Practice Essentials
Goodpasture syndrome is an eponym that has been used to describe the clinical entity of diffuse pulmonary hemorrhage (as seen in the images below) and acute or rapidly progressive glomerulonephritis. Goodpasture disease is a term used to describe glomerulonephritis, with or without pulmonary hemorrhage, and the presence of circulating anti–glomerular basement membrane (anti-GBM) antibodies. The definitions of these terms have not been consistent, however. [1] Anti-GBM disease, a more precise term, should be used to refer to either of the two distinct clinical manifestations of this disorder.
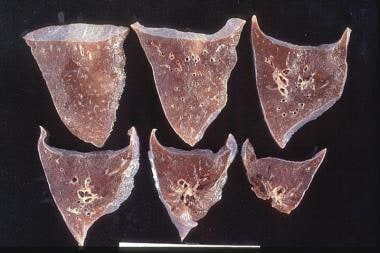 Goodpasture syndrome. A 45-year-old man was admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
Goodpasture syndrome. A 45-year-old man was admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
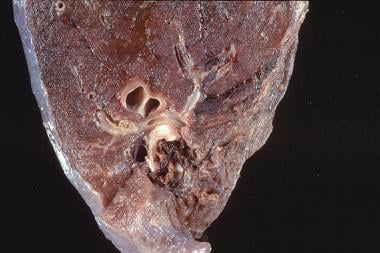 Goodpasture syndrome. Close-up view of gross pathology in a 45-year-old man admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
Goodpasture syndrome. Close-up view of gross pathology in a 45-year-old man admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
Goodpasture syndrome (ie, anti-GBM disease) is an uncommon disorder of complex pathogeneses. Early recognition and treatment of this syndrome are critical because the prognosis for recovery of renal function depends on the initial extent of injury.
The three principles of therapy are as follows (see Treatment) [2] :
Rapidly remove circulating antibody, primarily by plasmapheresis
Stop further production of antibodies using immunosuppression with medications
Remove offending agents that may have initiated the antibody production
Go to Pediatric Anti-GBM Disease (Goodpasture Syndrome) for complete information on this topic.
See Autoimmune Disorders: Making Sense of Nonspecific Symptoms, a Critical Images slideshow, to help identify several diseases that can cause a variety of nonspecific symptoms.
Background
Ernest Goodpasture first described this disorder in 1919. He reported a case of pulmonary hemorrhage and glomerulonephritis during an influenza epidemic. In 1955, Parkin [3] described 3 cases of lung hemorrhage and nephritis that occurred in the absence of arteritis. In 1958, Stanton and Tang [4] reported a series of young men with pulmonary hemorrhage and glomerulonephritis, similar to Goodpasture's original description.
In the 1950s, Krakower and Greenspon [5] identified GBM as the antigen. In 1967, Lerner, Glassock, and Dixon [6] confirmed that the antibodies taken from the diseased kidneys produced nephritis in experimental animals. The discovery of anti-GBM antibodies led to the understanding of the pathogenesis of Goodpasture syndrome.
Pathophysiology
Anti-GBM disease is an autoimmune disorder characterized by autoantibodies directed against the glomerular/alveolar basement membrane. The autoantibodies bind to their reactive epitopes in the basement membranes and activate the complement cascade, resulting in tissue injury. This is a classic type II reaction in the Gell and Coombs classification of antigen-antibody reactions. This binding of antibodies can be visualized as the linear deposition of immunoglobulin along the glomerular basement membrane and, less commonly, the alveolar basement membranes, by direct immunofluorescent techniques.
The basement membranes are complex structures that support layers of endothelium and epithelium. The principal component of basement membrane is type IV collagen, which acts as a support structure and is composed of building blocks that are linked end-to-end. The building blocks are composed of three alpha subunits of collagen, which form a triple helix. Type IV collagen can be expressed as six different chains, alpha1 to alpha6. The alpha chain itself has three structural domains, as follows:
- A short 7S domain at the amino terminus
- A triple helix of three alpha chains, which classically consists of two alpha1 chains and one alpha2 chain, and which ends at the carboxyl terminus
- A noncollagenous domain
In most patients, the autoantibody in Goodpasture syndrome is directed against a 28-kd monomeric subunit present within the noncollagenous domain of the alpha3 chain of type IV collagen (alpha3[IV]NC1). [7, 8] Two conformational epitopes of anti-GBM antibodies have been defined at residues 17-31 and 127-141 of alpha3(IV)NC1, which were named as EA and EB, respectively. The P14 peptide, which has been identified as one of the major linear epitopes recognized by sera from patients with anti-GBM disease, contains the amino acid sequence of EB, as well as one of the T cell epitopes found in anti-GBM disease. [9] Autoantibodies may also be directed against other alpha chains.
