Overview
For some time, electroencephalography (EEG) has been employed clinically as a measure of brain function in the hope of determining and differentiating certain functional conditions of the brain. It is used in patients with cognitive dysfunction involving either a general decline of overall brain function or a localized or lateralized deficit. This article primarily addresses the clinical use of EEG in the evaluation of dementias and encephalopathies. In addition, aspects of digital EEG and other newer developments are discussed briefly.
Definition of dementia
Criteria from Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) should be used in the diagnosis of dementia. Clinical dementia is a fairly broad-based decline of brain function, and most definitions center on the patient's intellectual decline and memory dysfunction. This is, however, a fairly simplistic approach, in that dementia encompasses much more than these fundamental deficits. Many dementias have specific distinguishing features.
The process that constitutes normal aging is still an ongoing debate. As our understanding and testing procedures develop, more people are being classified as suffering from some type of dementia.
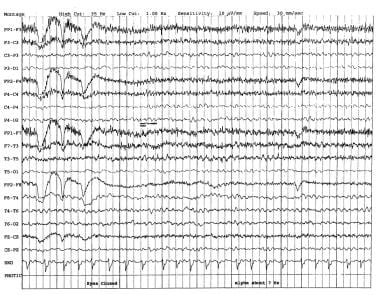
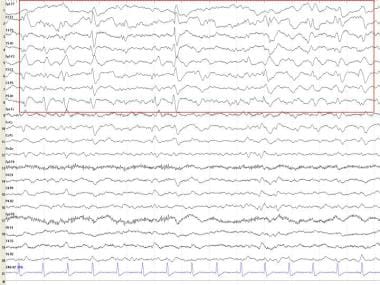
In 1998, Widagdo et al performed a quantitative EEG (QEEG) study of age-related changes during cognitive tasks. [1] This study revealed no conclusive differences between the young and the elderly. Cognitive decline, unlike normal aging, is associated with alterations in the temporospatial characteristics of EEG. The diagnosis of the initial stages of dementia is based mainly on neuropsychological testing and clinical suspicion. The EEG findings are nonspecific (see the image below).
EEG findings in dementia
In early dementia, the resting alpha frequency declines. Most authors agree that the lower limit of normal alpha frequency is 8 Hz (cycles per second). Medications can slow the posterior dominant rhythm; therefore, medication effect should always be excluded. In assessing the frequency of the alpha rhythm, alerting maneuvers are essential in order to ensure that the patient is in the best awake state and not drowsy. Computerized methods, such as EEG spectral analysis [2] , coherence, and complexity (ie, correlation dimension), have been demonstrated to correspond to cognitive function. [3]
Stevens et al recorded EEGs during 2 resting conditions (eyes closed and eyes opened) and 2 tasks (mental arithmetic and a lexical decision), with the aim of determining which temporal and spatial EEG descriptors change with cognitive decline and normal aging. [4] The EEGs were analyzed by using EEG microstates. The primary findings were a significant increase in the number of ultrashort EEG microstates and a reduction in the average duration of EEG microstates in cognitively impaired and demented patients.
Cognitive impairment was associated with a reduction or loss of EEG reactivity. [4] In contrast, no alterations in temporal or spatial EEG descriptors were found in normal aging. Cognitive tasks did not add to the information already obtained during the resting states. The reduction in EEG microstate duration correlated with loss of cognitive function.
Therefore, temporospatial analysis of the EEG record is a useful indicator of cortical dysfunction in dementia and correlates with the degree of cognitive impairment. Apparently, temporospatial analysis may be useful in distinguishing patients with dementia from those experiencing normal aging. Whether these data contribute significant additional information to the clinical data in evaluating dementia is unclear.
Definition of encephalopathy
Encephalopathy represents a brain state in which normal functioning of the brain is disturbed temporarily or permanently. Encephalopathy encompasses a number of conditions that lead to cognitive dysfunction. Some of these conditions are multifactorial, and some have an established cause, such as hepatic or uremic encephalopathy. Because the EEG patterns in most dementias and encephalopathies demonstrate few specific features, they are discussed together.
Some notable exceptions include Creutzfeldt-Jakob disease (CJD) and subacute sclerosing panencephalitis (SSPE); however, no specific patterns exist for most dementias and encephalopathies. Other conditions, such as hepatic and renal encephalopathies, carry distinguishing features; nevertheless, similar patterns may be seen in a fairly wide range of illnesses under certain conditions.
EEG findings in encephalopathy
In general, the most prominent feature of the EEG record in encephalopathies (if there is a change) is slowing of the normal background frequency. Over the course of the disease if serial EEGs are performed, disorganization of the record may develop gradually. Reactivity to photic or other type of external stimulation may be altered. If a QEEG is performed, it may show a frequency shift or decreased interhemispheric coherence of background frequencies. Some conditions are associated with an increase in seizure frequency, and in such cases, epileptic activity may be recorded. [5]
In a given context, EEG can play a clinically useful role, especially because functional MRI, positron emission tomography (PET), and single-photon emission computed tomography (SPECT) are either still in an experimental stage or require special settings that are not widely available.
Use of digital EEG data
Although the following sections frequently cite digital EEG data, these data primarily represent digital analysis of clinical EEG recordings. The referenced data are presumed to be based on an EEG recording that is read by a clinician; presently, recording is done with computerized technology for ease and also for availability for further analysis. Various mathematical transforms are available after the initial clinical interpretation (eg, coherence, Fourier transform, wavelets, and microstates; see Digital EEG). These allow further comparisons with norms and control groups but should be interpreted in conjunction with the primary EEG reading.
Dementia
Alzheimer disease
Electroencephalography (EEG) is the only clinical diagnostic instrument that directly reflects cortical neuronal functioning. Although the EEG may be normal or minimally disturbed in a number of patients in the initial stages of Alzheimer disease (AD), an abnormal EEG usually is recorded later in the course. A large percentage of patients with moderately severe to severe AD exhibit abnormal EEGs.
In 1981, Stigsby reported diffuse increases of delta and theta frequencies in AD, as well as decreases in the alpha and beta frequency ranges. Frontal slowing was also seen and was more prominent anterior to the sylvian fissure, whereas decreased blood flow was more prominent posterior to the sylvian fissure. These findings may be explained by the observation that EEG reflects the functional decline of the anterior structures, whereas the flow decrease correlates more with the structural damage to the parietal lobe. Frontal slowing probably reflects the loss of functioning of the frontal cholinergic system. [6]
Wada et al showed that EEG coherence provides a measure of functional correlation between 2 EEG signals. [7] They examined intrahemispheric EEG coherence at rest and during photic stimulation in 10 patients with dementia of the Alzheimer type. In the resting EEG, patients with AD had significantly lower coherence than gender and age-matched healthy control subjects in the alpha-1, alpha-2, and beta-1 frequency bands.
EEG analysis during photic stimulation demonstrated that the patients had significantly lower coherence irrespective of the stimulus frequency. [7] The changes in coherence from the resting state to the stimulus condition showed significant group differences in the region of the brain primarily involved in visual functioning. The patients had significantly lower coherence of their EEG reactivity to photic stimulation at 5 and 15 Hz over the posterior head regions.
Thus, patients with AD may have an impairment of interhemispheric functional connectivity in both nonstimulus and stimulus conditions, which suggests a failure of normal stimulation-related brain activation in AD.
Jelic et al found a positive correlation between levels of tau protein in the cerebrospinal fluid (CSF) and the EEG alpha/delta ratio. In a subgroup with high CSF tau levels, a strong relationship between EEG alpha/theta and alpha/delta power was found. No such correlation was found in healthy controls and mildly cognitively impaired individuals with elevated CSF tau levels. [8]
Locatelli et al used EEG coherence to evaluate the functionality of cortical connections and to obtain information about synchronization of regional cortical activity in patients with suspected AD. [9] Alpha coherence was decreased significantly in 6 patients. Significant delta coherence increase was found in a few patients between frontal and posterior regions. The group with AD demonstrated a significant decrease of alpha-band coherence in the temporal-parietal-occipital areas; this was expressed to a greater extent in patients with more severe cognitive impairment.
The investigators theorized that these abnormalities could reflect 2 different pathophysiologic changes, as follows [9] :
-
The alpha coherence decrease could be related to alterations in corticocortical connections
-
The delta coherence increase suggests lack of influence of subcortical cholinergic structures on cortical electrical activity
Strik et al found that the microstates of the resting EEG of patients presenting with mild or moderately severe dementia of the Alzheimer type demonstrated a significant anteriorization of the microstate fields, and the duration of sustained microstates was reduced. [10] These differences were more important than the diffuse slowing. The measurements of microstates may be useful in the early diagnosis of AD. [10]
Muller et al conducted a study comparing single-photon emission computed tomography (SPECT) and quantitative EEG (QEEG) and concluded that whereas QEEG might be as useful as SPECT brain scanning in staging the disease, the correlation with clinical status was weak. [11]
Akrofi et al, employing an automated coherence-based pattern recognition system involving multiple discriminant analysis (MDA) and k-means clustering coherence features from EEG obtained from 56 subjects, were able to distinguish patients with AD from patients with mild cognitive impairment (MCI) and from age-matched controls. This suggests that patients with AD may have a greater number of damaged cortical neurons than patients with MCI and that MCI may be an intermediate step in the development of AD. [12]
Siennicki-Lantz et al studied the relation of cerebral white-matter lesions to AD and found that cerebral blood flow (CBF) in white matter correlated with systolic blood pressure and multichannel EEG in senile dementia of the Alzheimer type. [13]
The presence and functional significance of white-matter lesions in the aging brain or in dementia and the relation of these lesions to blood pressure are unsettled issues. White-matter lesions occur in both cerebrovascular disease and AD. Probably, the white-matter lesions in hypertensive patients are not related to AD but are simply coexisting with it. Their influence on the overall expression of the degree of dementia is unclear; however, it seems intuitively plausible that the lesions should be causing additional cognitive dysfunction.
Siennicki-Lantz et al observed significantly lower white-matter CBF (WMCBF) in patients with AD than in controls. [13] This was more obvious in the posterior cerebral region (ie, the parietal-temporal-occipital area). QEEG from the posterior cerebral regions correlated with WMCBF. Systolic blood pressure was significantly lower in the AD group and was correlated positively with WMCBF in the posterior and anterior brain regions.
