Overview
The interactions between seizures and the autonomic nervous system (ANS) are very complex. We approach these interactions from various angles.
Ictal phase
Abnormal neuronal electrical activity corresponding to a seizure can involve central centers for the regulation of autonomic activity. A seizure can present with autonomic symptoms initially, during its propagation, or during the aftermath. These manifestations can involve the cardiovascular, pulmonary, gastrointestinal, urogenital, or endocrine systems. Hence it is essential for clinicians to have a better understanding of the autonomic manifestation of seizures.
The probable paths of propagation of the epileptic electrical activity are as follows. The ictal impulse involves either or both temporal and frontal areas. The insular cortex is then involved in both temporal and frontal seizures, and the hippocampus is involved in temporal seizures. This activity is then spread through the limbic system with involvement of the amygdala, hypothalamus, and thalamus. These in turn stimulate the ANS nuclei in medulla, including the nucleus tractus solitarius (NTS) and ambiguus nuclei. Both sympathetic and parasympathetic efferent discharges are then generated. See the image below.
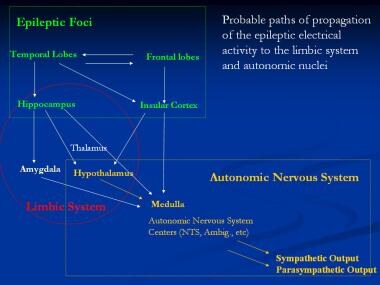 Probable paths of propagation of the epileptic electrical activity to the limbic system and autonomic nuclei. Ambig = ambiguus; NTS = nucleus tractus solitarius.
Probable paths of propagation of the epileptic electrical activity to the limbic system and autonomic nuclei. Ambig = ambiguus; NTS = nucleus tractus solitarius.
Intetrictal phase
Patients with epilepsy experience long-lasting changes in the regulation of the ANS and their target organs (eg, the heart). For example, the patient might experience frequent surges of catecholamines during the seizures. This in turn can cause fibrosis as permanent anatomical changes in the myocard. Cardiac arrhythmia can then arise due to these anatomical changes, independent of seizures.
Role of the ANS in sudden unexpected death in epilepsy (SUDEP)
In addition to contributing to the clinical presentation, autonomic involvement of organs due to epilepsy can contribute to sudden unexpected death in epilepsy (SUDEP). Heart rate variability shows increased sympathetic activity that is shown to be more prominent in patients with SUDEP. Therefore heart rate variability (HRV) can be considered a biomarker for predicting SUDEP. [1] Most of the current knowledge has originated from recordings of biological functions in individuals who suffered SUDEP. Also contributing are the pathological findings in heart and lungs, but more specifically in the brain autopsies of these individuals. Anatomical changes seen in these brains can explain partially the cardiovascular and pulmonary events that would lead to SUDEP. [2, 3]
Syncope vs seizure
Syncope can be a seizure imitator and seizures can cause loss of consciousness due to bradyarrhythmia and hypotenstion.
Role of antiseizure medications
Certain autonomic changes, especially cardiovascular, are due to antiseizure medications (ASM). For example, vimpat can cause EKG and cardiac arrhythmia.
Clinical implications
HRV measurement can be offered to all persons with epilepsy to detect individuals at risk of SUDEP.
Vagus nerve stimulation (VNS) is shown to improve the autonomic dysregulation with time [4, 5] and therefore potentially decrease risk of SUDEP. A similar effect can be documented with ASM use. [6]
Methodology
The regulatory effect of the sympathetic and parasympathetic nervous system on the end organs can be evaluated and quantified. To understand the effect of epilepsy on the autonomic nervous system (ANS), the autonomic functions in patients with epilepsy can be evaluated both during seizures and in their baseline state, also known as the interictal state.
The autonomic regulation or cardiovascular function plays the most crucial role of the autonomic functions and has been the most evaluated part of the ANS. Changes in heart rate and blood pressure are the basis of these functions. Heart rate variability (HRV) during rest and during certain standardized tests and blood pressure (BP) response to certain challenges comprise the basis.
For the evaluation of the HRV, ECG is evaluated by computerized programs. The distances between 2 QRS complexes are calculated. The statistical changes of these beat-to-beat intervals are evaluated in the so-called time domain. The frequency domain measures the occurrence of various parameters in certain frequencies. [7, 8]
The RR interval, in other words the beat-to-beat fluctuations of the heart rate also known as NN intervals, has been evaluated using certain statistical indicators. SDNN, the standard deviation of NN intervals, and RMSSD, root mean square of successive differences, are 2 commonly measured parameters.
The RR intervals can also be evaluated in the frequency domain. Fast Fourier spectral analysis is used by algorithms that imply the spectral analysis of heart rate values in various frequencies, as follow:
-
The so-called high frequency (HF: 0.15-0.4 Hz.) is an indicator of the vagal modulation of the HR
-
The low frequency range (LF: 0.04-0.15 Hz.) and the LF/HF power represent a complex interplay between sympathetic and parasympathetic modulation of HR
-
Very low frequency range (VLF: 0-0.04 Hz.) probably represents the sympathetic regulation as well, but is less clearly defined
HRV at baseline is mainly related to the response of the parasympathetic nervous system to breathing. During inspiration, a negative vacuum effect is created. To maintain cardiac output, the vagal nerve tone is lowered by autonomic centers, so that the heart rate is increased and the cardiac output can remain stable. During the expiratory phase of breathing, the heart rate is lowered by an increased vagal tone. A test battery to evaluate the autonomic regulation includes the following:
-
HRV during rest and during deep breathing is an indicator of the parasympathetic function
-
HRV and BP during Valsalva maneuver evaluates both the S and PS
-
HRV and BP during both standing and tilt-table evaluates mainly the sympathetic response
-
HRV during ice water immersion, hand grip, and arithmetic tasks evaluates the sympathetic response
In order to evaluate the cardiovascular autonomic functions at their baseline, these tests can be arranged in an ANS laboratory and compared with age- and sex-matched healthy individuals.
The evaluation of autonomic changes during a seizure creates a challenge, however. During a typical EEG, the ECG is recorded as well. Researchers can identify the seizures during a recording and can then investigate the HRV both during the seizures and during the preictal and postictal phases. The data can be analyzed both using the time domain and frequency domain techniques.
A recent trend has been to evaluate other parameters during an EEG recording, including 3-channel ECG, pulse oximetry, respiration, and beat-to-beat BP.
In addition to methods to evaluate cardiovascular reflexes, extensive neurophysiology methodology has been developed to evaluate the regulation of sweating, sympathetic skin density, bladder function, erectile function, gastrointestinal motility, and pupillary reaction. The scope of these methodologies is beyond this article.
This article deals with the relationship between seizures and the ANS and is divided into the following sections:
-
Ictal Autonomic Changes
-
Interictal Autonomic Changes
-
Autonomic Functions and SUDEP
-
AEDs and Autonomic Changes
-
Seizure Versus Syncope
See also the following:
Ictal (Peri-ictal) Autonomic Changes
Autonomic phenomena can constitute the initial seizure manifestation, or can result from propagation of the hypersynchronized electrical impulse to autonomic central nuclei. Simple partial seizures with autonomic manifestations have an ictal focus involving autonomic nervous system (ANS) centers without impairing awareness. The ANS centers can be involved secondarily in complex partial (CP), absence, generalized tonic, and generalized tonic-clonic (GTC) seizures. Autonomic symptoms accompany all GTC seizures and one third of simple partial seizures.
