Practice Essentials
Helicobacter pylori (see the image below) is a ubiquitous organism that is present in about 50% of the global population. Chronic infection with H pylori causes atrophic and even metaplastic changes in the stomach, and it has a known association with peptic ulcer disease. [1] The most common route of H pylori infection is either oral-to-oral or fecal-to-oral contact. [2]
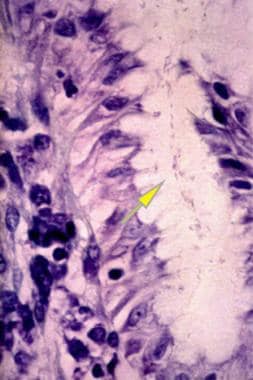 Helicobacter Pylori Infection. This image shows an antral gland of the stomach with a large Giemsa-stained colony of Helicobacter pylori in the lumen (arrow) at 250X power. Courtesy of Pantaleo Bufo, University of Foggia, Italy.
Helicobacter Pylori Infection. This image shows an antral gland of the stomach with a large Giemsa-stained colony of Helicobacter pylori in the lumen (arrow) at 250X power. Courtesy of Pantaleo Bufo, University of Foggia, Italy.
Signs and symptoms
In general, patients infected with H pylori are asymptomatic, and no specific clinical signs and symptoms have been described. When signs and/or symptoms are present, they may include the following:
-
Nausea
-
Vomiting
-
Abdominal pain
-
Heartburn
-
Diarrhea
-
Hunger in the morning
-
Halitosis (bad breath)
See Presentation for more detail.
Diagnosis
Testing
In patients with suspected H pylori infection, the following laboratory studies may aid in the diagnosis:
-
H pylori fecal antigen test: Very specific (98%) and sensitive (94%); positive results obtained in the initial stages of infection; can be used to detect posttreatment eradication
-
Carbon-13 urea breath test: Concentration of the labeled carbon is high in breath only when urease is present in the stomach, a reaction possible only with H pylori infection
-
H pylori serology: High (>90%) specificity and sensitivity; useful for detecting a newly infected patient but not a good test for follow-up of treated patients
Staging
There is no staging system for H pylori infection, but the following steps in the disease process are well described:
-
Chronic gastritis
-
Atrophic gastritis
-
Intestinal metaplasia: May evolve into dysplasia
-
Gastric adenocarcinoma: Consider ultrasonography and esophagogastroduodenoscopy (EGD) in patients with gastric MALTomas (mucosa-associated lymphatic tissue lymphomas) for more precise staging of the disease
Imaging studies
Imaging studies are not helpful in the diagnosis of H pylori infection. However, they may be useful in patients with complicated disease (eg, ulcer disease, gastric cancer, MALToma).
Procedures
-
EGD: Often necessary in patients with symptoms of peptic ulcer disease to view the condition of the mucosal lining of the stomach and duodenum
-
Biopsy plus EGD: To obtain biopsy specimens from the gastric antrum and corpus and to perform a histologic examination on the obtained specimens
-
Echography plus EGD: Mandatory in patients with positive biopsy results for gastric MALTomas to allow a more precise staging of the disease
See Workup for more detail.
Management
Only treat patients with a positive test result for H pylori infection. It is important to consider possible antibiotic resistance when selecting the treatment regimen.
Pharmacotherapy
Several triple therapy regimens for the treatment of H pylori infection in patients with gastric and duodenal peptic ulcer disease are used.
In the setting of any history of treatment with macrolides or fluoroquinolones, avoid clarithromycin- or levofloxacin-based regimens, respectively, because of the higher risk of resistance. Consider amoxicillin, tetracycline, and rifabutin as subsequent therapies in refractory H pylori infection as resistance to these antibiotics is rare.
If first-line therapy with bismuth quadruple therapy is ineffective, use shared decision making to select second-line options between (a) levofloxacin- or rifabutin-based triple-therapy regimens with a high-dose dual proton pump inhibitor (PPI) and amoxicillin, and (b) an alternative bismuth-containing quadruple therapy.
When using metronidazole-containing regimens, consider adequate dosing of metronidazole (1.5–2 g daily in divided doses) with concomitant bismuth therapy to improve success of eradication therapy.
All the eradication treatments have a high incidence of certain adverse effects (eg, nausea, metallic taste). If skin rash, vomiting, or diarrhea occurs, discontinue treatment.
Other medications used in the management of H pylori infection include the following:
-
Antidiarrheals (eg, bismuth subsalicylate)
-
Proton pump inhibitors (eg, lansoprazole, omeprazole)
-
H2-receptor blockers (eg, ranitidine, famotidine)
Surgical option
Surgical intervention is not required for patients with H pylori infection, but it may be a consideration for patients with severe complications, such as cancer.
See Treatment and Medication for more detail.