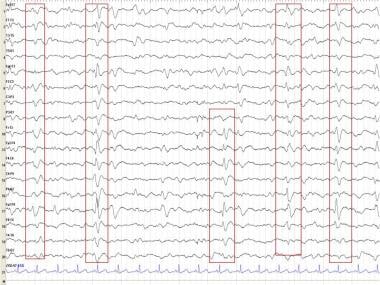
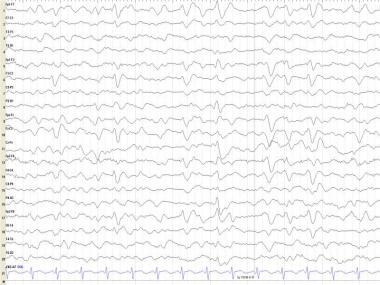
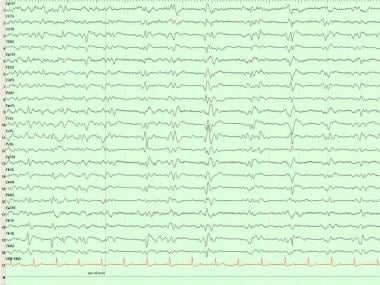
Epileptiform activity may occur more frequently in patients with AD than in the general population; clinical tonic-clonic seizures can occur. Bilateral synchronous periodic epileptiform discharges (BiPEDs) (see the first image below), such as triphasic waves (TWs) (see the second image below), may be recorded in AD, usually in the late stages (see Triphasic Waveforms).
These findings are not specific for AD because they most often are observed in metabolic disorders, particularly hepatic encephalopathy and other degenerative diseases, such as Creutzfeldt-Jakob disease (CJD). Although there is a good correlation between severity of EEG abnormalities and cognitive impairment, epileptiform discharges or TWs are not predictors of seizures. EEG often can be useful for excluding a superimposed reversible metabolic etiology of dementia and for confirming CJD when the dementia is rapidly progressive.
To investigate the relation between QEEG band powers and CBF, Rodriguez et al studied 42 patients with suspected AD and 18 healthy elderly controls and attempted to differentiate patients with AD from the controls by measuring QEEG and CBF. [14] Regional CBF and QEEG were correlated, especially in the right hemisphere. Significant correlations were found between Mini Mental State Examination (MMSE) scores and relative power of the 2- to 6-Hz and 6.5- to 12-Hz bands on either side and between MMSE scores and left regional CBF; the correlation between MMSE scores and right regional CBF was less strong.
Used together, QEEG and regional CBF had a sensitivity of 88% and a specificity of 89%, with a total accuracy of 88.3%. [14] . QEEG alone showed an accuracy of 77% in the whole group and 69% in those with mild AD; regional CBF alone had an accuracy of 75% in the whole group and 71% in those with mild AD. This study suggests that QEEG and regional CBF measurements, when used together, are reasonably accurate in differentiating AD from healthy aging.
Scheriter et al used clinical examinations, QEEG, neuropsychological testing and neuroimaging to see if distinctions could be made between patients with AD, mixed dementia (vascular), and controls; they found that as would be expected, patients with mixed dementias had more subcortical lesions with increased slow frequency power, which suggested subcortical pathology. [15]
The QEEG high-frequency power was normal in mixed dementia and decreased in AD, probably reflecting the cortical pathology seen in AD. [15] Hachinski scores and neuropsychological testing showed little difference between mixed dementia and AD. QEEG and neuroimaging may be of great use in diagnosing and differentiating these dementia types.
A study that presented a frequency band analysis of AD EEG signals suggested that optimized frequency bands may improve existing EEG-based diagnostic tools for AD, though additional testing on larger AD datasets will be required to verify the effectiveness of the proposed approach. [16]
Oscillatory brain dynamics in AD appear to differ according to age at onset. Young AD patients present with more severe slowing of spontaneous oscillatory activity than old AD patients, which is most pronounced in the posterior brain areas. This finding supports the hypothesis that early-onset AD presents with a distinct endophenotype. [17]
The apolipoprotein E (ApoE) sigma-4 allele is a risk factor for late-onset AD and may have an impact on cholinergic function in AD. Because the cholinergic system has an important role in modulating EEG, impairment of this system may have some relation to the EEG slowing that is characteristic of AD progression.
Lehtovirta et al studied the relation of ApoE to EEG changes. [18, 19] The QEEG of 31 patients with AD was recorded at the early stage of the disease and after a 3-year follow-up. Patients with AD were divided into several subgroups according to the ApoE sigma-4 allele (ie, 2 sigma-4, 1 sigma-4, and 0 sigma-4). These subgroups did not differ in clinical severity or duration of dementia.
The AD patients carrying the sigma-4 allele had more pronounced slow-wave activity than AD patients without the sigma-4 allele, although the disease progression rate did not change. [18, 19] These differences in EEG may suggest differences in the degree of the cholinergic deficit in these subgroups.
The typical electrophysiologic correlates of myoclonus in AD are similar to those of cortical reflex myoclonus, with a focal, contralateral negativity in the EEG preceding the myoclonic jerk. The electrophysiologic correlate of polymyoclonus that can be seen in AD and other pathologic states is a bifrontal negativity in the EEG that precedes the myoclonic jerk. This new type of electrophysiologic correlate of myoclonus may reflect activity of a subcortical generator.
Dementia with Lewy bodies
In a study comparing patients with dementia with Lewy bodies (DLB) and patients with AD, Briel et al found that 17 of the total 19 records from the patients with DLB) were abnormal. [20] Thirteen showed loss of alpha activity as the dominant rhythm, and half had slow wave transient activity in the temporal lobe areas. This slow wave transient activity correlated with a clinical history of loss of consciousness. The patients with AD were less likely to show transient slow waves and tended to have less marked slowing of dominant rhythm.
The greater slowing of the EEG in DLB than in AD may be related to a greater loss of choline acetyltransferase found in DLB. Temporal slow wave transients may be a useful diagnostic feature in DLB and may help to explain the transient disturbance of consciousness, which is characteristic of DLB. [21, 22]
Pick disease
Pick disease, which is a frontotemporal dementia, is much less common than AD. The age of onset is earlier than that of AD. The EEG is less abnormal than in AD, especially in the early stages. Posterior alpha rhythm is more preserved. Theta and delta are increased. Frequency analysis may demonstrate a difference at a time when simple visual reading may not pick up a clear abnormality. The major feature of Pick disease is a decline in judgment and insight with relative early preservation of memory.
Because EEG correlates poorly with the clinical symptoms, impressive EEG changes are not observed in this condition. Blood flow measurements correlate with thinking processes; Ingvar demonstrated these changes in 1977. [23] Stigsby demonstrated a decrease in anterior blood flow in patients with Pick disease. [6] Because the anterior cholinergic system is relatively preserved in Pick disease, the EEG changes are not prominent frontally.
Gemignani et al studied sleep in Pick disease with a longitudinal polysomnographic and fluorodeoxyglucose positron emission tomography (FDG-PET) study, [24] documenting sleep fragmentation, short rapid-eye-movement (REM) latency, and a severe reduction of slow wave sleep, with relatively preserved non-REM (NREM)-REM sleep cycles. PET scan revealed severe cerebral glucose metabolic reductions in the frontal and temporal areas.
Postmortem study showed severe neuronal loss, spongiosis, and gliosis most marked in cortical layers I, II, V, and VI. [24] In vivo, neurometabolic and postmortem neuropathologic data are consistent with and indicative of a severe dysfunction of intra- and transhemispheric regional connectivity and of corticothalamic circuits. These findings suggest that the decreased cortical and subcortical connectivity may have been the main pathophysiologic mechanism responsible for delta sleep reduction and the cognitive decline.
Huntington disease
Huntington disease is a genetic condition characterized by movement disorder (primarily chorea), cognitive impairment, and psychotic features. The degree of such symptoms varies widely. The EEG changes show gradual and progressive slowing over time. The amplitude also attenuates as the disease progresses. About 30% of the patients have very-low-voltage EEGs, with amplitudes below 10 μV. Hyperventilation as a rule does not increase the background voltage as it usually does in healthy subjects. About 3% of the patients show epileptiform activity; they tend to be juvenile cases.
The EEG has not been proven to be of any predictive value in identifying future affected family members. Genetic testing is far more useful.
Progressive supranuclear palsy
Progressive supranuclear palsy (PSP) causes decreased ocular motility, rigidity, dementia, impaired postural reflexes, and, histologically, midbrain atrophy and abnormal tau deposition. Usually, the degree of dementia is not severe. The EEG in PSP may initially be normal but eventually shows increasing delta and theta activity, as was the most common finding reported by Fowler and Harrison in 1986. These authors found that the delta often was rhythmic with frontal accentuation.
Gross et al found a decrease in background frequency of 6-7 Hz and delta activity over the temporal regions. [25] Su and Goldstein et al found initial EEG patterns to be normal in 8 of 12 (67%) of patients with PSP. [26] With disease progression, they found background slowing and frontal intermittent rhythmic delta activity (FIRDA) (see below) in this population.
Through the use of QEEG recordings in 6 patients with PSP compared with controls, Montplaisir et al found slowing over the frontal lobes in the waking state, with neuropsychological testing confirming this frontal lobe dysfunction. Abnormalities of sleep architecture with REM sleep abnormalities were seen as well.
Corticobasal degeneration
Corticobasal degeneration (CBD) is a neurodegenerative disorder and tauopathy characterized by progressive dementia and asymmetrical rigidity and limb apraxia. Tashiro et al found prominent focal slowing on EEG in the anterior and temporal head regions in early CBD in 8 of 10 patients studied. [27] Frontal intermittent rhythmic delta activity was also observed but was not found to be specific to CBD.
Roche et al evaluated 5 patients with CBD, of whom none had REM behavioral disorder (as is often seen in many neurodegenerative disorders) or excessive daytime sleepiness. All 5 patients with CBD had insomnia, 4 had periodic limb movements or restless legs syndrome, and 2 had sleep respiratory disorders. [28]
Parkinson disease
The EEG is frequently normal in Parkinson disease (PD). In advanced cases, however, marked slowing is noted. Sleep may be markedly abnormal with frequent awakenings, prolonged sleep latency, reduced REM sleep, periodic leg movements and increased frequency of REM behavioral disorder.