In a first comprehensive study, Van Buren et al investigated autonomic functions in 13 patients during 20 epileptic attacks of temporal lobe (TL) origin. Simultaneously with electroencephalography (EEG), they recorded autonomic phenomena as represented by changes in electrocardiography (ECG), blood pressure, respiratory movements, skin temperature and resistance, esophageal pressure, and gastric pressure. They reported the occurrence in a majority of the patients of a fairly stereotyped pattern of initial decrease in skin resistance and swallowing, followed by cessation of respiration and gastric motility, and then tachycardia, hypotension, and decrease in pulse amplitude. They concluded that this pattern was indicative of propagation of the electrical activity through spatially separated autonomic centers. [9] Similar observations were reported in patients who had seizures induced by electroconvulsive therapy (ECT). [10]
Evaluation of ECG and heart rate variability is the most crucial part of the investigation of autonomic changes during any certain time epoch. In most EEG recordings, a parallel ECG is also recorded that can be evaluated for heart rate variability. Through these evaluations, the autonomic cardiac regulations can be assessed. The time-wise correlation of these cardiac seizures allows the investigator to correlate any changes in heart rate variability in the preictal, ictal, or postictal period.
The differential diagnosis of ictal autonomic phenomena includes organic diseases of the viscera (eg, carcinoid, pheochromocytoma), hypoglycemia, panic attacks, and primary autonomic system dysfunctions.
Below, Table 1 summarizes autonomic symptoms and signs accompanying seizures. These manifestations of ANS involvement will be discussed in detail in this section.
Table 1. Autonomic Symptoms and Signs Associated With Seizures (Open Table in a new window)
Symptoms and Signs |
Remarks |
Cardiac/thoracic Palpitations, chest pain, tachycardia, bradycardia, arrhythmia, hypotension, hypertension |
More common in right temporal mesial foci; potential SUDEP with arrhythmia |
Respiratory Apnea, hyperventilation, hypoxia, cough |
Particularly in temporal foci, hippocampal, and insular involvement; potential SUDEP with apnea |
Gastrointestinal/abdominal Ascending sensation (dyspepsia), pain, hunger, borborygmi, nausea, vomiting, belching, urge to defecate, fecal incontinence |
Particularly in temporal mesial foci; vomiting in occipital and opercular foci; pain especially in children |
Urinary Incontinence, urgency |
Detrusor muscle contraction in absence seizures and external sphincter relaxation in GTC |
Genital Genital sensations, erection, orgasm |
Genital sensation in sensory cortex; sexual arousal in limbic and temporal cortex |
Cutaneous Flushing, erythema, cyanosis, blanching, pallor, piloerection |
Can be unilateral |
Pupillary Mydriasis, miosis, hippus |
Can be unilateral; must be distinguished from cerebral herniation |
Secretory Perspiration, salivation, lacrimation |
Frequent in GTC |
GTC = generalized tonic-clonic seizure; SUDEP = sudden unexpected death in epilepsy. |
|
Cardiovascular manifestations
Alteration of the heart rate during a seizure is a well-known phenomenon. Jackson and his associates first described autonomic symptoms in seizures caused by mesial temporal lobe lesions. [11] Early works of Gastaut, [12] White et al, [13] and Van Buren [9] documented the correlation of temporal lobe partial epileptic activity with cardiovascular phenomena. Many of the earlier studies were based on observations of autonomic phenomena during seizures induced by ECT or epileptogenic substances. Many anecdotal reports and case series evaluated autonomic phenomena in unprovoked seizures; however, only a limited number of studies have used simultaneous recordings of EEG and ECG. Table 2, below, reviews several studies of ictal cardiac manifestations with simultaneous EEG and ECG recordings in unprovoked seizures.
Table 2. Review of Selected Studies on Ictal Cardiac Manifestations in Unprovoked Seizures (Open Table in a new window)
Series |
Event # |
Seizure Types |
Seizure Origin |
Tachycardia, % of Events |
Bradycardia, % of Events |
No Change |
Arrhythmia, % of Events |
Van Buren (1958) [9] |
13 |
SP, CP |
T |
93% |
7% |
0% |
- |
Marshall et al (1983) [14] |
12 |
CP |
T |
64% |
- |
- |
- |
Blumhardt et al (1986) [15] |
74 |
CP |
T |
92% |
- |
- |
42% |
Smith et al (1989) [16] |
93 |
CP |
T |
74% |
5% |
20% |
- |
Epstein et al (1992) [17] |
27 |
SP, CP |
T |
100% |
- |
- |
- |
Liedholm and Gudjonsson (1992) [18] |
9 |
CP |
T |
- |
100% |
- |
1 arrest |
Nashef et al (1996) [19] |
47 |
CP, GTC |
T, F |
91% |
11% |
- |
- |
Reeves et al (1996) [20] |
23 |
CP, GC |
T |
- |
100% |
- |
- |
Schernthaner et al (1999) [21] |
92 |
CP |
T, F, O |
83% |
3% |
- |
- |
Nei et al (2000) [22] |
51 |
CP, GTC |
T |
- |
- |
- |
39% |
Zijlmans et al (2002) [23] |
281 |
SP, CP, GC |
? |
73% |
7% |
- |
0.5% |
Leutmezer et al (2003) [24] |
145 |
CP |
T, ex-T |
87% |
1% |
13% |
- |
Mayer et al (2004) [25] |
20 |
CP |
T |
98% |
0% |
- |
- |
Rugg-Gunn et al (2004) [26] |
377 |
- |
- |
- |
2.1% |
- |
4 patients |
Odier et al (2009) [27] |
1277 |
- |
F, T, PO |
76% |
8% |
- |
1 arrest |
Moseley et al (2011) [28] |
218 |
- |
- |
57% |
2% |
- |
QTc changes |
CP = complex partial; ex-T = extratemporal; F = frontal; GC = generalized clonic; GTC = generalized tonic-clonic; O = occipital lobe; PO = parieto-occipital; SP = simple partial; T = temporal. |
|||||||
In 2016 Van der Lende et al [29] published a comprehensive review of the relevant literature of ictal cardiac phenomenon captured in video EEG of patients who experienced seizures in epilepsy monitoring units. They identified seven distinct patterns of (post)ictal cardiac arrhythmias: ictal asystole (103 cases), postictal asystole (13 cases), ictal bradycardia (25 cases), ictal atrioventricular (AV)-conduction block (11 cases), postictal AV-conduction block (2 cases), (post)ictal atrial flutter/atrial fibrillation (14 cases), and postictal ventricular fibrillation (3 cases). Ictal asystole had a mean prevalence of 0.318% (95% CI 0.316% to 0.320%) in people with refractory epilepsy who underwent video EEG monitoring. Ictal asystole, bradycardia, and AV-conduction block were self-limiting in all but one of the cases and seen during focal dyscognitive seizures. Seizure onset was mostly temporal (91%) without consistent lateralization. They noticed ictal arrhythmias were typically transient and self-limiting. Postictal arrhythmias were mostly found following convulsive seizures and often associated with (near) SUDEP. The contrasting clinical profiles of ictal and postictal arrhythmias suggest different pathomechanisms. They conclude postictal rather than ictal arrhythmias seem of greater importance to the pathophysiology of SUDEP.