Background
In 1983, Warren (a biologist) and Marshall (a clinician) described Helicobacter pylori (HP). At first, they named the bacterium Campylobacter pyloridis. Later, it was named Campylobacter pylori. Since then, a large number of reports have been produced on H pylori and its pathogenetic potential.
In fact, although peptic ulcer disease is the most studied disease related to H pylori infection, this bacterium is seemingly involved in the pathogenesis of several extragastric diseases, such as mucosa-associated lymphoid tissue lymphomas (MALTomas), coronaritis (inflammation of coronary arteries), gastroesophageal reflux disease (GERD), iron deficiency anemia, skin disease, and rheumatologic conditions. However, at present, many of these associations remain largely uncertain, and the debate to confirm or refute causality related to these associations is still open.
The association of chronic H pylori infection with alterations in the gastric mucosal cell proliferation is recognized worldwide. In addition, H pylori can produce and release several bioactive factors that may directly affect the stomach's parietal cells, which produce hydrochloric acid, and enterochromaffinlike (ECL) cells (ie, G cells and D cells), which produce gastrin and somatostatin, respectively. Evidence suggests that H pylori inhibits D cells and stimulates G cells. H pylori has some control mechanisms that are able to switch on or off the transcription of different genes when needed. Two histology images are presented below.
 Helicobacter Pylori Infection. This image shows an antral gland of the stomach with a large Giemsa-stained colony of Helicobacter pylori in the lumen (arrow) at 250X power. Courtesy of Pantaleo Bufo, University of Foggia, Italy.
Helicobacter Pylori Infection. This image shows an antral gland of the stomach with a large Giemsa-stained colony of Helicobacter pylori in the lumen (arrow) at 250X power. Courtesy of Pantaleo Bufo, University of Foggia, Italy.
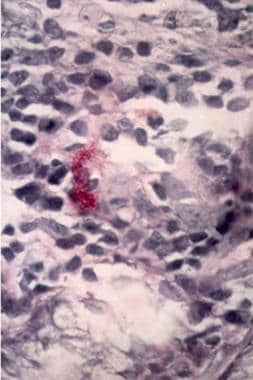 Helicobacter Pylori Infection. A lamina propria of the stomach is shown with two mast cells overlapping each other. Note the upper part reveals the degranulating process with the release of granules of inflammation mediators (Giemsa staining, 250X). Courtesy of Pantaleo Bufo, University of Foggia, Italy.
Helicobacter Pylori Infection. A lamina propria of the stomach is shown with two mast cells overlapping each other. Note the upper part reveals the degranulating process with the release of granules of inflammation mediators (Giemsa staining, 250X). Courtesy of Pantaleo Bufo, University of Foggia, Italy.
A strong association has been reported between H pylori infection and gastric lymphoma and adenocarcinoma of the body and antrum of the stomach. Some cofactors may play a key role in determining such diseases. Whether H pylori eradication can decrease the risk of cancer is unknown.
H pylori infection occurs more frequently in developing countries than in industrialized countries. H pylori strains differ in their potential to cause diseases. Although anyone can develop a microscopic gastritis, only a minority of infected persons develop ulcers or other diseases. H pylori gastritis is considered an infectious condition, even in the setting of asymptomatic patients and regardless of whether a peptic ulcer or gastric cancer is present. [1]
Some Helicobacter -like organisms (HLOs) have been detected by specific polymerase chain reaction tests. The first of these HLOs was described in ferrets and is called Helicobacter mustelae. Helicobacter hepaticus has been described in Syrian hamsters. These HLOs are useful for researching H pylori infection modalities.
Pathophysiology
The most common route of H pylori infection is either oral-to-oral (stomach contents are transmitted from mouth to mouth) or fecal-to-oral (from stool to mouth) contact. [2] Parents and siblings seem to play a primary role in transmission.
In a susceptible host, H pylori results in chronic active gastritis that may lead, in turn, to duodenal and gastric ulcer disease, gastric cancer, and MALTomas. H pylori infection causes chronic active gastritis, which is characterized by a striking infiltration of the gastric epithelium and the underlying lamina propria by neutrophils, T and B lymphocytes, macrophages, and mast cells. Mast cells, usually responsible for the immune response balance, may be important effector cells in the pathogenesis of gastritis. However, H pylori does not seem to invade the gastric mucosa, although evidence suggests that the mucus layer provides a niche wherein the bacterium is protected from gastric secretions.
The release of host cytokines after direct contact of H pylori with the epithelial cells of the gastric lining could recall the inflammatory cells in the infected area. One study demonstrated that the gastric epithelium, when infiltrated by neutrophils and macrophages in the lamina propria, highly expresses two neutrophil chemotactic factors: gro-alpha and interleukin-8. In addition, the interferon-gamma inducible protein–10 (IP-10) and the monokine induced by interferon-gamma (MIG), 2 selective chemotactic factors for T lymphocytes, are expressed by the endothelium and mononuclear cells of the gastric mucosa in patients with H pylori -related gastritis. According to the same study, gro-alpha and interleukin-8 may have a central role in neutrophils trafficking from the vessels to the mucosal epithelium, while IP-10 and MIG determine T lymphocyte recruitment into the mucosa.