Wszolek et al studied patients with rapidly progressive familial parkinsonism and dementia with pallidopontonigral degeneration (PPND). [29] The patients had PPND linked to chromosome 17q21-22; 11 EEGs of 9 patients were studied. EEGs revealed normal findings early in the disease and diffuse slowing that became more prominent with disease progression.
Serizawa et al, using QEEG to compare PD patients with age-adjusted controls, also found diffuse slowing in the patients with PD. [30] Electromyography (EMG) and nerve conduction studies (NCSs) showed no abnormalities. Visual evoked potentials (VEPs) and sensory evoked potentials (SEPs) were normal. The clinical neurophysiologic study findings were consistent with a cortical and subcortical degenerative process.
With clinical deterioration, progressive decline is seen in the mean parietal frequency and background rhythms. Theta and theta-delta mixture may be recorded bilaterally in the posterior head regions. After stereotactic surgery, focal theta or delta slowing may be observed.
Korsakov syndrome
Obraztsova et al, in a study involving 32 patients (21 with reversible and 11 with chronic Korsakov syndrome of traumatic origin) and 20 healthy controls, found that EEG beta activity (13-20 Hz) in the frontobasal and brainstem locations had negative prognostic significance in Korsakov syndrome. Most typically, patients with Korsakov syndrome have abnormal EEGs with slowing in the theta and delta frequencies. [31]
Vascular Dementia
Binswanger disease
Binswanger disease usually demonstrates slowing of background and a nonspecific pattern. Kuroda et al reported other patterns, describing a 72-year-old patient with von Recklinghausen disease who exhibited akinetic mutism within 6 months of the onset of dementia. Encephalography (EEG) demonstrated periodic synchronous discharges (PSDs), suggesting Creutzfeldt-Jakob disease (CJD). Computed tomography (CT) of the brain identified diffuse cerebral atrophy. Autopsy findings revealed diffuse subcortical white matter disease and marked arteriosclerotic changes of the subcortical arterioles. [32]
The cortex was relatively spared, and the pathologic diagnosis confirmed Binswanger disease. Binswanger disease, therefore, can present with PSDs and should be included in the differential diagnosis of dementia. On the other hand, Dzialek et al described a group of 15 patients with Binswanger subcortical atherosclerotic encephalopathy who showed a different EEG appearance. The EEG records were pathological in most cases, with varying degrees of slow activity that was distributed symmetrically. [33]
Circulatory encephalopathy
Atherosclerosis
Plachinda et al studied the correlations of cognitive disorders and the EEGs of elderly patients with circulatory encephalopathy. They explored the possibilities of using EEG for evaluating intellectual-mnemonic disorders in elderly patients with cerebral atherosclerosis. Ninety-five patients (aged 60-74 y) with atherosclerotic encephalopathy but without stroke were included in the study. Statistical analysis of the data demonstrated a correlation between psychological test results and EEG readings and computerized EEG data. [34]
In cerebrovascular disease, focal slowing is far more frequent than in nonvascular dementia; therefore, EEG can be useful in distinguishing the 2 conditions.
Multi-infarct dementia
No specific EEG pattern is associated with multi-infarct dementia. Some background slowing may be observed, especially in advanced disease. These changes are less prominent and do not show the progressive course observed in Alzheimer disease (AD).
Iznak et al used quantitative EEG (QEEG) to reveal the specific features of and study amplitude-frequency parameters in patients with mild dementia of different origins compared with healthy elderly individuals. [35] They found that alpha rhythm was suppressed in AD and vascular dementia and that alpha rhythm was slower and theta activity higher in AD. Patients with AD were characterized by desynchronized EEG.
Transient global amnesia
A variety of records have been reported from normal to even epileptiform potentials in transient global amnesia (TGA). Nonepileptiform activity, such as bitemporal delta or bioccipital theta, has been reported. Kushner described patients with normal activity, one with occasional epileptic activity, and one with asymmetric alpha depression, while 2 patients had intermittent rhythmic slowing. [36] TGA caused by a seizure is uncommon, and is believed to be caused by a vascular etiology or spreading depression.
Hereditary Encephalopathies
Action myoclonus
Action myoclonus consists of arrhythmic muscular jerking induced by voluntary movement. It can be made worse by attempts at precise or coordinated movement (ie, intention myoclonus) and may be elicited by sensory stimuli. The effective stimulus for action myoclonus is thought to be feedback from muscle afferents, although it may be related to activity in the motor cortex relayed to the reticular formation preceding or coinciding with voluntary movement.
The condition usually is associated with diffuse neuronal diseases, such as posthypoxic encephalopathy, uremia, and the various forms of peripheral neuroepithelioma, although action myoclonus may be limited to one limb in some cases of focal cerebral damage. It is caused by hyperexcitability of the sensorimotor cortex (ie, cortical reflex myoclonus) or reticular formation (ie, reticular reflex myoclonus), or both. Autopsied cases have failed to reveal a clear pathology. Theories include loss of inhibitory mechanisms involving serotonin and possibly gamma-aminobutyric acid (GABA) transmitters.
Myoclonus may be seen in degenerative disorders of the nervous system. It may be associated with tonic-clonic seizures or dementia. Myoclonus has been described in cases with Lafora inclusion bodies and cerebral storage diseases, as well as system degenerations: cerebellodentatorubral, pyramidal, extrapyramidal, optic, auditory, posterior columns and gracile and cuneate nuclei, spinocerebellar pathways, motor neurons of cranial nerves and anterior horns, and muscle fibers.
Action myoclonus usually responds to sodium valproate or clonazepam, and some patients with posthypoxic action myoclonus may improve with serotonin precursors.
Generalized myoclonus
Generalized myoclonus in comatose survivors after CPR implies a poor prognosis despite improvement of the critical care of patients. [37] This transient, self-limited phenomenon reflects dysfunction of lethally damaged neurons. Propofol often controls myoclonus but does not change the underlying condition. Clinical, pathologic, and electroencephalographic (EEG) findings indicate that these patients die of severe hypoxic-ischemic damage. Abnormal EEG patterns, especially burst suppression EEG (BS-EEG) and alpha-coma EEG, are seen in these patients. The EEG abnormalities include BS-EEG, generalized epileptiform discharges, alpha-coma EEG. [38]
Unverricht-Lundborg disease
Unverricht-Lundborg disease (ULD) (ie, Baltic myoclonus) is an autosomal recessive progressive myoclonic epilepsy syndrome. ULD is found sporadically worldwide, but is common in Finland. The myoclonus is severe and generalized seizures occur that are difficult to control. Progressive background slowing, generalized spike and wave and polyspike and wave complexes, and focal occipital spikes are found on EEG. [39]
Mitochondrial encephalopathy with lactic acidosis and stroke (MELAS) and myoclonus epilepsy with ragged red fibers (MERRF)
Isozumi et al described a 50-year-old woman with subacute dementia and myoclonus in whom computed tomography (CT) revealed brain atrophy and EEG revealed periodic synchronous discharges (PSDs). She initially was thought to be suffering from Creutzfeldt-Jakob disease (CJD) but dramatically recovered over 5 months. On the basis of further investigations, the final diagnosis was mitochondrial encephalomyopathy. In general, the EEG changes were described as background slowing, multifocal epileptiform discharges, and photosensitivity.
Post stereotactic surgery
Patients developed EEG slowing of different degrees about 50% of the time.
Alpers disease
Alpers disease, an autosomal recessive inherited disorder consisting of progressive neuronal degeneration of childhood with liver disease, has been studied by Boyd et al. [40] The onset is in early childhood and consists of intractable fits, progressive dementia, and brain atrophy. EEG studies have been carried out on 12 children with this condition. The EEGs were similar and demonstrated abnormal patterns with high-amplitude slow activity as well as lower amplitude polyspikes. The flash visual evoked potential (VEP) was usually abnormal and often asymmetrical. In the appropriate clinical setting, the neurophysiologic features may aid the clinician in the diagnosis of this disease.
Adrenoleukodystrophy
Multifocal paroxysmal discharges, hypsarrhythmic pattern, and prominent arrhythmic delta are present in temporal-occipital areas. Epileptic discharges usually do not occur in adrenoleukodystrophy.
Zellweger syndrome
This syndrome is characterized by diffuse slowing.
Infantile neuroaxonal dystrophy
This condition is characterized by a high-voltage, 14- to 22-Hz activity that is not reactive to environmental stimuli.
Pantothenate kinase-associated neurodegeneration (PKAN)
In PKAN, formerly known as neurodegeneration with brain iron accumulation type I, the EEG is normal to slow.
Neuronal ceroid lipofuscinosis
In the infantile form, the EEG is slow and early, and posterior spikes may be present. Photic response is excessive and evokes high-voltage spikes that are polyphasic. The EEG abnormalities in the juvenile form are not as marked.
Gaucher disease
In patients with type III disease, posterior spikes and sharp waves, diffuse spike and waves, and photomyoclonic and photoparoxysmal responses may be present.
Metachromatic leukodystrophy
Diffuse slowing progresses to high-voltage generalized delta activity. Epileptic activity is rare; however, hypsarrhythmia may be observed.
Tay-Sachs disease
EEG is generally slow. Generalized or multifocal spikes accompany the seizures.
Rett syndrome
Rett syndrome is a slowly progressive encephalopathy that occurs only in girls and is characterized by early deterioration of higher brain function with dementia, autistic behavior, loss of purposeful use of the hands, and deceleration of head growth. Al-Mateen et al reported 15 cases of Rett syndrome. [41] When affected girls are aged 2-4 years, epilepsy may develop with minor motor seizures. Additional features may include an extrapyramidal disorder with dystonia and choreoathetosis and lactic acidemia. A precise biochemical marker of this disorder has not been identified.
According to McIntosh et al, Rett syndrome consists of a progressive encephalopathy and psychomotor deterioration in young girls who have appeared clinically normal until age 6-18 months. [42] The incidence is similar to that of phenylketonuria and autism in females. When the child is at least 6 months old, head growth decelerates in association with severe dementia, autism, apraxia, stereotypic handwashing movements, and loss of previously acquired skills. Other signs include breathing dysfunction, seizures, EEG abnormalities, and growth retardation. It appears to be sporadic in occurrence.
