 4-6 Hz (atypical), [15] or less than 2.5 Hz (slow). Both typical and atypical discharges occur in idiopathic/genetic syndromes, and slow spike-wave complexes usually are seen in symptomatic or cryptogenic epilepsies, in which epilepsy results from a widespread insult to the central nervous system. Those interpreting the results should recognize that, during sleep, even classic or atypical spike-wave complexes usually become slower (often less than 2 Hz), occur in isolation rather than in bursts, and can include polyspikes.
Distinction From Ictal Discharges
An unsettled controversy in EEG concerns whether IEDs are fundamentally distinct from ictal discharges or in fact represent just very brief seizures. The answer to this question may vary with seizure and syndrome type. Sometimes, it is difficult to call the EEG activity interictal versus ictal.
Focal ictal vs interictal patterns
Although experimental evidence exists indicating that focal neurological dysfunction corresponds to isolated interictal discharges, EEG partial seizure patterns rarely resemble repetitive spikes or sharp waves. Partial/focal seizures typically show a complex evolution, sometimes beginning with repetitive activity in the alpha or beta band, followed by slowing to the theta and then delta range as the discharge increases in amplitude and spreads topographically. [3] Furthermore, seizures typically are followed, but not preceded, by an increase in IED frequency that lasts for hours to days. [16, 17] A good EEG technician can assess the patient during the EEG changes; with video EEG, the patients clinical state may help in determining the interictal or ictal nature.
Some fMRI studies have shown that blood volume (and by implication, blood flow and metabolism) may either increase or decrease in association with isolated IEDs, whereas focal ictal discharges are associated with increased blood flow and metabolism and is the basis of ictal single photon emission computerized tomography (SPECT) studies. [18, 19] Ictal SPECT, when subtracted from iterictal SPECT, can provide subtractcon ictctal SPECT coregistered on MRI (SISCOM), which has been a very useful study in localizing seizure-onset zone.
Periodic discharges
Repetitive IEDs generally indicate a higher risk of seizures than sporadic discharges. When continuous or nearly so, that is, without intervening background activity, such patterns constitute electrographic seizures. When a more distinct interval is noted between discharges (see following image), the pattern is termed periodic (see Focal EEG Waveform Abnormalities) and may constitute a transitional phase between interictal and ictal events. This transitional nature may be confirmed by clear evolution into electrographic seizures, or by gradual resolution, usually over several days. [20]
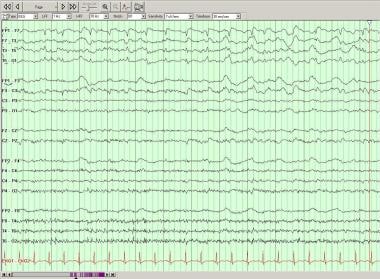 Periodic lateralized epileptiform discharges (PLEDs) over the left temporal region on this longitudinal bipolar montage.
Periodic lateralized epileptiform discharges (PLEDs) over the left temporal region on this longitudinal bipolar montage.
In cases in which associated epilepsia partialis continua is noted (see Epilepsia Partialis Continua) or with clinical signs that resolve, along with the periodic EEG pattern, shortly after administration of antiepileptic drugs, an ictal state can be assumed.
Generalized ictal vs interictal patterns
Absence: With respect to generalized spike-wave complexes, morphologies of ictal and interictal discharges are essentially identical, and the only difference is one of duration and clinical signs. Studies show that for clinical absences to be detectable, the spike-wave burst must last at least 3-5 seconds. [8] In experimental situations, however, a delay in reaction time can be observed in association with even one spike-wave complex. [21] Clearly, then, the ictal-interictal distinction is somewhat arbitrary in this instance.
Myoclonic: Myoclonic seizures are usually extremely brief, lasting substantially less than 1 second, and typically correlate with isolated polyspike-wave complexes, but can occur repetitively and evolve into generalized seizures. [15] This highlights the similarity between ictal and interictal discharges in generalized epilepsies.
Transient cognitive impairment associated with interictal discharges
As already mentioned, both generalized and focal discharges in isolation can be demonstrated to impair sensitive cognitive tasks. Left-sided discharges may affect verbal, right-sided discharges visuospatial, and posterior discharges visual function. [22, 23, 24] This transitory cognitive impairment may be related more to the aftercoming slow wave, presumably an inhibitory event, than to the discharge itself. [23] Recent evidence suggests that medications that suppress interictal discharges may improve behavior in children with mild epilepsy. [25] Valproate, lamotrigine, and levetiracetam probably suppress spikes more than carbamazepine or phenytoin; this is particularly true for generalized spike-wave discharges, which can be aggravated by carbamazepine and perhaps phenytoin. [26, 27, 28, 29, 30, 31]
Clinical Correlations
Frequency in people without seizures
By adhering to rigorously conservative criteria for interictal discharges, the specificity of EEG in the diagnosis of epilepsy in conjunction with the clinical history can be maintained at or above 90%, comparing favorably to most other tests in clinical medicine. [32] The interpretation of rarely detected IEDs in the EEGs of people without a clinical history of seizures is open to question in individual cases.