ECG changes
The spectrum of ECG changes during epileptic activity is extensive. Erickson studied ictal ECG changes systematically for the first time. [30] He reported ictal tachycardia and T-wave flattening. Initial bradycardia followed by tachycardia has been documented in as many as 64% of patients with petit mal and 100% of those with GTC seizure attacks. [31, 32, 33] Tachycardia is reported in 74-92% of patients with complex partial seizures. [15, 16, 25, 34]
Persistent bradycardia is less common than tachycardia and is documented in 3-7% of patients with complex partial seizures. [9, 21] Ictal cardiac rhythm and conduction abnormalities are reported in 5-42% of patients with partial seizures. Rhythm abnormalities include atrial fibrillation, sinus arrhythmia, atrial and ventricular premature depolarizations, bundle-branch block, torsade de pointes, asystole, ST-segment and T-wave abnormalities, and QT prolongation. [21, 22, 35, 36, 28]
In addition to brief recordings of ECG, a prolonged recording via a so-called loop recorder implanted in subcutaneous tissue has been used. By recording ECG for 1 month, this method can give invaluable insight into cardiac rhythm changes both during seizures and at baseline. In a small group of refractory epilepsy, Rugg-Gunn et al recorded prolonged ECG in 20 patients in a hospital in the United Kingdom and shed a light on the severity of life-threatening cardiac arrhythmias in these patients. [26] Devices were programmed to record automatically if bradycardia (< 40 bpm) or tachycardia (>140 bpm) were detected.
More than 220,000 patient-hours were monitored over 24 months, during which ECGs were captured on implantable loop recorders in 377 seizures. In 16 patients, median heart rate during habitual seizures exceeded 100 bpm. Ictal bradycardia (< 40 bpm) occurred in 8 (2.1%) recorded events in 7 patients; 4 patients (21%) had bradycardia or periods of asystole with subsequent permanent pacemaker insertion, and of these 4, 3 (16% of total) had potentially fatal asystole. [26]
Tachycardia and tachyarrhythmias
Most studies that have documented EEG and ECG recordings report tachycardia in 64-93% of complex partial seizures, mostly of temporal lobe origin. [9, 14, 21] Schernthaner et al assessed ECG recordings that were recorded simultaneously with EEG during 107 seizures and reported ictal tachycardia (heart rate increase >10 bpm) in 83% of seizures. [21] Heart rate changes usually occurred several seconds before seizure onset as recorded on scalp EEG. Tachycardia occurred significantly more often in seizures with onset in the temporal lobe.
Few investigators have evaluated the cardioregulatory mechanisms in children with epilepsy. Mayer et al showed tachycardia in 98% of children suffering complex partial seizures of temporal lobe origin and, as such, more frequently than in adults. [25]
Using prolonged ECG recording via loop recorders, Rugg-Gunn et al evaluated the ECG during 3,370 seizures, of which tachycardia occurred in most. [26] Onset of ictal tachycardia was typically of short duration with, for example, about 20 seconds between the onset of tachycardia and the attainment of the maximum rate. Characteristically, the heart rate returned to baseline within 1–2 min. [26] The lateralization of seizure focus, and the type of seizures (simple partial, CP, GTC) did not play a statistically significant role in the frequency and the extent of tachycardia. No tachyarrhythmias were recorded. [26]
For related information, see Atrial Tachycardia.
Bradycardia and cardiac arrest
Sustained cardiac bradyarrhythmias and asystole associated with seizures are reported less frequently in the literature than tachyarrhythmias and are most likely secondary to parasympathetic autonomic dysfunction. Cardiac bradyarrhythmias and arrest have been documented in both generalized and complex partial seizures. [37] Nashef et al reported bradycardia in most patients who experienced central apnea during seizure. [19] In their study of 90 seizure attacks, Schernthaner et al reported ictal bradycardia (heart rate decrease >10 bpm) in 3% of seizures. [21] In this study, bradycardia was observed only in seizures of frontal lobe origin. [21] Similar findings have been documented in 3-11% of patients with complex partial seizures, mostly those of temporal lobe origin. [9, 15, 24, 38, 39]
The localization of the epileptic focus with prominent bradycardia has been shown to be in the left temporal area in some studies. [40] This finding might need further studies to clarify, as there is also evidence supporting a bilateral origin of ictal events. [41, 42]
Reeves et al documented the syndrome of ictal bradycardia in 27 patients with simultaneous EEG and ECG recordings. [20] Diagnosis was made after documentation of bradycardia/asystole, syncope, and EEG evidence of preceding epileptic activity. Patients suffered prolonged decreases in heart rate that began during the seizure but persisted after the seizure stopped. Simultaneous EEG showed generalized slowing, possibly secondary to cerebral hypoperfusion as well as to postictal effects. [20] Although this phenomenon could potentially have a fatal outcome, no cases of death by this mechanism have been documented. Eighty-seven percent of the seizures originated from temporal lobe foci, and the remainder from frontal and occipital lobes. [20] Complete atrioventricular block has been documented during partial epileptic attacks.
Ictal bradycardia is postulated to be the cause of loss of consciousness resembling syncope in some patients. This might impose a diagnostic challenge (see Seizure Versus Syncope). [18, 36, 43, 44, 45]
Rugg-Gunn et al evaluated prolonged ECG recorded over 1 month in a small group of refractory patients and delineated similarities between this patient population and the population at high risk for sudden unexpected death in epilepsy (SUDEP). [26] A loop recorder was implanted in 20 patients and the heart rate changes were evaluated in 327 seizures over 22,000 patient-hours. Ictal bradycardia (< 40 bpm) was rare, occurring in 8 (2.1%) recorded events in 7 patients. Four patients (21%) had bradycardia or periods of asystole with subsequent permanent pacemaker insertion, and 3 of these 4 (16% of the total) had potentially fatal asystole. [26] The type of antiepileptic medications was not significant.
ECG could be interpreted during 377 seizures. [26] The authors reported only rare (0.24%) bradycardias with heart rate less than 40 bpm. However, these occurred in 4 of the 20 patients (21%). Three of the 4 patients (16% of the total) proved to require a permanent pacemaker placement. [26] The seizure focus in patients with ictal bradycardias proved, as in most other studies, to be in the temporal areas (75%), and in temporal lobes (75%). The type of antiepileptic medications was not significant.
Heart rate variability
Heart rate variability (HRV) during a seizure can be calculated from ECG recorded simultaneously with EEG. HRV before, during, and after the seizure can be an indicator of the sum of sympathetic and parasympathetic input to the heart. Novak et al documented rapid parasympathetic withdrawal approximately 30 seconds before seizure onset and a sympathetic activation peak at seizure onset. [46]
In a group of patients with secondarily generalized complex partial seizures, Delamont et al reported an increase in parasympathetic activity before the seizure to above normal values as well as a significant fall to previously established normal values following the seizure. [47] The investigators proposed that preictal elevation of cardiac parasympathetic activity may be a marker for secondary generalization of seizures.