The EEG may demonstrate slowing, a variety of nonspecific patterns, and epileptiform discharges. The epileptic activity may include multifocal spikes, slow-wave spikes, and paroxysmal delta slowing with spikes that may appear in sleep; in certain cases, however, sleep may attenuate the EEG abnormalities. Background flattening occurs to some degree, corresponding with the stage of dementia and cognitive decline. Rolandic spikes may be elicited by noise.
Infectious Encephalopathies
Creutzfeldt-Jakob disease
In Creutzfeldt-Jakob disease (CJD), electroencephalography (EEG) shows a fairly typical repetitive pattern of bilateral synchronous periodic epileptiform discharges (BiPEDs; see the first image below) such as triphasic waves (TWs; see the second image below) approximately 1-1.5 seconds apart. These usually are present during wakefulness and disappear during sleep.
Periodic synchronous discharges (PSDs) seem to be the EEG hallmark of CJD; however, a number of atypical EEG presentations have been reported without these waveforms.
Aoki et al reported giant spikes with photic stimulation. [43] These photic-stimulated giant spikes simultaneously suppressed PSDs. Necropsy exhibited extensive gray and white matter lesions. Both lateral geniculate bodies and pregeniculate bodies were involved preferentially. The superior colliculus, optic nerve, and optic tracts were not affected. The cortices of the occipital lobes were damaged severely. The Gennari line was spared. The lesion of the lateral geniculate body appeared to be associated with the unusual EEG feature.
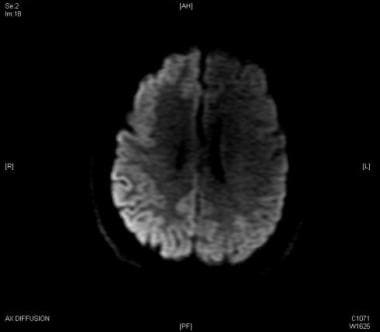
These findings indicate that the visual pathway may be involved in the generation of PSD in CJD (see the image below).
The EEG findings and the evolution of clinical signs were investigated by Hansen et al in 7 patients with CJD who underwent serial EEG recordings. [44] At the onset (mean 8.7 weeks) of periodic slow-wave complexes (PSWC), 5 patients already had progressed to akinetic mutism characterized by loss of verbal contact and movement disorders (ie, myoclonus, exaggerated startle reaction, or focal dyskinesia started in 5 patients).
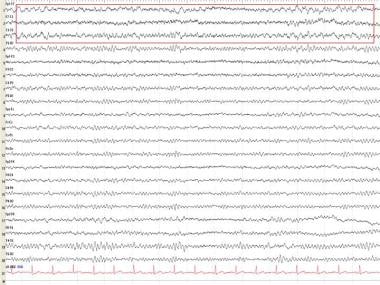
When akinetic mutism commenced (average 7.5 weeks), runs of frontal intermittent rhythmic delta activity (FIRDA), like that shown below, were found in all cases. These were later replaced by PSWC in 6 patients. Occurrence of PSWC often related negatively to external stimuli and sedative medication. [44]
These data help in the selection of EEG recording dates to detect PSWC in patients in whom CJD is suspected. The survival time is short after the onset of PSWC (average 8 weeks). In earlier disease stages, FIRDA-like EEG activities should be regarded as compatible with the diagnosis of CJD and should encourage further EEG studies for the demonstration of PSWC in a more advanced stage of CJD.
EEG characteristics of CJD and its differential diagnosis were studied by Steinhoff et al, who found some nonspecific EEG findings and also typical PSWC in the course of the disease, [45] obtaining a sensitivity of 67% and a specificity of 86%. With the exception of one familial variant of CJD, PSWC are usually absent in all other human prion diseases.
The authors presented a pathophysiologic hypothesis on the development of PSWC based on the assumption that the specific periodicity of PSWC results from a still functionally active but greatly impaired subcortical-cortical circuit of neuronal excitability. [45] They stressed the use of clinical signs, laboratory data, and EEG correlation and suggested that the clinical diagnosis of CJD should be reconsidered if repeated EEG recordings fail to reveal PSWC under technically adequate conditions. Some patients with CJD presented with visual blurring, diplopia, and visual loss—ie, the Heidenhain 5 variant.
Focal EEG abnormalities as described in the Heidenhain variant of CJD are uncommon. Lee et al reported a 73-year-old man presenting with visual symptoms, right hemianopia, and rapidly progressive dementia. Myoclonus was synchronous with generalized periodic epileptiform discharges on EEG (see the image below).
In addition, periodic focal sharp waves were present at the left occipital region. Diffusion-weighted magnetic resonance imaging (MRI) of the brain showed slightly increased signal intensity in the occipital parasagittal area, left more than right. The 14-3-3 protein was detected in the cerebrospinal fluid (CSF). The patient died within 5 months of presentation. [46]
Subacute spongiform encephalopathy
Aguglia et al described 20 patients with subacute spongiform encephalopathy and periodic paroxysmal activities in the EEG. Evolution of clinical and EEG abnormalities were analyzed in all 20 (16 pathologically confirmed). Illness duration was less than 4 months in 65% and greater than 17 months in 10%. The early clinical stage was characterized by gradual gait disturbances, mental deterioration, and sensory or autonomic changes. In 10 EEG recordings from 7 patients examined in the early clinical stage, no periodic discharges were present. [47]
Early periodic paroxysmal activity appeared within 12 weeks of the onset of the disease in 88% of the patients who underwent EEG recordings. This early periodic paroxysmal activity usually occurred at an intermediary stage, when the patients demonstrated marked worsening of the clinical picture. Focal, segmental, and/or generalized myoclonic jerks were observed in 15%, 53%, and 100% of cases at prodromal, intermediary, and terminal stages, respectively. Different kinds of periodic paroxysmal activity were observed:
-
Biphasic or triphasic periodic complexes
-
Periodic complexes with multiphasic configuration
-
Periodic polyspiking discharges
Abnormal "pacing" by slowly repeated flashes was found in 4 patients presenting with visual hallucinations or cortical blindness. Burst-suppression activity was observed frequently in the terminal stage in decorticate patients.
AIDS dementia
EEG abnormalities usually precede brain atrophy on computed tomography (CT) of the brain. Generalized or multifocal slowing may be observed. Computerized EEG is abnormal in most cases. About one half of patients who have normal neurologic findings on physical examination exhibit abnormal EEGs.
Thomas et al described a 40-year-old HIV-positive, right-handed homosexual man who was admitted for progressive mental deterioration coexisting with permanent, segmental, middle-amplitude, arrhythmic, asynchronous, and asymmetrical myoclonic jerks. EEG demonstrated frontocentral bursts of rhythmic triphasic 1.5- to 2-Hz sharp waves similar to the characteristic periodic pattern of CJD. Biological investigations were negative, thus ruling out a metabolic encephalopathy. [48]
Dramatic neurological improvement occurred shortly after initiation of intravenous and then oral zidovudine, which produced absolute EEG normalization. This unusual electroclinical presentation of the AIDS dementia complex underlines the fact that this condition presents a diagnostic challenge, particularly in individuals in whom HIV infection has not been diagnosed previously.
Canafoglia et al described a case of a HIV-seropositive patient with ataxia and upper limb rhythmic myoclonus. [49] Electromyographic (EMG) recordings of the forearm muscles correlated with frontocentral rhythmic activity on EEG. This movement disorder should be considered a rhythmic variant of cortical myoclonus. HIV infection may have caused a dysfunction in the central nervous system pathways similar to that occurring in genetically determined conditions characterized by cortical myoclonus.
Sinha et al described various electrophysiologic abnormalities in HIV encephalopathy. [50]
Polich et al found greater frontal delta power in HIV cases than in control subjects. [51]
Ferrari et al described 2 patients with HIV type 1 infection who presented new-onset epilepsia partialis continua (EPC) as an early manifestation of progressive multifocal leukoencephalopathy (PML). [52] PML represents an increasingly recognized cause of new-onset seizures in both seropositive and seronegative patients.
Diehl et al followed 117 HIV patients with EEG. Serial EEGs on 117 HIV patients without any clinical signs of secondary neuromanifestations were studied in order to document EEG changes in the course of HIV infection. Clinical signs of HIV-associated encephalopathy presented in 18 patients at the first examination and 23 at reexamination. Significant slowing of background activity occurred in the course of the disease. The results of this study indicated progressive central nervous system (CNS) dysfunction with worsening of the immunostatus. [53]
Chronic rubella encephalitis
This condition is characterized by myoclonus, mental deterioration, ataxia, and chorea, with diffuse slowing on EEG. Intermittent rhythmic delta activity (IRDA) has been described. Periodic activity with spikes and slow-wave spikes may occur.
Viral encephalitis
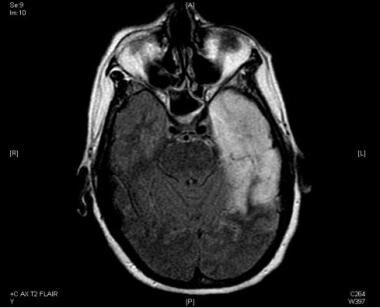
Viral encephalitis frequently causes EEG abnormalities. If the cortical gray-matter involvement is predominant, more polymorphic delta activity is observed, while with subcortical involvement, a rhythmic pattern (IRDA) is more common. In herpes simplex encephalitis (HSE; see the first image below), temporal intermittent rhythmic delta slowing (TIRDA; see the second image below), nonrhythmic temporal slowing, and frontotemporal slowing are characteristic; a periodic pattern may develop as the disease evolves.