Another hypothesis states that H pylori may recall immune cells from afar because of its own molecules, such as urea or lipopolysaccharide (LPS). Outer-membrane permeability is a function mediated by LPS. Despite the presence of bacterial LPS in biologically active quantities in the gastric mucosa, the mechanisms by which it may recall the immune cells are still unknown. According to one hypothesis, H pylori may induce the production of autoantibodies against the host's gastric lining.
The LPS of H pylori shows certain blood group antigens, such as Leb, Lex, Ley, and H-type I. Such antigens are thought to represent important virulence factors involved in the adhesive process of the germ. Leb constitutes an adhesin, and differences exist in the Le compositions of adherent and nonadherent bacteria. This, perhaps, accounts for a relationship between adhesion and Le expression. Hage and colleagues identified the BabA protein (Blood group antigen-binding Adhesin) in H pylori that interacts with gastric mucus binding Leb antigens, confirming the relationship. [5] As a consequence, H+ bridges may be formed, strongly anchoring the bacterium to the gastric mucosa.
In addition, any Le antigen shows phase variation leading to the spontaneous and random switching on and off of the expression of these antigens. For example, the H-type I antigen seems to be the result of a reversible singular nucleotidic deletion/insertion in a tract of a glycosyl transferase gene. The LPS of the H pylori also seems to influence tumoral proliferation of ECL cells, stimulating the intracellular polyamine biosynthesis pathway and ornithine decarboxylase activity by the activation of a CD14 receptor on the ECL cell.
In 1997, Tomb and coworkers completely sequenced the H pylori genome, and some differences were found in gene encoding factors that are likely to interact with the host, such as surface proteins. [6] Two of the most important genes of H pylori are VACA and CAGA. The VACA gene codes for the Vac-A cytotoxin, a vacuolating toxin. Most H pylori strains (60%), by unexplained causes, do not produce this protein. The CAGA gene codes for the Cag-A protein, which seems to stimulate the production of chemotactic factors for the neutrophils by the gastric epithelium of the host. A certain proportion of H pylori strains (40%), by unexplained causes, does not produce this protein.
After the exposure to CAGA -positive H pylori strains, an increase in catalase, glutathione peroxidase, and superoxide dismutase activity has been reported. This increase is associated with fewer DNA adducts and reduced susceptibility of the gastric cells to the irreversible injuries from reactive oxygen species (ROS) compared with exposure to CAGA -negative H pylori strains. Such alterations of the ROS scavenging enzymes may partly account for the increased risk of gastric cancer in individuals with H pylori infection.
A relationship among CAGA/Cag-A, VACA alleles, and the Le subtype of H pylori strains has been reported, as has a link between these and the redox status of the gastric mucosa. For example, H pylori is able to induce apoptosis in epithelial cells and T lymphocytes. The CAGA -positive strains of H pylori seem to be able to increase FASL expression in T lymphocytes (up-regulation of FASL on such cells is redox-sensitive), which facilitates a selective killing of the T lymphocytes. This molecular mechanism may have a key role in the persistence of CAGA -positive strains.
In addition, H pylori up-regulates caspases 3, 6, 8, and 9. Caspases 3 and 9 in epithelial cells are fundamental in inducing apoptosis. The expression of some bacterial genes is acid-regulated, as reported for the FILA gene (responsible for the H pylori motility) that codes for a sigma factor required for transcription of the flagellin gene FLAA. [7] Flagella and urease are very important for the colonization of the gastric mucosa by the bacterium.
Etiology
Note the following:
-
H pylori infection causes atrophic and even metaplastic changes in the stomach.
-
The bacterial adhesion appears to result in tyrosine phosphorylation and is specific for gastric cells.
-
The adhesion of H pylori to the gastric cells causes a direct decrease in the mucosal levels of glutathione, a fundamental molecule in the maintenance of the cellular redox status and in the molecular regulation of host immune responses. However, the LPS of H pylori may induce the production of autoantibodies that are able to worsen the atrophy in the corpus mucosa and cause a concomitant increase in parietal cell antibodies. Such events are accompanied by a decrease in anti-H pylori immunoglobulin titers. This process leads to a scenario of severe atrophy without bacterial colonization combined with high levels of autoantibodies against gastric parietal cells.
-
A number of reports show the close association between H pylori infection and low-grade gastric MALTomas.