Al-Aweel et al evaluated HRV in frequency domain and demonstrated an increase in immediate postictal low-frequency oscillations. [48] This is yet another indicator of postictal autonomic instability. Increased sympathetic and decreased vagal HR modulation often precede the electroclinical onset and ictus of temporal lobe seizures. The postictal period is characterized by decreased vagal HR modulation that persists for considerably longer after secondarily generalized seizures.
Decreased HRV is known to increase the vulnerability of cardioregulatory centers, leading to an increase in ventricular automaticity, and potentially to arrhythmia (see Mechanism, below).
Subjective sensations
Cardiac and thoracic sensations are another aspect of cardiovascular involvement. Patients may report palpitations or irregular heartbeats during a seizure attack. Patients have reported subjective awareness of heart pounding in the absence of ECG changes. [49] A very early study reported angina during a seizure attack [50] ; and Devinsky et al reported atypical angina as the primary epileptic manifestation in 5 patients. [38]
Mechanism of ictal arrhythmogenic potentials and the ANS
In an animal model, Lathers et al investigated the "lockstep phenomenon," concluding that this phenomenon might explain propagation of the electrical impulse to central ANS regulatory centers, thus provoking arrhythmogenic potentials. [51] The investigators recorded cardiac autonomic neuronal discharges in anesthetized cats, and under EEG monitoring, seizures were induced by intravenous injection of pentylenetetrazol. Cardiac postganglionic sympathetic and vagal discharges were synchronized with both ictal and interictal discharges. [51] Premature ventricular contractions, ST/T changes, and conduction blocks occurred during interictal spikes.
In a study by Goodman et al, hypertension and bradycardia were induced after temporal lobe seizures in kindled rats, with the results indicating that amygdaloid kindled seizures activate both branches of the ANS. [52] Bradycardia was mediated by activation of the parasympathetic system, whereas the pressor response was caused by an increase in peripheral resistance due to alpha-adrenergic receptor activation. In a similar model of kindling in rats, bradycardia lasted up to a week postictally. [53] In a rat study, stimulation of the thalamus caused seizures and subsequently a variety of cardiac arrhythmias as well as hypotension in association with both ictal and interictal discharges. [54]
Zaatreh et al evaluated the association between the baseline interictal epileptiform discharges and autonomic output in humans and demonstrated: (1) brief bradycardia in the heartbeat after right hemispheric interictal discharges and (2) brief tachycardia after left hemispheric interictal discharges. [55] The time span between 2 heartbeats in the ECG (RR interval) was measured during interictal discharges in the EEG and with the RR interval immediately after the interictal activity; 200 right-sided and 200 left-sided interictal discharges were compared. With the activity on the right, 116 had an RR prolongation (brief bradycardia), whereas only 17 had RR shortening (brief tachycardia). [55] However, on the left side, in 100 cases, an RR shortening was noted, and, in 31 cases, an RR prolongation was noted.
Most ictal cardiovascular events have been reported in temporal lobe complex partial seizures. Frontal lobe seizures are known to cause bradyarrhythmias more often than temporal lobe seizures. Oppenheimer et al reported that stimulation of the left anterior insula causes bradycardia and depressor responses [35] ; the investigators documented tachycardia and pressor responses with right insular stimulation. These findings have been confirmed by other researchers. [39] Inactivation by injection of amobarbital caused reverse results; ie, heart rate increased after left hemisphere inactivation and decreased after right hemisphere inactivation. [56] In addition, the time lag between ictal spikes and the induced cardiac changes was documented to be longer with frontal lobe than with temporal lobe seizures. [21]
Generally, tachycardia occurs before EEG changes or early during the attack. In one study, heart rate changes occurred several seconds before seizure onset as recorded by scalp EEG in 76.1% of seizures and by invasive EEG in 45.7% of seizures. [21] Bradycardia, however, is usually a late manifestation and is of shorter duration. After combined parasympathetic and sympathetic activation, rapid parasympathetic withdrawal occurred approximately 30 seconds before the seizure and sympathetic activation peaked at seizure onset. [46]
Stimulation of both human insular cortices causes changes in heart rate and blood pressure. [35] Neuronal discharges in human mesial temporal structures, amygdala, and hippocampus are synchronized with the cardiac cycle and, to some extent, with the respiratory cycle. [57] Seizures that have their foci in the temporal lobe propagate easily to the centers in the brain that regulate the activities of the ANS (ie, amygdala and hippocampus). This phenomenon was easier to explain after Leutmezer et al showed the ictal tachycardia preceded EEG seizure onset by 14 seconds in the temporal lobe and by 8 seconds preceding EEG seizure onset in extratemporal origins of seizures. [24] . One study by Behbahani et al [58] showed clearly that patients with a right-sided focus showed more reduction in parasympathetic activity and more increase in sympathetic activity. As a result, a right-sided seizure can cause more tachycardia and a left-sided seizure can cause more bradycardia.
In both animal models and humans, stimulation and recording studies implicate the amygdala in the control of heart rate, blood pressure, and respiration. [59] The amygdala receives both direct and indirect projections from the ANS afferents and projects into the hypothalamus and brainstem centers for ANS homeostasis. In a study of 27 temporal lobe seizures monitored by depth and subdural electrodes, Epstein et al documented that limbic ictal involvement is essential for cardioregulatory changes. [17] Restricted amygdaloid seizure activity, however, was generally insufficient to alter heart rate. The investigators postulated that ictal heart rate changes depend on the volume of the brain involved and not on duration of the attack.
The general level of sympathetic activation is another contributory factor to cardiovascular changes. Increases in sympathetic discharge and plasma catecholamines peak 30 minutes after tonic-clonic seizures. [60] High-level spinal anesthesia blocked the initial tachycardia and hypertension accompanying a few generalized seizures. [13] In addition, myocardial fibrosis has been reported to develop in patients with repetitive exposure to catecholamine toxicity. These areas of degeneration and fibrosis can, in turn, serve as new foci for tachyarrhythmias.
Summary of ictal cardiovascular events
Studies contributing to our understanding of ictal cardiovascular events are based on recordings from ictal events in either animal models or patients with epilepsy, as well as knowledge of central autonomic regulation. Although involvement of the amygdala in the electrical event is postulated to cause most of the autonomic changes, propagation to the whole limbic system seems to be necessary.
Provoked temporal lobe seizures in kindled rats can activate both branches of the ANS. A variety of cardiac arrhythmias and hypotension have been documented with both ictal potentials and interictal spikes in these models; this is described as the "lockstep phenomenon." Most ictal cardiovascular events have been reported in temporal lobe complex partial seizures. Frontal lobe seizures are known to cause bradyarrhythmias more often than tachyarrhythmias. In frontal lobe seizures, time lags between seizure spikes and the induced cardiac changes were longer than in seizures with temporal lobe foci. This is thought to be due to the longer distance of frontal lobe from the limbic system.
Stimulation of left anterior insula in humans can cause bradycardia and depressor responses, whereas stimulation of right insular cortex induces tachycardia and pressor response.
Careful examination of HRV before ictal events indicates combined parasympathetic and sympathetic activation; rapid parasympathetic withdrawal occurred approximately 30 seconds before the seizure, and sympathetic activation peaked at seizure onset. [46] This is accompanied by tachycardia before EEG changes or early during the attack. Bradycardia, however, is usually a late manifestation, and is of shorter duration.