Hsieh et al found abnormal EEG findings and abnormal neuroimaging in three fifths of children (n=26) with HSE aged 1-6 years correlated with poorer outcomes. [54]
In a retrospective review of EEG in HSE, Al Shekhlee et al found periodic lateralized epileptiform discharges (PLEDs) (see the image below) or focal temporal slowing to be present in 90% of the PCR-positive group (PCR testing for the herpes virus from spinal fluid being the most sensitive and specific test for the diagnosis of HSE) at symptom onset as compared with 30% of the PCR-negative group. [55]
The investigators found that the sensitivity of the EEG recording for these focal and epileptiform findings decreases after 48 hours. The MRI results were consistent with HSE in 86% of those with HSE-positive PCR results obtained 48 hours from symptom onset. They found the EEG to be of important diagnostic use when obtained within the first 24-48 hours of HSE symptom onset. [55]
Serial EEGs usually capture PLED activity, but in the later stages of the disease course, the EEG may revert to normal.
St Louis encephalitis
Wasay et al, using EEG and MRI to study patients, found that of the 9 patients who were examined with EEG, all 9 had seizures or other abnormalities, and 1 had nonconvulsive status epilepticus. The MRI findings in 2 of the 9 patients showed edema. One of the 9 patients had HIV coinfection. [56]
Subacute sclerosing panencephalitis
Subacute sclerosing panencephalitis (SSPE) is a progressive neurodegenerative disorder caused by defective measles virus replication in the brain as a consequence of measles immunization.
The EEG may provide an important clue regarding SSPE and demonstrates bilaterally synchronous, high-amplitude spike or slow-wave bursts that often correlate with clinical myoclonus. As SSPE progresses, the background activity becomes suppressed, resulting in a burst-suppression pattern. Neuroimaging studies demonstrate nonspecific abnormalities or diffuse atrophy, although T2 prolongation can be detected by MRI symmetrically in the cerebral white matter or multifocally in the subcortical white matter or cortex.
Flaherty et al described a 17-year-old boy with SSPE discovered when he presented with confusion after a mild head injury. The EEG strongly suggested the diagnosis. Repeated CT scans of the head were normal. The boy had a 3-year history of decreased vision, associated with a focal pigmentary retinopathy. On assessment, the patient demonstrated visual agnosia and early dementia. MRI demonstrated symmetrical demyelination of the white matter, particularly in the occipital lobes. The typical EEG findings and the presence of measles antibodies in the CSF confirmed the diagnosis of SSPE. [57]
SSPE should be considered in young patients who have persisting cognitive dysfunction that is not proportional to the severity of the initial trauma. A focal pigmentary retinopathy, especially with macular involvement, should raise the possibility of SSPE, even if neurologic symptoms are absent initially. The longest interval (to date) between the visual symptoms and onset of neurological signs of SSPE was reported by the author.
Koppel et al reported on the relation of SSPE and HIV. At one time largely eliminated from the United States by nearly universal measles vaccination, SSPE has reemerged in HIV-infected children. Two children with SSPE were described. The first was HIV-positive and presented with seizures and encephalopathy at the age of 21 months. The second developed myoclonus and dementia at 4 years of age; she was not infected with HIV, but her mother had AIDS. MRI brain scans were nonspecific. EEG was characteristic of SSPE, showing high-voltage PSWCs and background slowing. Brain biopsy and high measles antibody titers in the CSF confirmed the diagnosis of SSPE. [58]
Metabolic Encephalopathies
Metabolic disorders
Anoxic encephalopathy
Hypoxia causes diffuse slowing on the electroencephalogram (EEG). The acute and prolonged anoxia of cardiac arrest exhibits no changes initially. In 7-10 seconds, slow waves appear. This is followed by rhythmic, high-voltage delta activity; subsequently, attenuation and EEG flattening occurs. As a rule, irreversible brain damage results in 4-8 minutes.
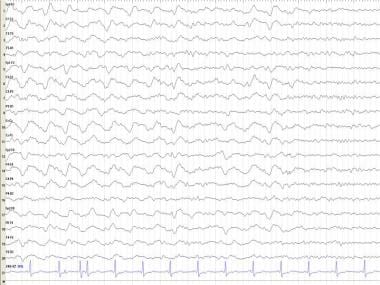
In some cases, establishing the completeness and duration of anoxia is difficult. Certain patterns carry a poor outcome: flat EEG, burst-suppression patterns, and burst suppression patterns with epileptiform discharges (see the image below) nearly always carry a poor prognosis. Postanoxic EEGs may exhibit a variety of abnormal patterns: triphasic activity, alpha coma pattern, repetitive complexes, and bilateral PLEDs. [59]
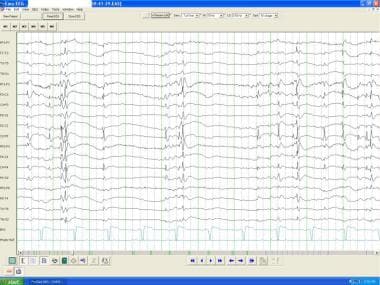 Anoxic encephalopathy. Burst suppression pattern with bursts of spike and wave and polyspike wave discharges with voltage suppression.
Anoxic encephalopathy. Burst suppression pattern with bursts of spike and wave and polyspike wave discharges with voltage suppression.
Takahashi et al reported a 47-year-old man admitted to the hospital for depression, who suddenly developed cardiopulmonary arrest of unknown etiology and entered a chronic vegetative state as a result of anoxic encephalopathy. Periodic synchronous discharges (PSDs) were present for as long as 5 months. The wave pattern, periodicity, and duration of appearance of PSDs were similar to those of PSDs seen in Creutzfeldt-Jakob disease (CJD). The PSDs were prolonged gradually, with a course similar to that of the discharges observed in CJD. The mechanism of occurrence is considered to be similar to that of PSDs in CJD. [60]
Fernandez-Torre et al described the clinical and electroencephalographic features of a comatose patient with severe anoxic encephalopathy who experienced acute reflex myoclonus precipitated by passive eye opening/closure and painful stimulation. Acute stimulus-sensitive postanoxic myoclonus is an underdiagnosed epileptic condition. Shortly after the anoxic insult, the diagnosis should be based on EEG evaluation and various types of stimulation. These should include passive eye opening/closure and painful stimuli. [61]
Comatose intensive care patients
Young et al investigated the usefulness of continuous EEG monitoring. Twenty percent of patients recorded seizures. The study suggests that continuous EEG monitoring may be more valuable for detection of seizures in patients with acute structural brain lesions (ASBLs) than in patients with metabolic encephalopathies. [62]
Hyponatremic encephalopathy
Usually, nonspecific slowing is observed in hyponatremic encephalopathy. A variety of other patterns have been described: triphasic waves (TWs); burst of high-voltage rhythmic delta; central, high-voltage, 5- to 7-Hz rhythm; and sensory stimulation-induced, high-voltage delta activity. Epileptic activity is very rare, even in cases of clinical seizure.
Kameda et al reported a case of frontal intermittent delta activity (FIRDA) in the EEG of a patient with pituitary adenoma, hyponatremic encephalopathy, and major depression. The pituitary adenoma is thought to be a major factor for FIRDA in this case. Complicating factors included diffuse encephalopathy and use of antipsychotic drugs; FIRDA remained in the EEG after these factors diminished. The size of the pituitary adenoma that was proposed to be associated with FIRDA in the EEG recording was not noted. FIRDA may be associated with a small pituitary adenoma less than 10 mm in size. [63]
Hypocalcemia and hypercalcemia
Paresthesias, tetany, muscle spasm and, rarely, seizures may occur in hypocalcemia. EEG findings include theta and polymorphic delta slowing, polyspikes, sharp waves, and paroxysmal activity. Hypercalcemia is associated with renal failure, neoplasms, bone destruction, parathyroid hormone (PTH)-releasing tumors, and hypervitaminosis D. Muscle weakness, polydipsia, polyuria, nausea, anorexia, and coma may develop. EEG changes appear when serum calcium level is approximately 13 mg/dL; slowing and intermittent rhythmic delta activity is seen. Photic driving may be prominent, and TWs may be recorded.
When serum calcium is normalized, the EEG usually improves but not immediately. A hypercalcemic condition can be observed in association with hyperthyroidism. Confusional state and EEG alterations, among which diffuse monomorphic delta rhythms were remarkable, were shown by Juvarra. [64] As soon as normalization of calcium serum level was achieved, rapid clinical and EEG improvement ensued.
Endocrine conditions
Adrenal disease
EEG pattern is nonspecific.
Cushing disease
EEG changes are uncommon.
Addison disease
Nonspecific slowing and diffuse theta and delta may be seen in a disorganized manner.
Pheochromocytoma
No particular EEG pattern has been noted.
Hypoglycemia
The EEG resembles changes described with hypoxia; hyperventilation response is exaggerated and FIRDA may be observed. If prolonged coma ensues, the EEG changes persist and may become permanent. In most cases of hypoglycemia, a generalized disorganization of record occurs; in patients with long-term diabetes, the EEG is usually mildly to moderately diffusely disorganized and slow.
Hyperglycemia
Similar slowing is the rule; however, epileptic activity may be observed with clinical seizure.
Wang et al described hyperglycemia with occipital seizures. They described acute and follow-up visual evoked potential (VEP) and magnetic resonance imaging (MRI) findings of a patient with hyperglycemia-related visual SE of occipital origin. Occipital seizures and hemianopsia can be caused by hyperglycemia and may be accompanied by special MRI and VEP findings. [65]
Glutaric aciduria type I
Neurophysiologic abnormalities are frequently seen in organic acidemias. Yalnizoglu et al studied EEG, VEP, and brain-stem auditory evoked response (BAER) in 7 children with glutaric aciduria type I (GA1). [66] Three of the 7 patients showed abnormal EEG findings; 2 showed asymmetry with intermittent occipital delta slowing in 1 hemisphere. This finding probably indicates underlying cerebral dysfunction and is not a specific feature. One patient showed high amplitude bursts of beta in the occipital regions with left predominance while on clonazepam and baclofen.
Hyperthyroidism
This has a nonspecific pattern, including slowing and FIRDA. Depending on the severity of the thyroid dysfunction, seizures and epileptiform discharges can be seen. [39]
Hypothyroidism
Low-voltage theta is the rule with reduced photic driving response.
Nutritional deficiency syndromes
Pyridoxine deficiency causes severe, and at times, fatal convulsions in infants. The underlying metabolic problem has been suggested to be insufficient gamma-aminobutyric acid (GABA) synthesis. Thiamine deficiency causes diffuse slowing in Wernicke encephalopathy. Malnutrition results in EEG slowing, proportional and corresponding to the clinical alertness of the patient.