As summarized by Niedermeyer, [3] several large studies in the 1940s, especially of presumably healthy military populations, found discharges in 0.3-9%. Generalized spike-wave complexes, likely the most reliably detected abnormality, were found in 0-2.7% of patients in several studies in the following 2 decades; this finding was most frequent among younger patients, an unknown percentage of whom had a family history of epilepsy. Focal discharges were found in 0-6.4%.
Among more than 3000 children aged 6-13 years, Cavazutti et al found discharges in 3.5%, generalized in about one third. [33] Over varying follow-up periods, discharges disappeared in most, and 6% developed clinical seizures, many of whom had positive family histories.
In general, then, detection of definite epileptiform discharges, especially in patients who have had one or more suggestive clinical episodes, has a very high positive predictive value for epilepsy. IEDs also can appear in patients without epilepsy who are taking drugs that lower the seizure threshold, such as clozapine [34] ; these are not truly false positives, however, since these patients are at increased risk of having a seizure. A similar argument could be applied to family members of patients with genetic epilepsies.
Frequency in people with seizures
Perhaps an even more important clinical problem relates to the sensitivity and the negative predictive value of the test: how strongly does a normal or nonepileptiform EEG argue against the presence of epilepsy?
The percentage of patients with epilepsy who have IEDs on a routine or prolonged EEG varies in relation to whether sleep was recorded, timing of last seizure, age of patients studied, and type of epilepsy (as well as exclusion of patients with nonepileptic seizures). [35, 36] The sensitivity of a single EEG has been estimated to be in the range of 50%, though estimates have ranged from as low as 10% to as high as 77%. After one or more repeat records, at least some of which include sleep, sensitivity rises to the 80-90% range. Even after several days of continuous recording, a small minority of patients with intractable epilepsy, perhaps 2-3%, do not have IEDs detectable on surface recording. This percentage may be higher among patients with well-controlled epilepsy, who typically do not undergo this type of investigation, and in certain types of partial-onset epilepsies of extratemporal neocortical origin.
In general, IEDs are most likely to appear in the recordings of children, within hours or days of a seizure, and in association with idiopathic or symptomatic generalized epilepsies.
Activation techniques, prolonged recording, spike detection
Most study results agree that sleep recordings increase the sensitivity of EEG, and some suggest that sleep deprivation can increase the yield beyond its effect on promoting sleep. [37] The other commonly used activation procedures, hyperventilation (HV) and intermittent photic stimulation (IPS), occasionally elicit IEDs that do not appear at other times. Both HV and IPS are more effective in inducing generalized epileptiform IEDs than focal IEDs.
Since medications typically have only minor effects on IEDs, delaying or missing medications is not recommended as an activation technique, since it exposes patients to seizure risk without commensurate diagnostic benefit. In the past, some people used medications to activate IEDs; however, this practice is no longer used.
Increasing the time of recording clearly makes detection of IEDs more likely, the ultimate extension of this concept being long-term EEG monitoring (with or without video) for several days (see Ambulatory EEG and EEG Seizure Monitoring).
Computerized digital recordings allow spike and seizure detection programs. These algorithms rely on mathematical representations of IED morphology, including sharpness of the peak and interruption of background rhythms. Spike-detection programs typically are designed to overdetect possible spikes (false positive) and thus require manual screening review of the detected spikes. The study requires manual review as well because spike detection misses some spikes (false negative). Computerized ictal detection programs are also plaqued by false positives and false negatives, thus requiring manual review. Although the spike and seizure programs are continually improving, it is a complement to manual review of the study.
Responsive neurostimulation (RNS), which is an implantable device with intracranial electrodes to treat refractory seizures that uses spike and seizure detection programs, is quite a remarkable device that is already helping patients. RNS is generating incredible amounts of data and is the epitome of personalized medicine as the device's seizure detection is finetuned to each patient's seizure patterns.