Massive sympathetic discharge can be the cause of potentially fatal ictal arrhythmias. Moreover, damage to the myocardium caused by frequent increases in plasma catecholamines can produce areas of degeneration and fibrosis that can serve as new foci for tachyarrhythmias in the interictal state.
Further evaluation of autonomic cardiovascular activity can reveal information about the focality, propagation, and nature of seizure activity.
The changes in autonomic functions during partial seizures and generalized seizures are well-established facts now. Obtaining ECG tracings is easier and more readily available than EEG. Increased ANS activity can even potentially serve as a biomarker to indirectly detect a seizure. The ANS is more activated during a complex partial seizure than during a psychogenic nonepileptic seizure. [61]
The postictal state represents a period during which the patient has not completely regained his or her baseline. Clinically, lethargy, psychomotor slowing, confusion, and even depression and paranoia can be detected. The EEG often shows slowing, which can be focal or diffuse, depending on the nature of the seizure. This period has gained much attention in the evaluation of autonomic functions in the aftermath of a seizure. Theoretically, because the electrophysiological state of balance has not yet been reached, the tendency to have abnormalities in autonomic tone can still persist. In a few studies, a correlation between the degree of EEG slowing and the extent of hypotension and autonomic dysregulation has been established. [62]
Respiratory manifestations
Apnea, hypoventilation, and hyperventilation occur during and after a GTC seizure. Berger first documented a series of autonomic phenomenon, including apnea, at seizure onset. [63] Apnea in the context of a partial seizure can occur with bradycardia [64] or without bradycardia. [65] Nashef et al reported apnea in 38% of a group of patients with various types of seizures. [19] All of these patients had central apnea, but obstructive apnea also was documented in about 30%. [19] Oxyhemoglobin saturation (SpO2) dropped to less than 85% in 10 patients with partial seizures of temporal lobe origin. [19, 66]
In one multicenter study, Lacuey et al [67] evaluated 312 seizures in 126 patients. Ictal central apnea (ICA) occurred exclusively in focal epilepsy (51/109 patients [47%] and 103/312 seizures [36.5%]) (P < .001). ICA was the only clinical manifestation in 16/103 (16.5%) seizures, and preceded EEG seizure onset by 8 ± 4.9 s, in 56/103 (54.3%) seizures. ICA ≥ 60 s was associated with severe hypoxemia (SpO2 < 75%). Focal onset impaired awareness (FOIA) motor onset with automatisms and FOA nonmotor onset semiologies were associated with ICA presence (P < .001), ICA duration (P = .002), and moderate/severe hypoxemia (P = .04). Temporal lobe epilepsy was highly associated with ICA in comparison to extratemporal epilepsy (P = .001) and frontal lobe epilepsy (P = .001). Isolated postictal central apnea was not seen; in 3/103 seizures (3%), ICA persisted into the postictal period. ICA is a frequent, self-limiting semiological feature of focal epilepsy, often starting before surface EEG onset, and may be the only clinical manifestation of focal seizures. However, prolonged ICA (≥ 60 s) is associated with severe hypoxemia and may be a potential SUDEP biomarker. ICA is more frequently seen in temporal than extratemporal seizures, and in typical temporal seizure semiologies. ICA rarely persists after seizure end.
The respiratory brainstem control centers are interlinked closely with the cardiomodulatory centers. As such, the potential mechanisms for ictal cardiovascular changes already described also may apply to respiration. [68]
Gastrointestinal/abdominal manifestations
Ascending abdominal sensations are among the most common early symptoms of partial seizures. These phenomena are associated particularly with seizures arising from mesial temporal foci. [69, 70, 71, 72] Dyspepsia, pain, hunger, borborygmi, nausea, vomiting, belching, urge to defecate, and fecal incontinence also have been reported. Abdominal pain is common, especially in children. Vomiting also can occur in seizures with opercular, inferior temporal, or occipital foci. [73] Afferent autonomic fibers play a key role in the epigastric sensations, whereas efferent pathways cause belching, vomiting, and defecation. [49]
Panayiotopoulos syndrome has been classified as a common benign childhood epilepsy with frequent autonomic symptoms, especially vomiting. Panayiotopoulos syndrome is the diagnosis when at least 5 of the following 8 diagnostic criteria are present [74] :
-
Infrequent seizures (up to 5)
-
Prolonged seizures (>5 min)
-
Ictal vomiting
-
Ictal eye deviation
-
Ictal autonomic manifestations
-
Ictal behavioral disturbances
-
Gradual suppression of consciousness during seizures
-
Convulsions
Urinary and genital manifestations
Incontinence and urgency frequently accompany seizures. Incontinence is caused by external sphincter relaxation in GTC seizures and by detrusor muscle contraction in absence seizures.
Erotic feelings, genital sensations, and orgasm are rare ictal phenomena. During a seizure, genital sensations result from stimulation of postcentral sensory cortex. Sexual arousal is reported in seizures with limbic and temporal lobe involvement. Orgasm can be reached in seizures that involve the hypothalamus. [75]
It is speculated that the great Russian writer Dostoevsky (1821-1881) suffered from a rare form of temporal lobe epilepsy termed ecstatic epilepsy, also called Dostoevsky epilepsy. It is reported that sexual fantasies and, rarely, even orgasms were reached by him during his seizures. [76] Dostoevsky alleged (via one of his characters) that when he had a seizure, the gates of Heaven would open and he could see row upon row of angels blowing on great golden trumpets. Then 2 great golden doors would open and he could see a golden stairway that would lead right up to the throne of God.
Erectile dysfunction with intact libido in men with epilepsy has been known to researchers since the 1950s. [77] Hyperprolactinemia resulting from complex partial seizures has been postulated to contribute to male sexual dysfunction in epilepsy. [78]
Cutaneous manifestations
Unilateral or bilateral flushing, erythema, cyanosis, blanching, or pallor can be manifestations of temporal lobe seizures. Piloerection as a seizure manifestation has been reported. [79] Piloerection often accompanies epigastric sensations, and blanching may accompany nausea. In an animal model, piloerection occurred after stimulation of the amygdala. [68]
Pupillary manifestations
Mydriasis, miosis, and hippus can occur as manifestations of a partial seizure and can be unilateral. [80]
Interictal (Baseline) Autonomic Changes
Many researchers have evaluated the autonomic nervous system (ANS) during the interictal period.
The autonomic cardiovascular reflexes are the most substantial part of autonomic functions. A standardized test battery is used in ANS laboratories. Electrocardiographic (ECG) patterns and blood pressure are measured continuously while the patient is undergoing certain physical, postural, and mental changes. With the help of specially designed software programs the heart rate and blood pressure variability are calculated from the data obtained from ECG and blood pressure measurements.
Standard tests for this evaluation include assessment of heart rate variability (HRV) during rest, deep breathing, Valsalva maneuver, face immersion in cold water, and gravitational challenge with the passive tilt-table test. [81, 82, 83, 84] The results are evaluated in both time and frequency domains. HRV during rest, deep breathing, and Valsalva maneuver provides indications of cardiovascular parasympathetic function. Blood pressure variability during Valsalva maneuver, isometric exercise, and orthostatic challenge (ie, tilt table) reflects sympathetic functions.