Toxic agents
Aluminum toxicity
Flaten et al reported a wide range of toxic effects of aluminum. This element has been demonstrated in plants and aquatic animals in nature, in experimental animals by several routes of exposure, and under different clinical conditions in humans. [67] Aluminum toxicity is a major problem in agriculture, affecting perhaps as much as 40% of arable soil in the world. In fresh waters acidified by acid rain, aluminum toxicity has led to fish extinction. Aluminum is a very potent neurotoxin. Subtle neurocognitive and psychomotor effects and EEG abnormalities have been reported at plasma aluminum levels as low as 50 μg/L.
Infants and patients with impaired renal function could be particularly susceptible to aluminum accumulation and toxicity. Evidence exists to suggest that aluminum may be the causative agent in the development of dementia in patients with chronic renal failure who are on dialysis (ie, dialysis dementia). The EEG may become abnormal months before the full-blown dementia develops. Aluminum also is associated with dialysis encephalopathy, which often is accompanied by osteomalacia and anemia. Such effects also have been reported in certain patient groups without renal failure.
Aluminum accumulation occurs in the tissues of workers with long-term occupational exposure to aluminum dusts or fumes. Such exposure may cause neurologic effects.
In dialysis dementia, the EEG abnormalities usually are diffuse slowing, although TWs may occur. When seizure develops, high-voltage spike and slow-wave complexes and paroxysmal bursts with a frequency of 2-4 Hz have been observed. Polymorphic frontally dominant delta often is observed. The background slowing usually correlates with severity of mental status impairment. Subcortical dysfunction may be present with FIRDA.
Carmofur
Carmofur, an antineoplastic derivative of 5-fluorouracil, has been reported to cause subacute leukoencephalopathy. Kuzuhara described 3 individuals who developed subacute leukoencephalopathy after carmofur (l-hexylcarbamoyl-5-fluorouracil) administration. [68] Initial symptoms were unsteady gait and dementia, developing several weeks or months after administration of carmofur. Symptoms increased gradually even after stopping the drug.
Severe encephalopathy with confusion, delirium, or coma developed. Symptoms were usually reversible but occasionally resulted in death. The EEG demonstrated marked slowing. CT scan of the brains of severely intoxicated patients showed marked hypodensity of the entire cerebral white matter. [68] Carmofur must be discontinued immediately if any psychomotor symptoms develop.
Cefepime
Status epilepticus and encephalopathy have been reported with use of cephalosporins in patients with renal failure. Maganti et al reported the case of a 79-year-old patient with normal renal function who developed subtle mental status changes during cefepime therapy for urinary tract infection. EEG showed nonconvulsive status epilepticus (NCSE). [69]
Lead
The heavy metal lead is usually absorbed into the body by ingestion and/or inhalation. Symptoms from acute poisoning may range from lethargy, seizures, coma, and death to cognitive impairment, delirium, ataxia and distal motor neuropathy with chronic exposure. [70, 71] Stewart et al found lead to be linked to neurodegeneration with cumulative exposure leading to decreased brain volumes and white matter lesions. [72] Treatment includes chelation therapy and removing the source of the lead exposure from the patient's environment (see Lead Encephalopathy).
Lithium
Encephalopathy, confusional states, and nonconvulsive status epilepticus due to lithium toxicity and overdose is well documented. [73] In a report by Bellesi et al, a patient presented in nonconvulsive status epilepticus with a normal serum lithium level that resolved with benzodiazepine administration and withdrawal of the lithium. After 2 months , the lithium was restarted with therapeutic levels attained. The patient again was found to be in NCSE with 3-4 Hz diffuse spike and wave discharges. The lithium was stopped, never to be readministered. [74]
Manganese encephalopathy
Excess manganese (Mn) can cause several neurotoxic effects. Herrero Hernandez et al described an epileptic syndrome due to manganese intoxication in a 3-year-old boy. EEG showed progressive encephalopathy. The patient developed epileptic status. Chelating treatment promptly succeeded in reverting epileptic symptoms. [75]
Neuroleptic encephalopathy
Treatment of psychiatric patients often necessitates overlapping neuroleptic medication. Lambreva et al reported a 60-year-old woman suffering from schizoaffective disorder who temporarily received 3 neuroleptics, together with lithium. She developed neurotoxic encephalopathy with symptoms of a malignant neuroleptic syndrome. The authors recommended frequent EEG controls for early detection of neurotoxicity.
Tiagabine
Tiagabine hydrochloride (TGB) (an antiepileptic medication) is a selective GABA reuptake inhibitor that is used as an add-on therapy for partial seizures. The risk of NCSE can be elevated by TGB (comedication 7.8% vs TGB alone 2.7%). [76] Kellinghaus et al reported 3 cases of recent increase in TGB doses causing nonconvulsive status epilepticus with the EEG of one of the patients demonstrating rhythmic delta waves. [77]
Vinton et al reported TGB-induced NCSE in 3 patients with focal lesional epilepsies—1 with initiation of the medication and 2 with recent dose escalation. All of these patients had continuous high amplitude and generalized 2-4 Hz delta activity with intermingled spikes seen with episodes of unresponsiveness. After dose reduction or withdrawal of the TGB and administration of intravenous (IV) clonazepam, EEG and clinical signs normalized. [78]
Valproate and topiramate encephalopathy
Panda et al reported 2 children with encephalopathy and slowing of EEG background activity, which promptly reverted to normal along with clinical improvement following withdrawal of valproate (VPA). [79]
According to Segura-Bruna et al, VPA-induced hyperammonemic encephalopathy (VHE) is VPA treatment that results in elevated serum ammonium levels, which leads to a decreased level of consciousness, cognitive slowing, vomiting, drowsiness, lethargy, and increased seizure frequency. If VHE is suspected, serum ammonium levels should be evaluated and the existence of a possible urea cycle enzyme deficiency, such as ornithine carbamoyltransferase deficiency, should be considered. Generalized slowing in the theta and delta frequencies, FIRDA, and TWs can be found on EEG. These findings and other clinical symptoms usually resolve after VPA is withdrawn. [80]
Cheung et al proposed a novel term, topiramate-valproate induced hyperammonemic encephalopathy, to describe the clinical features of patients on concomitant topiramate and valproate therapy. With this specific encephalopathic syndrome those on the above therapy may display excessive sleepiness or somnolence, increased seizure activity, hyperammonemia, and an absence of TWs on EEG. [81]
Liver transplantation
The EEG in hepatic encephalopathy may consist of slow waves and TWs; epileptic activity may be observed. Adams et al studied patients after liver transplantation; 17 (33%) of 52 patients who underwent 56 consecutive orthotopic liver transplants had serious postoperative neurologic complications. [82]
Seizures were described in 13 (25%) patients; of these, 50% had onset of seizures within the first week. In 3 patients, the seizures were associated with postoperative metabolic encephalopathy and fatal progressive neurological deterioration. Cyclosporine was thought to be causing the seizures in some of these patients. In others, electrolyte disturbances, steroid treatment for graft rejection, and cerebral infarction could have contributed to the occurrence of seizures. [82]
Autoimmune Encephalopathy
Scleroderma
CNS involvement and psychiatric manifestations can occur in systemic sclerosis (ie, scleroderma). Hietaharju et al evaluated CNS and psychiatric involvement in 32 patients. Severe CNS or psychiatric symptoms were present in 5 patients (16%), including encephalopathy, psychosis, anxiety disorder, grand mal seizures, and transient ischemic attacks. In addition, abnormal VEPs were recorded in 5 of 32 patients (16%), suggesting optic neuropathy. EEGs were mainly normal or showed only slight nonspecific changes. [83]
Anti-NMDAR encephalopathy
Anti– N -methyl-d-aspartate receptor (NMDAR) encephalitis is a neuroimmune syndrome in patients with autoantibodies recognizing extracellular epitopes of NMDAR, and the autoantibodies attenuate NMDAR function through the internalization of NMDAR.” [84]
This autoimmune encephalitis is characterized by headache, confusion, memory deficits, psychiatric disturbance, including psychosis, dyskinesias, coma, autonomic instability, and seizures. [84, 85, 86] Anti-NMDAR encephalopathy is often due to ovarian teratomas in young women, with children less likely to have the above tumors. [84] Many patients need ICU admission and close monitoring because of autonomic instability, coma, and seizures. [84, 86] Extreme care must be taken when evaluating the abnormal movements often seen in anti-NMDAR encephalitis. In one study, false impressions of status epilepticus rather than encephalopathy were reported in 6% of the cases reviewed. [86]
Anti-NMDAR encephalitis can sometimes be reversed with immunotherapy, including steroid treatment, intravenous immunotherapy, and surgery (removal of the teratoma). When able, physical, occupational, speech, cognitive, and behavioral therapies are often required. Recovery can be slow, with cognitive and motor deficits sometimes significant. [84]
EEG patterns seen in this autoimmune encephalopathy can include focal or hemispheric slowing and often diffuse background slowing. [87] With more prolonged hospitalization, a unique “delta brush”–type pattern has been observed in up to 30% of adults with anti-NMDAR encephalitis in one study of 23 patients. [88] This pattern can be continuous or become rhythmic and is often quite refractory to anticonvulsant therapy. Focal nonconvulsive or convulsive status epilepticus can be seen as well, requiring video or continuous EEG monitoring for closer surveillance. [85, 86]
Hashimoto myoclonic encephalopathy
Ghika-Schmid et al reported 2 patients with subacute diffuse encephalopathy characterized by confusion, myoclonic encephalopathy, and mild akineto-rigid extrapyramidal signs in one case and by apathy, memory deficit, and partial complex seizures in the other. Hashimoto thyroiditis with high titers of antithyroglobulin antibodies was diagnosed in both patients, who were not responsive to anticonvulsant medication but exhibited rapid neurologic improvement following steroid treatment. On neuropsychological examination, predominant frontotemporal dysfunction was noted. [89]
EEG activity was remarkable for its rhythmic delta activity, which was unresponsive to, or even paradoxically increased by, anticonvulsant treatment. Atrophy with temporal predominance was observed on MRI. These observations support the idea that this potentially treatable dementia and movement disorder should be classified as a separate clinical entity.