Use in medical treatment decisions
As already discussed, detection of IEDs after a transient neurological event greatly increases the likelihood that a seizure was responsible; in most cases, IEDs can be classified as generalized or focal, providing valuable information with respect to syndrome classification and treatment. In the case of a single unprovoked seizure, the risk of recurrence is approximately 20-80% depending on whether the cause is cryptogenic or symptomatic. This risk is increased by a history of previous neurological insult, especially if accompanied by an acute symptomatic seizure and by detection of IEDs, especially in children. [38, 39]
EEG also can contribute to answering the reverse question, ie, whether medications should be stopped after a 2-year or longer period of seizure freedom after the diagnosis of epilepsy is established. [40] For patients with idiopathic generalized epilepsy, EEGs tend to normalize when complete seizure control is attained, and lack of IEDs suggests a decreased risk of relapse when medications are withdrawn. However, the type of idiopathic epilepsy syndrome is most important in predicting the chance for remission (eg, good for childhood absence and poor for juvenile myoclonic epilepsy). For patients with partial epilepsy, or in whom IEDs were not seen before treatment, the value of a negative study is less clear.
Although the utility of following EEG results in patients with epilepsy has not been established, some evidence suggests that discharge frequency does reflect seizure frequency and epilepsy duration. [41]
Use in epilepsy surgery
In patients considered for surgical treatment of intractable epilepsy, a consistently well-localized IED provides extremely valuable localizing information, especially if neuroimaging shows a potential epileptogenic lesion, including mesial temporal sclerosis. Occasionally during video EEG, interictal data may be more plentiful than ictal recording(s) for localizing the epileptogenic region, although the ictal recordings are the criterion standard. The role of newer techniques for identifying IEDS, such as EEG-fMRI and MEG, remains to be established. [42, 43, 44] . As electrical fields also generate magnetic fields, one can record EEG and MEG spikes. The MEG source analysis (solving the inverse problem) when merged with MRI is called magnetic source imaging, which gives a reasonable localization of the IED; however, again, for surgical planning the ictal recordings are usually most informative. Like MEG source analysis, EEG source analysis can be done with much cheaper equipment setup but is limited to interictal spikes as opposed to ictal recordings.
When patients require invasive recording using depth or subdural electrodes, ictal onsets are of more value, since IEDs often are seen at multiple locations, including some that are remote from the site of seizure onset. Intracranial recording performed intraoperatively, termed electrocorticography (ECoG), sometimes is used to tailor lesionectomies or temporal lobectomies, although its value is not established. Consensus is growing that sporadic spikes are not indications for resection of the underlying cortex, whereas repetitive spikes are (see following image).
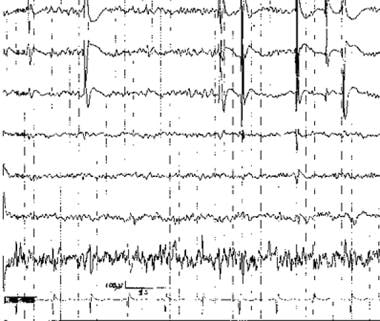 Irregularly repetitive spikes recorded at the distal end of a subtemporal strip (upper channels) during intraoperative electrocorticography (ECoG). Channel above ECG has artifact from poor electrode contact.
Irregularly repetitive spikes recorded at the distal end of a subtemporal strip (upper channels) during intraoperative electrocorticography (ECoG). Channel above ECG has artifact from poor electrode contact.
After surgery, detection of IEDs on routine follow-up studies can suggest an increased risk of relapse, [45, 46, 47] and may influence decisions concerning whether and when to withdraw medications. For those not amenable to rescetive sugery, the RNS device depth or strip electrodes are used in seizure detection and treatment.
Conclusions
As some spikes and sharps are cleary epileptiform while others are not, correct interpretation can be difficult and takes an experienced EEGer to call the activity correctly and then the clinical correlation by the clinician. Computerized spike and seizure programs are availble to interepret massive data, but also used to detect and treat seizures with the RNS device. Epileptiform discharges on EEG are transients, which is of fundamental importance in understanding the physiology of epilepsy, and in its diagnosis, classification, prognosis, and treatment.
-
Schematic of an interictal epileptiform discharge (IED), upper tracing, vs nonspecific sharp transient, lower tracing. Note interruption of the background, asymmetric contour with descending limb falling below the baseline, and aftercoming slow wave associated with the IED; sharpness of the peak does not distinguish the 2 waveforms.
-
Right anterior temporal spikes. Note the typical contour and well-defined field of the interictal epileptiform discharges (IEDs) in each segment, detected at different times during long-term monitoring. Phase reversals are noted at T2 in the coronal montage (top 7 channels) and at F8 in the longitudinal bipolar derivations (below EKG channel 8).
-
Generalized spike-wave complexes in a patient with idiopathic generalized epilepsy. Note phase reversals at F3 and F4 of both the spike and slow wave components.
-
Periodic lateralized epileptiform discharges (PLEDs) over the left temporal region on this longitudinal bipolar montage.
-
Irregularly repetitive spikes recorded at the distal end of a subtemporal strip (upper channels) during intraoperative electrocorticography (ECoG). Channel above ECG has artifact from poor electrode contact.