In general, the interictal HRV studies suggest that sympathetic overactivity happens in most forms of epilepsy. Specifically, the autonomic dysregulation is more prominent in temporal lobe epilepsy and in refractory epilepsy. [85] Furthermore, during sleep, some individuals might be more prominently affected than during wakefulness. [86]
Evaluation of autonomic cardiovascular reflexes in patients with epilepsy indicates dysfunction of both the sympathetic component [46, 87, 88, 89, 90] and to a higher level; the parasympathetic division. [46, 87, 89, 91, 92, 93] Furthermore, hypofunction of autonomic cardiovascular reflexes is postulated to be more prominent in patients who also are at a high risk for sudden unexpected death in epilepsy (SUDEP), including those with more refractory seizure disorders. [87, 90] A study of HRV during sleep in 11 children with epilepsy confirmed the above findings. [94]
A more recent evaluation method for HRV, fractal correlation properties, evaluated the approximate entropy (ApEn) of the RR intervals. Using this method, Ansakorpi et al assessed the HRV in patients with temporal lobe epilepsy. [93] After comparison of 24-hour recordings of ambulatory electroencephalography (EEG), the investigators showed that, in 19 patients with refractory temporal lobe epilepsy, the HRV was more significantly impaired relative to the 25 patients with well-controlled temporal lobe epilepsy. [93]
A recent meta-analysis evaluated the contents of 30 references. The common finding in patients with epilepsy when compared with an age- and sex-matched general population was a decrease in HF HRV, a marker of vagal regulation of the heart. [95] Other markers of sympathovagal imbalance, including SDNN and RMSSD in the time domain, showed similar results across the studies.
Table 3. Review of Some Studies on Interictal Autonomic Cardiovascular Reflexes in Patients with Epilepsy (Open Table in a new window)
Case Series |
Subjects |
Time Domain |
Freq Domain |
Results |
||||||||
Seizure patients |
Norm |
HRV DB |
HRV rest |
HRV VM |
HRV Tilt |
BP Tilt |
Low Freq (S) |
High Freq (PS) |
||||
N |
Type |
Focus |
||||||||||
Kalviainen et al (1990) [91] |
15 |
BM |
— |
14 |
— |
— |
— |
N |
— |
— |
— |
PS dysfunction |
Devinsky et al (1994) [96] |
24 |
CP |
T |
40 |
inc |
— |
— |
— |
— |
— |
— |
AED induced |
Massetani et al (1997) [87] |
65 |
Mix |
T |
50 |
— |
— |
— |
— |
— |
dec |
dec |
S and PS dysfunction |
Drake et al (1998) [92] |
20 |
GTC |
— |
20 |
inc |
— |
— |
— |
— |
— |
— |
PS dysfunction |
Tomson et al (1998) [88] |
21 |
JME |
— |
21 |
— |
N |
— |
— |
— |
N |
N |
N |
Tomson et al (1998) [88] |
21 |
CP |
T |
21 |
— |
dec |
— |
— |
— |
dec |
N |
N |
Isojarvi et al (1998) [89] |
84 |
CP |
— |
50 |
dec |
N |
N |
dec |
N |
— |
— |
S and PS dysfunction |
Novak et al (1999) [46] |
12 |
CP |
T |
— |
— |
— |
— |
— |
— |
dec |
dec |
S and PS dysfunction |
Ansakorpi et al (2000) [90] |
38 |
CP |
T |
38 |
N |
dec |
N |
dec |
N |
— |
— |
S and PS dysfunction |
Ferri et al (2002) [94] |
11 |
SP/CP |
— |
11 |
— |
dec |
— |
— |
— |
dec |
dec |
Sleep S and PS dysfunction |
El-Sayed et al (2007) [97] |
25 |
— |
— |
50 |
dec 49% |
dec 8% |
dec 28% |
— |
— |
— |
— |
S and PS dysfunction |
Hallioglu et al (2008) [98] |
92 |
— |
— |
83 |
— |
dec |
— |
— |
— |
— |
dec |
PS dysfunction only in patients without AED |
Harnod et al (2009) [99] |
25 |
CP |
F |
25 |
— |
— |
— |
— |
— |
— |
dec |
PS dysfunction |
Chroni et al (2009) [100] |
71 |
— |
— |
71 |
dec |
dec |
dec |
dec |
— |
— |
— |
PS dysfunction |
Yildiz et al (2011) [101] |
37 |
— |
— |
32 |
— |
dec |
— |
— |
— |
— |
dec |
PS dysfunction |
Ansakorpi et al (2011) [102] |
36 |
— |
— |
36 |
— |
dec |
— |
— |
dec |
— |
dec |
PS dysfunction |
El-Rashidey et al (2015) [6] |
60 | Mix | 20 | dec | - | - | - | - | inc | dec | decresaed PS increased S both improving after ASM treatment |
|
Sivakumar et al (2016) [103] |
91 | IGE | - | 25 | increased PS during sleep | |||||||
AED = antiepileptic drugs; ApEn = approximate entropy; ASM= antiseizure medications; BM = Baltic myoclonus epilepsy; BP = blood pressure; CP = complex partial; DB = deep breathing; dec = decreased; Freq = frequency; GTC = generalized tonic-clonic; HRV = heart rate variability; IGE = idiopathic generalized epilepsy; inc = increased; JME = juvenile myoclonic epilepsy; mix = different types of epilepsies; N = within normal limits; PS = parasympathetic; rest = at rest; S = sympathetic; SP = simple partial; T = temporal lobe; tilt = during tilt-table test, VM = Valsalva maneuver |
||||||||||||
The mechanism of dysfunction of the ANS in epileptic seizures is likely to be multifactorial. Interictal spikes have been shown to cause arrhythmias in animals. [51] Also, the autonomic control centers may have undergone physiologic or anatomic alterations. Interictal hypometabolism sometimes seen in the area adjacent to the epileptic focus on positron emission tomographic (PET) scanning studies, could, for example, underlie such functional changes. [104]
Autopsies of patients with epilepsy who experienced sudden unexpected death (ie, SUDEP) have shown fibrosis of the cardiac conductive system in 33%. [105, 106] Repetitive exposure to catecholamines is known to cause myocardial fibrosis. These fibrotic areas can act, per se, as new foci for cardiac arrhythmias. Also, antiepileptic drugs (AEDs) may play a role in modification of ANS functions. In a study of the cardiovascular reflexes in 24 patients with epilepsy, Devinsky et al documented increased HRV in this group, attributed at least partially to carbamazepine. [96] Other researchers have reported similar findings. [88, 89]
Decreased HRV is known to increase the vulnerability of the cardioregulatory centers, leading to an increase in ventricular automaticity, in turn predisposing to arrhythmias. This is particularly crucial, because autonomic cardiac arrhythmias may contribute significantly to the phenomenon of SUDEP.
In addition to using ECG for evaluation of arrhythmias and HRV, other methods have been used to evaluate the autonomic innervations of the cardiac muscle. Single photon emission computer tomography (SPECT) scanning using iodine-123 (123 I)-meta- iodobenzylguanidine (MIBG) has been used for this purpose. MIBG-SPECT scanning is an established method to quantify cardiac postganglionic norepinephrine uptake. In one study, cardiac MIBG uptake was significantly decreased compared with healthy individuals, suggesting altered postganglionic sympathetic activity. [107] Also, the HRV of these patients was compared with the control group, which showed a comparable decline. Carbamazepine had no effect on the cardiac norepinephrine uptake.