Kothbauer-Margreiter et al reported 6 patients with Hashimoto thyroiditis and associated encephalopathy and compared with 14 well-documented cases identified in the literature. [90] Encephalopathy typically affects patients when they are euthyroid and in an appropriate clinical situation; antithyroid autoantibodies are the main indicators of the encephalopathy. Since clinical features of Hashimoto encephalopathy are nonspecific, other etiologies, such as infectious, metabolic, toxic, vascular, neoplastic, and paraneoplastic causes, must be considered.
Two types of initial clinical presentation can be differentiated:
-
A vasculitic type with strokelike episodes and mild cognitive impairment
-
A diffuse progressive type with dementia, seizures, psychotic episodes, or altered consciousness
These types may overlap, particularly over the long term in untreated patients. A strong female predominance existed in the study by Kothbauer-Margreiter et al ; 18 of the 20 patients were women. The EEG was abnormal in 90% of cases; it showed nonspecific changes. The condition is steroid-responsive.
Chronic Traumatic Encephalopathy
Chronic traumatic encephalopathy (CTE) evolved from the term "dementia pugilistica,” which describes the dementia found in many boxers. CTE is a progressive degenerative disease that causes dementia and depression, particularly in athletes subjected to multiple concussions or sub-concussive blows to the head. It has also been called “sports-related concussion syndrome,” but can be seen in many forms of non-sports-related brain trauma as well. The neuropathology has evolved to specify a unique type of tauopathy found in perivascular spaces at the depth of sulci and other features not typically seen in neurodegenerative tauopathies. Currently, there are no treatments for CTE and the disease can only be diagnosed by direct tissue examination, including full autopsies. Little research has been done on the evolving EEG patterns noted in these traumatic brain injury patients that develop this encephalopathy dementia-type complex. [91, 92]
The athlete post-career adjustment (AP-CA) model was developed to better evaluate athletes with risk of CTE. The AP-CA consists of four elements: neurotrauma, chronic pain, substance use, and career transition stress. Any of these elements can account for a significant number of CTE symptoms. Additionally, depression may be present, which can be a chronic, lifelong comorbid condition. Neurotrauma is a necessary condition for the development of CTE symptomology. [93]
Traumatic brain injury (TBI) has long been associated with the development of dementia and may be a risk factor for other neurodegenerative disorders that can be associated with dementia. TBI results in white matter tract and neural network disruptions, as well as amyloid pathology and other neurodegenerative proteinopathies. [94] Risk factors for TBI-associated dementia include the following:
-
Any blast or blunt physical force to the head as long as there is violent head displacement
-
Decreased cognitive and/or neuronal reserve and the related variable of older age at TBI
-
The presence of apolipoprotein E ɛ4 alleles, a genetic risk factor for AD
Triphasic Waveforms
Triphasic waves (TWs; see the image below) were initially described by Foley et al in hepatic encephalopathy. They later were described in other metabolic states and brain tumors. [95] Most electroencephalographers now agree that TWs are a relatively nonspecific pattern observed in a number of metabolic conditions, degenerative dementias, and anoxia. In a bipolar montage, TWs usually comprise a high-voltage, positive wave followed by a smaller negative deflection; they usually are bilaterally synchronous and maximal frontally. A fronto-occipital (anteroposterior) phase lag varies from 25 to 140 ms; this is expressed less in referential montages.
TWs have not been reported in children. Generally, the TW pattern carries a poor prognosis with a high mortality if it occurs in association with rapid neurologic and clinical deterioration.
However, TWs in a psychiatric population described by Blatt and Brenner carried a different prognosis. In a large retrospective study consisting of 15,326 electroencephalograms (EEGs) performed from 1983 to 1992 in a psychiatric institute, 83 EEGs (62 patients—13 men and 49 women aged 59-90 years, with a mean age of 74 years) had TWs.
All 62 patients were awake, though they often were confused. Most (n=56) had dementia, usually severe; 15 also had delirium. Six patients had no dementia. Infrequent etiologies included neuroleptic malignant syndrome (n=1) and hepatic encephalopathy (n=1); in 4, the cause was uncertain, although all were receiving lithium. [96]
EEG features analyzed included frequency of background rhythms, distribution of the TWs, periodicity, and epileptiform abnormalities. [96] Background rhythms were slow in all but 7 patients. TWs were maximal posteriorly in 47 patients and anteriorly in 6 and were diffuse in 9. Neuroimaging studies demonstrated prominent posterior abnormalities in only 1 individual. Periodicity was prominent in 4 patients; in 2, the TWs were maximal anteriorly. Interictal epileptiform activity was present in 6 patients, a history of seizures in 8, and myoclonus in 4. TWs are uncommon in psychiatric populations; they occur primarily in elderly and severely demented patients.
Aguglia et al discussed nonmetabolic causes of TWs and described 2 patients with TWs on their EEGs in the absence of metabolic disturbances. [97] One patient had coma associated with cerebellar hematoma, the other had mild dementia associated with idiopathic calcifications of the basal ganglia and healthy auditory brainstem responses and subcortical and cortical SEPs. Neurologic examination revealed no asterixis in either patient.
The literature on nonmetabolic causes of TWs also was reviewed, and the clinical and anatomic reports of 10 patients were analyzed. [97] Seven patients had focal brainstem-diencephalic lesions: craniopharyngioma (2), thalamic gliomas (3), or pontine stroke (2). Three patients suffered from diffuse subcortical or multifocal encephalopathies: Binswanger encephalopathy (1), cerebral carcinomatosis (1), or multifocal cerebral lymphoma (1).
From the clinical point of view, patients with nonmetabolic diseases causing TWs presented with either disturbance of higher cerebral functions with no asterixis or sudden onset of coma. Aguglia et al concluded that TWs may result from focal brainstem/diencephalic lesions or from diffuse subcortical or multifocal encephalopathies in the absence of concomitant metabolic abnormalities. [97] Nonmetabolic causes of TW should be suspected in patients presenting with neurologic disturbances not associated with asterixis.
TWs also were evaluated by Sundaram et al, and their clinical correlates and morphology were assessed. [98] Of 63 consecutive patients with TWs, 26 (41%) had various types of metabolic encephalopathies, and 37 patients (59%) had nonmetabolic encephalopathies, usually senile dementia. TWs were not found to be specific for any single type of metabolic encephalopathy. Etiology was linked more closely to level of consciousness at recording than to any morphologic or distributional feature of the TWs themselves. Thus, all 31 alert patients had nonmetabolic encephalopathies, and all 13 comatose patients had metabolic encephalopathies.
The second, positive component (wave II) most often had the highest voltage, while equally maximal waves I and II occurred next most commonly. [98] In these patients, TWs most often were expressed maximally anteriorly. Among patients with metabolic encephalopathies, a posterior-anterior delay or lag of the wave II peak occurred more commonly than did the better known anterior-posterior lag. Lags occurred with both metabolic and nonmetabolic conditions but were more common with the former. No difference in quantity or mode of appearance existed between the metabolic and nonmetabolic groups when matched for consciousness level.
Prognosis for patients with either metabolic or nonmetabolic encephalopathies was unfavorable. [98] Only 4 of 24 patients with metabolic encephalopathy and 1 of 35 patients with nonmetabolic encephalopathy were well at follow-up more than 2 years later. Forty percent of EEGs with sharp and slow-wave complexes (slow spike waves) had sporadically appearing TWs. The relative amplitudes of the 3 components differed from those of TWs in other conditions; equally maximal waves II and III were the most usual form.
Digital EEG
As stated in the assessment report of the American Academy of Neurology and the American Clinical Neurophysiology Society, digital electroencephalography (EEG) is an established substitute for recording, reviewing, and storing a paper EEG record. In this sense, digital EEG simply replaces and improves the paper record, much as word processing has improved letter writing over what could be done by hand or even by a typewriter. However, routine digital recording of the clinical EEG does not add new information that was not present in the paper record.
Once the paper recording is made, various options are available for further analysis. Some processing methods, such as different montage displays and digital filtering, simply enhance the visibility of the record. Others, such as calculating the mean band frequencies and different band-energy spectra, may bring into the forefront information that was already there in the paper record but is too tedious and time-consuming to calculate without use of a computer.
Spike recognition is an important enhancement and a great time saver but needs careful review by the interpreting physician. Lately, technologic developments have enabled the authors to record long-term monitoring on small storage devices, making the diagnosis of syncope, seizures, and sleep disorders much easier.
EEG brain mapping visualizes a selected electrical event in the brain and maps its geographic distribution. Attempts have been made to standardize some aspects of brain mapping; however, no clear uniform recommendation has yet emerged. Although frequency bands are fairly well standardized, different ways of calculating the data exist. Normative values are being developed; however, most brain maps are not time locked to an event or brain state; therefore, comparisons of frequency bands are difficult to accomplish across groups or disease states.
A clear definition for the clinical correlation of the brain maps is still needed; therefore, EEG brain mapping and other advanced quantitative EEG (QEEG) techniques should be used only by physicians highly skilled in clinical EEG and only as an adjunct to traditional EEG interpretation.
These tests may be clinically useful only for patients who have been carefully selected on the basis of their clinical presentation. Certain QEEG techniques are considered established as an addition to the digital EEG and include screening for possible epileptic spikes or seizures, long-term EEG monitoring or ambulatory recording, and operating room (OR) and intensive care unit (ICU) monitoring.
Continuous EEG monitoring by frequency trending helps to detect early intracranial processes in the OR or ICU (eg, screening for possible epileptic seizures in high-risk ICU patients). QEEG frequency analysis may be a useful adjunct to interpretation of the routine EEG. In a number of conditions (eg, postconcussion syndrome, head injury, learning disability, attention disorders, schizophrenia, depression, alcoholism, and drug abuse), QEEG remains investigational. On the basis of available clinical and scientific evidence and expert opinions, QEEG is not currently useful in civil or criminal cases.
QEEG is a derivative of regular EEG. The original data must be evaluated before any further mathematical translation of this same data set is done. A thorough understanding and firm knowledge of clinical EEG diagnosis may help prevent erroneous interpretations of digitally displayed mathematical constructs (eg, brain maps and coherence maps).