-
Giannakis and colleagues demonstrated that H pylori may adapt to gastric stem cells, influencing their biology and contributing to tumorigenesis of the stomach. [8]
Epidemiology
United States statistics
The frequency of H pylori infection may be linked to race and low socioeconomic status. White persons account for 29% of cases, and Hispanic persons account for 60% of cases.
International statistics
H pylori is a ubiquitous organism. At least 50% of all people are infected, [9] but an exact determination is not available, mostly because precise data are not available from developing countries. H pylori may be detected in approximately 90% of individuals with peptic ulcer disease; however, less than 15% of infected persons may have this disease.
Race-, sex-, and age-related statistics
The pathogenetic role of H pylori may differ depending on the geography and race. White persons are infected with H pylori less frequently than persons of other racial groups. The prevalence rate is approximately 20% in white persons, 54% in African American persons, and 60% in Hispanic persons.
No sex predilection is known; however, females have a higher incidence of reinfection (5%-8%) than males.
H pylori infection may be acquired at any age. According to some epidemiologic studies, this infection is acquired most frequently during childhood. Children and females have a higher incidence of reinfection (5%-8%) than adult males.
Prognosis
The prognosis is usually excellent, even in patients with complications, such as gastric MALToma. However, the prognosis becomes poor for patients who develop squamous cell esophageal cancer or gastric carcinoma.
The rate of reinfection is very low (1%-2%); however, children and females have a higher incidence of reinfection (5%-8%)
Mortality/morbidity
The mortality rate related to H pylori infection is not precisely known, but it seems to be minimal (ie, approximately 2%-4% of all infected people). Mortality is due to the complications of the infection, such as gastric ulcer perforation or MALTomas of the GI tract. Otherwise, the morbidity of H pylori infection can be very high.
Results from a recent meta-analysis by Lender and colleagues suggest that the reduction of H pylori infection in developed nations may be contributing to the rise in obesity in those countries. Using 49 studies, with data from 10 European nations, Japan, the United States, and Australia, the investigators found a significant inverse correlation between the rates of obesity/overweight and the prevalence of H pylori infection. Mean rates for obese and overweight individuals were 46.6% and 14.2%, respectively, whereas the mean prevalence of H pylori infection was 44.1% (range, 17%–75%). The authors acknowledge, however, that the study does not prove that the reduced prevalence of H pylori has directly impacted obesity rates. The association, they admit, could be more complex; it may be, for example, that hygiene factors that encourage H pylori infection may also somehow discourage obesity. [10, 11]
Complications include the following:
-
Gastric adenocarcinoma is the most severe consequence of an H pylori infection.
-
Gastric MALToma may be treated with H pylori eradication therapy and has a better prognosis than gastric adenocarcinoma. A significant difference exists between the therapeutic response of MALTomas restricted to the mucosa and other, more infiltrating lesions. The only predictive factor for disease regression seems to be the absence of nodal involvement.
-
H pylori infection is associated with squamous cell esophageal cancer.
-
H pylori may play an important role in idiopathic thrombocytopenic purpura. This is due to anti-CagA antibodies that cross-react with platelet antigens.
-
According to some reports, H pylori eradication may cause peptic esophagitis, probably due to a protective action of the bacteria on the cardia area.
Patient Education
Educate patients with a high risk for gastric cancer about clinical control methods and, if H pylori-positive, to begin eradication therapy. However, eradication regimens for H pylori are complex and might not be fully comprehended by patients; thus explore barriers to adherence and address these prior to prescribing therapy. [12] Explain the rationale for therapy, dosing instructions, expected adverse events, and the importance of completing the full therapeutic course. [12] It is crucial to educate patients about the adverse effects of the therapy to impress upon them the importance of compliance with the full regimen to prevent antibiotic resistance and relapse.
-
Helicobacter Pylori Infection. This image shows an antral gland of the stomach with a large Giemsa-stained colony of Helicobacter pylori in the lumen (arrow) at 250X power. Courtesy of Pantaleo Bufo, University of Foggia, Italy.
-
Helicobacter Pylori Infection. A lamina propria of the stomach is shown with two mast cells overlapping each other. Note the upper part reveals the degranulating process with the release of granules of inflammation mediators (Giemsa staining, 250X). Courtesy of Pantaleo Bufo, University of Foggia, Italy.
-
Helicobacter Pylori Infection. A transverse section of the gastric lamina propria is shown. In the lower part, an antral gland of the stomach is present with some Helicobacter pylori in the lumen (red-blue arrow). In the upper part, a mast cell (yellow arrow) is present (Giemsa staining, 250X). Courtesy of Pantaleo Bufo, University of Foggia, Italy.
-
Helicobacter Pylori Infection. An antral gland of the stomach is shown with a large colony of Helicobacter pylori in the lumen (Giemsa staining, 425X). Courtesy of Pantaleo Bufo, University of Foggia, Italy.