In a later study, this group showed the postganglionic sympathetic activity was more impaired in patients with documented ictal bradycardia when compared with either age-matched patients with temporal lobe epilepsy without ictal bradycardia or with controls. [108]
El-Rashidey et al compared the HRV in children with epilepsy before and after being treated with ASM. These values were compared to age- and sex-matched healthy children. They noticed an increased sympathetic and decreased parasympathetic tone compared to healthy children, as noted by previous investigators. Interestingly, these values improved after treatment with ASM. [6]
Autonomic Functions and SUDEP
Patients with epilepsy have a mortality rate that is 2-3 times that of the general population because of epilepsy-related deaths. The phenomenon of sudden unexplained death in epilepsy (SUDEP) may account for 8-17% of deaths in patients with epilepsy. [109] SUDEP is defined as sudden, unexpected, nontraumatic, nondrowning death in an individual with epilepsy, witnessed or unwitnessed, in which postmortem examination does not reveal an anatomic or toxicologic cause for the death. This phenomenon has been discussed in detail in the article Sudden Unexpected Death in Epilepsy.
The incidence of SUDEP is estimated to be 0.35 per 1000 person-years of follow-up in this population. Known associated demographic risk factors for SUDEP include male sex and an average age of 28-35 years.
Seizure-related risk factors include the following:
-
Symptomatic epilepsy
-
Generalized tonic-clonic (GTC) seizure
-
Onset at a younger age
-
Duration of longer than 10 years
-
Higher number of attacks
The following are proposed treatment-related risk factors [105, 106, 110, 111, 112, 113, 114] :
-
Subtherapeutic antiepileptic drug (AED) levels
-
Higher number of AEDS
-
Recent changes in AED regimen
-
History of surgical treatment for seizures
Although autopsy, by definition, fails to reveal the underlying cause of death, several autopsy reports confirm a variety of findings in the organs of the victims. In addition to the underlying cerebral pathology in patients with symptomatic epilepsy, cerebral edema, signs of hypoxia in the hippocampal area, and sclerosis of the amygdala have been reported. [105, 115, 116, 117] Mild to moderately severe pulmonary edema with protein-rich fluid and alveolar hemorrhage were seen in 62-100% of all specimens from SUDEP patients. [105, 116] Cardiac nonfatal pathologic findings, including fibrosis of the conductive system, have been reported in 33% of patients. [105, 106]
Various pathophysiologic events may contribute to SUDEP, as follows:
-
Cardiac arrhythmias, during the ictal and interictal periods, leading to arrest and acute cardiac failure may contribute significantly to SUDEP. [15, 20, 21, 22, 38, 39, 119, 120] Decreased baseline HRV is indicative of impaired autonomic cardiovascular reflexes in epilepsy. This can cause an increase in ventricular automaticity, in turn predisposing to arrhythmias. Catecholamine surges during repeated seizures can cause cardiac conduction system fibrosis and arrhythmias. Anatomic and functional changes in the cardiac and pulmonary function are evident and might be a direct or indirect consequence of autonomic dysregulation.
-
A recent seizure might cause central apnea, often associated with bradyarrhythmias.
These physiologic events might develop in combination (see the image below). For example, central apnea and cardiac bradyarrhythmias might have common central inducing mechanisms. A seizure has been reported to be the immediate terminal event in 30-80% of witnessed SUDEP cases. [116, 124] Cardiac arrhythmias are the main postulated mechanism in these cases. Dysfunctions of the autonomic cardioregulatory centers, on the other hand, would increase the automaticity and decrease the threshold for arrhythmias.
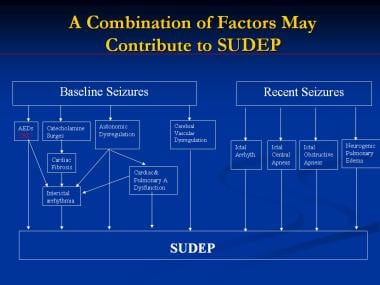 A combination of factors may contribute to sudden unexpected death in epilepsy (SUDEP). AEDs = antiepileptic drugs; arrhyth = arrhythmia; CBZ = carbamazepine.
A combination of factors may contribute to sudden unexpected death in epilepsy (SUDEP). AEDs = antiepileptic drugs; arrhyth = arrhythmia; CBZ = carbamazepine.
Systematic evaluation of autopsies of the brains of individuals who passed due to SUDEP have shown both nonspecific and specific anatomical changes. [2, 3, 125]
In one study, Muller et al attempted predicting if mesencephalic atrophy, seen on MRI in pateints with epilepsy and impaired HRV, is associated with future SUDEP. Two populations were studied: (1) Autonomic system function population (ASF, 18 patients with focal epilepsy, 11 controls) with HRV measurements and standardized 3 T MR exams; (2) SUDEP population (26 SUDEP epilepsy patients) with clinical MRI 1–10 years before SUDEP. In conclusion, epilepsy is associated with brainstem atrophy that impairs autonomic control and can increase the risk for SUDEP if it expands into the mesencephalon.
In summary, although autonomic dysfunction is known to be associated with epileptogenic activity, its importance as a contributory risk factor to potential fatal outcomes for this population is yet to be determined. Evaluation of autonomic cardiovascular and respiratory reflexes in patients with epilepsy can provide us with valuable information on the mechanism of SUDEP.
AEDs and Autonomic Changes
Antiepileptic medications (AEDs) can have a series of effects on the autonomic nervous system (ANS). Arrhythmia, hypotension, and respiratory depression are frequent adverse effects of these drugs, as shown in Table 4, below. However, these medications may have a stabilizing effect on cellular membranes, and thus have anti-arrhythmic properties. Table 4. Autonomic Effects of Antiepileptic Medications
Table. (Open Table in a new window)
Antiepileptic Medication |
Autonomic Manifestations |
Diazepam, lorazepam |
Hypotension, respiratory depression |
Carbamazepine, oxcarbazepine |
QRS prologation, Respiratory depression, cardiac arrhythmias, conduction defects, anticholinergic findings |
Ethosuximide |
Gastrointestinal disturbance |
Felbamate |
Gastrointestinal disturbance |
Lacosamide |
PR prolongation, syncope |
Lamotrigine |
QRS prolongation |
Phenobarbital, pentobarbital |
Hypotension, respiratory depression |
Phenytoin |
Arrhythmia, hypotension |
Valproic acid |
Hypotension, gastrointestinal disturbance |
Investigators of a few studies have reported that administration of carbamazepine decreases heart rate variability (HRV) and causes parasympathetic hypofunction. Tomson et al demonstrated that patients on carbamazepine had significantly lower values for a few parameters of HRV (ie, standard deviation of RR intervals, low frequency power, and low frequency/high frequency power ratio) than did their age-matched, healthy, drug-free controls. [88] Also, abrupt carbamazepine withdrawal can cause decreased HRV. [123]
Findings from these studies indicate a potential increase in cardiac arrhythmias associated with carbamazepine use and withdrawal. [88, 123] Carbamazepine can also predispose especially elderly patients to bradyarrhythmias by slowing conduction across the atrioventricular node. Similar effects have been noted in the case of lamotrigine. [126] Lacosamide, one of the newer antiepileptics on the US market, has been shown to cause QRS prolongation on ECGs, as well as syncope. However, this is not shown to contribute significantly to risk of SUDEP.