Ideally, only physicians properly trained in EEG and, in addition, sufficiently well trained in mathematics and computing science should use these new technologies. A substantial risk of erroneous interpretations exists if any of the elements required is missing. Clinical use of any of the EEG brain mapping or other QEEG techniques by practitioners who are not physicians highly skilled and properly trained in clinical EEG interpretation or who have not reviewed the original record should be unacceptable.
Conclusion
Electroencephalography (EEG) is regarded as a fairly nonspecific measure of clinical states. A limited number of abnormalities that can be recognized in widely varied disease states exist.
On the other hand, an abnormal EEG is a sensitive measure of brain function. When the patient has clinically symptomatic encephalopathy or moderate dementia, the EEG is almost always abnormal.
Accordingly, the clinical utility of EEG must be appreciated in a different way from that of some other diagnostic procedures. In medicine, clear correlations and specific answers are desired; however, depending on the modality and the underlying principle of measurement, this goal cannot always be achieved.
In addition to EEG, magnetic resonance imaging (MRI) scan is very sensitive in showing various lesions in the brain. However, it is difficult to know exactly what these lesions represent on the basis of mere appearance. When interpreting MRI images, the clinician relies to a large extent on other relevant clinical information.
EEG measures electrical field variations, and a number of clinical conditions can disturb the normal electrical field of the brain. Simple state or electrolyte changes may alter the appearance and time variation of the brain-generated electrical fields; hence, a large number of conditions cause the EEG to appear abnormal. In EEG practice, the clinician has to rely to a large extent on the clinical history and the neurologic examination findings to make a clinically meaningful conclusion.
In most instances, the correct question may be whether the EEG is normal or abnormal. The next step is to decide how an abnormal EEG would help the clinical diagnosis; therefore, the EEG can be used to confirm clinical observation or suspicion or to determine the extent of the abnormality for prognostic purposes (ie, in attempting to predict outcome). Sometimes, EEGs serve as a "proof" to families that the patient's brain function is indeed so greatly disturbed that recovery is doubtful. In such cases, the EEG helps resolve anxiety and supports a more correct ethical decision.
Newer EEG techniques offer a number of conveniences and also enhance communication between the electroencephalographer and other clinical specialists. However, they may not make the record more specific but merely render it more easily understandable. Computer analysis, on the other hand, may offer features that, although present in the regular record, are difficult or time-consuming to extract and display.
EEG has a definite role in evaluating changes in mental states. It can confirm or rule out nonconvulsive status epilepticus (NCSE). EEG changes are usually proportional to the degree of metabolic, hepatic, or renal encephalopathy. EEG often is abnormal in subdural hematoma, normal pressure hydrocephalus (NPH), and Creutzfeldt-Jakob disease (CJD).
Computer analysis of the EEG may help reveal subtle changes in Alzheimer disease (AD). This application is promising and, it is to be hoped, will soon become clinically useful and available. In cases of clinical dementia, a normal EEG with preserved alpha might help establish the diagnosis of Pick disease, whereas a slowed and shifted alpha frequency is seen in AD and progressive supranuclear palsy (PSP). Low-voltage, flat EEG and the appropriate clinical presentation may raise the suspicion of Huntington disease.
EEG study should be requested if clear clinical indications are present or if the clinician has a reasonable presumption that it may give clinically relevant information. Such information would be expected to alter the clinical decision-making process. It would also help the referring source in diagnosis and treatment.
Another role is to help the patient or family to understand the ongoing disease process. EEG frequently is ordered to evaluate patients with different degrees of mental and behavioral changes and encephalopathy or coma. Usually, nonspecific abnormalities are present that do not give definite information about the cause of the underlying process but do provide information on its location and severity; therefore, unless epileptic seizures are a consideration, the EEG does not give direct unequivocal information on the cause of the patient's condition.
Nevertheless, EEG may differentiate between a generalized and a focal abnormality. This may guide the clinician to further appropriate imaging studies. On the other hand, if the abnormality is generalized, the EEG can be used to characterize and monitor the disease process. With coma, the EEG may help in predicting the neurologic prognosis. EEG is an important diagnostic tool in dementias in which specific morphologic lesions are not apparent on imaging studies.
Personal perspective
EEG often is compared with MRI; when this comparison is made, it usually refers to clinical MRI rather than functional MRI. This comparison is puzzling, in that a primarily anatomic test is being compared with a functional one.
In general, MRI is good at telling us where the lesion is, whereas EEG is good at separating normal and abnormal primarily cortical function. The topologic usefulness of EEG is limited, although it may be improved with computerization. The purpose of MRI is to provide precise localization of a lesion, usually one that has passed a certain stage of evolution. The EEG, on the other hand, captures the changing electrical characteristics of a functioning brain, primarily those of the cortex.
Conditions can be identified with EEG that as a rule cannot be seen on the MRI; therefore, the use of these studies is not exclusive but complementary. The EEG may be used for the following purposes:
-
To exclude nonconvulsive status epilepticus
-
To identify focal interictal epileptiform activity to confirm clinical suspicion that seizures may contribute to the condition in question
-
To attempt to record functional disturbance in individuals whose brain MRI is "normal" but in whom brain dysfunction is clinically evident (eg, metabolic encephalopathies)
-
To attempt to record disease-specific patterns in the proper clinical setting, such as progressive myoclonic epilepsies, CJD, SSPE
-
To help a psychiatrist with the multitude of complex disorders masking as potential epilepsy or encephalopathy (eg, lithium intoxication may present with BiPEDs)
-
To identify focal or lateralized changes that suggest a structural cause to the encephalopathy
The truism that EEG is nonspecific and cannot diagnose etiology or localization well (eg, the cause of coma) is often cited. However, in general medical practice, nonspecificity is often not the question, because most of the referrals in general neurology are individuals in whom the cause is fairly clear, or reasonably suspected, on the basis of clinical history and laboratory chemistry. The questions from the clinician are whether the brain is involved and what is the extent of brain damage (if any). At present, for answering these questions, no clinical tool is more useful than the EEG.
Questions & Answers
Overview
How is EEG used in the evaluation of dementia and encephalopathy?
Which findings on EEG are characteristic of dementia?
Which findings on EEG are characteristic of encephalopathy?
What is the role of digital EEG data in the evaluation of dementia and encephalopathy?
What EEG findings are characteristic of Pick disease?
What EEG findings are characteristic of Huntington disease?
What EEG findings are characteristic of progressive supranuclear palsy (PSP)?
What EEG findings are characteristic of corticobasal degeneration (CBD)?
What EEG findings are characteristic of Alzheimer disease?
What EEG findings are characteristic of dementia with Lewy bodies (DLB)?
What EEG findings are characteristic of Parkinson disease (PD)?
What EEG findings are characteristic of Korsakov syndrome?
What EEG findings are characteristic of Binswanger disease?
What EEG findings are characteristic of atherosclerosis?
What EEG findings are characteristic of multi-infarct dementia?
What EEG findings are characteristic of transient global amnesia (TGA)?
What EEG findings are characteristic of action myoclonus?
What EEG findings are characteristic of generalized myoclonus?
What EEG findings are characteristic of Unverricht-Lundborg disease (ULD)?
What EEG findings are characteristic of mitochondrial encephalomyopathy?
What EEG findings are characteristic post stereotactic surgery?
What EEG findings are characteristic of Alpers disease?
What EEG findings are characteristic of adrenoleukodystrophy?
What EEG findings are characteristic of Zellweger syndrome?
What EEG findings are characteristic of infantile neuroaxonal dystrophy?
What EEG findings are characteristic of PKAN?
What EEG findings are characteristic of neuronal ceroid lipofuscinosis?
What EEG findings are characteristic of Gaucher disease?
What EEG findings are characteristic of metachromatic leukodystrophy?
What EEG findings are characteristic of Tay-Sachs disease?
What EEG findings are characteristic of Rett syndrome?
What EEG findings are characteristic of viral encephalitis?
What EEG findings are characteristic of Creutzfeldt-Jakob disease (CJD)?
What EEG findings are characteristic of subacute spongiform encephalopathy>?
What EEG findings are characteristic of AIDS dementia?
What EEG findings are characteristic of chronic rubella encephalitis?
What EEG findings are characteristic of St. Louis encephalitis?
What EEG findings are characteristic of subacute sclerosing panencephalitis (SSPE)?
What EEG findings are characteristic of anoxic encephalopathy?
What EEG findings are characteristic of comatose intensive care patients?
What EEG findings are characteristic of hyponatremic encephalopathy?
What EEG findings are characteristic of hypocalcemia and hypercalcemia?
What EEG findings are characteristic of adrenal conditions?
What EEG findings are characteristic of aluminum toxicity?
What EEG findings are characteristic of carmofur-related subacute leukoencephalopathy?
What EEG findings are characteristic of cefepime-related encephalopathy?
What EEG findings are characteristic of lead encephalopathy?
What EEG findings are characteristic of lithium encephalopathy?
What EEG findings are characteristic of manganese encephalopathy?
What EEG findings are characteristic of neuroleptic encephalopathy?
What EEG findings are characteristic of tiagabine toxicity?
What EEG findings are characteristic of valproate and topiramate encephalopathy?
What EEG findings are characteristic of hepatic encephalopathy?
What EEG findings are characteristic of systemic sclerosis?
What EEG findings are characteristic of anti-NMDAR encephalopathy?
What EEG findings are characteristic of Hashimoto myoclonic encephalopathy?
What EEG findings are characteristic of chronic traumatic encephalopathy (CTE)?
What is the role of EEG in the workup of dementia and encephalopathy?
How does EEG compare to MRI for the evaluation of dementia and encephalopathy?
-
EEG in dementia.
-
Bilateral periodic epileptiform discharges (BiPEDs).
-
Triphasic waves, maximum amplitude bilateral frontal.
-
Frontal intermittent rhythmic delta activity (FIRDA).
-
MRI axial diffusion weighted image: Cortical ribbon sign in CJD.
-
Generalized periodic epileptiform discharges (GPED).
-
MRI axial FLAIR with gadolinium; herpes encephalitis, left temporal.
-
Left temporal intermittent rhythmic delta (TIRDA).
-
Periodic lateralized epileptiform discharges.
-
Anoxic encephalopathy. Burst suppression pattern with bursts of spike and wave and polyspike wave discharges with voltage suppression.