However, few recent studies have shown that patients on AEDs have a less impaired regulation of the autonomic cardiovascular reflexes than other patients with epilepsy who are not being treated. This might be an indication that seizure control may help improve the cardiac autonomic function and the prognosis. [98]
Go to Antiepileptic Drugs for complete information on this topic. [127]
Seizure Versus Syncope
Loss of consciousness is a frequent reason for seeking medical care. The most frequent causes are cardiovascular (eg, arrhythmia, decreased blood pressure) or neurologic (eg, seizure, stroke). [128, 129] Loss of consciousness can be a diagnostic challenge both for neurologists and cardiologists. In 60% of cases, the cause is obvious from the clinical picture.
Syncope can occur with abrupt cessation of the cerebral perfusion for 6-8 seconds, or a reduction of only 20%. Clinical findings suggestive of a cardiovascular pathophysiology are dizziness or lightheadedness before the event and regaining consciousness, shortly after resuming the supine position. In patients with loss of consciousness caused by seizures, clonic jerks, and prolonged confusion after the event are more frequent. Below, Table 5 highlights the major findings in seizure and syncope that can help distinguish these 2 entities. Inability to make this distinction correctly may lead not only to misdiagnosis in approximately 40% of patients but also to treatment that is ineffective and potentially dangerous.
Table 5. Features That Distinguish Between Seizure and Syncope (Open Table in a new window)
Findings |
Seizure |
Syncope |
Prodrome |
Specific auras |
Symptoms of cerebral hypoperfusion - Lightheadedness, dizziness, dimming vision, and hearing Signs of sympathetic activation - Sweating, hyperventilation, anxiety |
Onset |
Rapid |
Gradual |
Vital functions |
Usually tachycardia |
Bradycardia or tachycardia, hypotension |
Position |
Can be in any position |
Usually in upright position |
Wakefulness |
Can be sleep related |
Almost never in sleep |
Motor activity |
Tonic-clonic activity, automatisms common |
Can have tonic posturing, clonic jerks, rarely automatisms |
Incontinence |
Common |
Uncommon |
Oral and head trauma |
Common |
Uncommon |
Return of consciousness |
Gradual |
Prompt |
Postictal confusion |
Common, lasts hours to days |
Seconds, rarely 1 minute or longer |
EEG during attack |
Typically shows ictal rhythmic activity |
Slowing, indicating cerebral hypoperfusion |
ECG during attack |
Can show tachycardia or arrhythmia |
Can show tachycardia, bradycardia, or arrhythmia |
Holter monitoring |
Unremarkable |
May show arrhythmia |
Tilt table |
No change |
Can induce syncope |
ECG = electrocardiography; EEG = electroencephalography. |
||
At one end of the spectrum, patients who lose consciousness because of acute cardiovascular failure, as in hypotension or arrhythmia, may have myoclonic jerks, tonic spasm, and urinary incontinence during the event. [130] These are probably induced by transient cerebral hypoperfusion, releasing motor centers in the upper brain stem from inhibition. In this group, interictal electroencephalography (EEG) may be normal. When these patients undergo evaluation of autonomic cardiovascular reflexes, they can show not only decreased heart rate variability (HRV) but also syncope during the tilt-table test, which may be accompanied by some myoclonic jerks if the tilt is not reversed promptly.
Simultaneous EEG recording during tilt-table testing typically shows no evidence of seizure but rather progressive slowing, sometimes progressing to flattening, a sequence typical of cerebral hypoperfusion. [131] When diagnosed correctly, these patients do not require antiepileptic medications; rather, they need further evaluation and treatment of their cardiovascular disorders.
At the other end of the spectrum, patients with epilepsy may have cardiac arrhythmias, including asystole, which in turn can cause loss of consciousness. Many studies, as mentioned above, have documented these cardiovascular events during seizures. When diagnosed appropriately, such patients may benefit more from antiepileptic medications than from treatment of their cardiac pathologies, although a pacemaker can be protective for those with ictal bradyarrhythmias whose seizures cannot be controlled. Ictal asystole with convulsive syncope mimicking secondary generalization is an interesting dilemma that can represent a challenging clinical diagnosis. [40]
In prolonged QT syndrome, defects in the potassium and sodium channels with superimposed cardiac sympathetic imbalance result in prolongation of the myocyte action potential and trigger ventricular arrhythmias. Patients with this syndrome, especially those with the familial form, have a high rate of seizures and sudden death. Both children and adults with history of loss of consciousness after a seizure require an electrocardiogram (ECG) to rule out this syndrome. [132, 133] Treatment with phenytoin can aggravate the associated arrhythmias.
Very little evidence exists to clarify the potential influence of ANS on cortical epileptogenic foci. The general level of excitement in some patients seems to contribute to their seizure susceptibility. The ANS also may be involved in certain forms of reflex epilepsy. In addition, cardiac arrhythmias potentially can cause cerebral hypoperfusion, leading in some cases to convulsive activity and rarely to actual cortical epileptic activity. [134]
Treatment Considerations
The implications of the interaction between epilepsy and the autonomic nervous system (ANS) are multiple.
Clinicians need to understand this interaction, so that patient events are properly interpreted. For example, if the prominent feature of the event is autonomic manifestations, this does not exclude a seizure as the primary pathology.
In the case of patients who are at high mortality risk owing to the cardiovascular or respiratory complications of seizures, proper measure should be attempted. The most widely encountered example is complex partial seizures that cause bradyarrhythmias and cardiac arrest. [135] A pacemaker is a reasonable choice in most of these patients. Use of continuous airway positive pressure (CPAP) machine in patients with potential apnea is another example. Finally, a vagal nerve stimulator is known to improve autonomic cardiac regulation. [136]
Certain medications can increase the risk of cardiac arrhythmias and hypotension. On an individual basis, the clinician might consider avoiding these treatments. Phenytoin, carbamazepine, and lamotrigine might be in this category.
On the other hand, treatment of epilepsy can improve impaired autonomic dysfunction. Vagus Nerve Stimulation (VNS) is shown to improve autonomic dysregulation with time [4, 5] therefore potentially decreasing the risk of SUDEP. Hirfanguro et al evaluated the HRV in 20 pediatric patients before and 6–12 months after VNS implantation. The cardiovascular system is under deep sympathetic influence in children with epilepsy. Although VNS seems to provide a substantial improvement by achieving increased parasympathetic effects in short-term therapy, the levels were still lower than those of healthy children after either short- or long-term therapy.
A similar effect can be documented with ASM use. [6]
In conclusion, a brief, easily accesible battery of tests aimed at evaluation of autonomic cardiac functions in individuals with epilepsy might detect patients at risk of SUDEP and trigger a more comprehensive preventive approach.
-
Probable paths of propagation of the epileptic electrical activity to the limbic system and autonomic nuclei. Ambig = ambiguus; NTS = nucleus tractus solitarius.
-
A combination of factors may contribute to sudden unexpected death in epilepsy (SUDEP). AEDs = antiepileptic drugs; arrhyth = arrhythmia; CBZ = carbamazepine.