Although basement membranes are ubiquitous, only the alveolar and glomerular basement membranes are affected clinically. The preferential binding to the alveolar and glomerular basement membranes appears to be caused by greater accessibility of epitopes and greater expansion of alpha3 collagen units. Furthermore, the alpha3 collagen chains of glomerular and basement membranes are structurally integrated in such a way that they are more accessible to the circulating antibodies.
Under normal conditions, the alveolar endothelium is a barrier to the anti–basement membrane antibodies. However, with increased vascular permeability, antibody binding to the basement membrane occurs in the alveoli. Therefore, for the deposition of antibody, an additional nonspecific lung injury that increases alveolar-capillary permeability is required.
A variety of factors that can result in increased alveolar-capillary permeability have been identified. [10] These include the following:
-
Increased capillary hydrostatic pressure
-
High concentrations of inspired oxygen
-
Bacteremia
-
Endotoxemia
-
Exposure to volatile hydrocarbons
-
Upper respiratory infections
-
Tobacco smoking
Strong evidence exists that genetics play an important role. Patients with specific human leukocyte antigen (HLA) types are more susceptible to disease and may have a worse prognosis.
There is an increased prevalence of HLA-DR15 (previously known as HLA-DR2) and DRB1*03, DRB1*04 and a decreased frequency of DRB1*01 and DRB1*07. [11, 12] Goodpasture disease is strongly associated with the DRB1*1501 and to a lesser extent the DRB1*1502 allele. Although a strong association exists between anti-GBM disease and HLA DRB1*1501, this allele is present in as many as one third of individuals in white populations. It is therefore clear that additional factors, either genetic or environmental, are required for disease expression.
Also of note, HLA-B7 is found more frequently and is associated with more severe anti-GBM nephritis.
Although anti-GBM disease is seen as a prototypic autoantibody-mediated disease, T cells have a vital role in disease initiation and progression. T cells enhance B-cell function and antibody production and may play a direct pathogenic role in kidney and lung injury. [13] Autoreactive T cells against alpha3(IV)NC1 are rare in healthy individuals, owing to thymic deletion during fetal development. Autoreactive T cells in patients may form as a consequence of exposure of neoepitopes, altered antigen processing allowing pathogenic epitopes to be presented, or altered T-cell regulation. [12]
Approximately 20%-40% of patients are double-positive for anti-GBM antibodies and antineutrophilic cytoplasmic antibodies (ANCAs). Coexistence of ANCAs (mostly myeloperoxidase [MPO-ANCAs]) with anti-GBM antibodies is thought to occur when the renal involvement in ANCA vasculitis leads to the exposure of antigens from the basement membrane and the formation of anti-GBM antibodies. [14]
Etiology
Like other autoimmune conditions, anti-GBM disease is thought to result from an environmental insult in a person with genetic susceptibility. The human leukocyte antigen (HLA) serotype HLA-DR15 has been strongly asssociated with anti-GBM disease. An initial insult to the pulmonary vasculature is required for exposure of the alveolar capillaries to the anti-GBM antibodies. Environmental factors that may lead to such exposure include the following:
-
Exposure to organic solvents or hydrocarbons
-
Smoking
-
Infection (eg, influenza A2) [15]
-
Cocaine inhalation
-
Exposure to metal dusts
-
Lymphocyte-depletion therapy, such as alemtuzumab
-
Extracorporeal shock wave lithotripsy [16]
COVID-19 and COVID-19 vaccinations have been associated with both new and recurrent cases anti-GBM disease. [17, 18, 15]
Epidemiology
Anti-GBM disease is an uncommon disorder. The incidence of anti-GBM disease is estimated to be 0.5-1.8 cases per million per year in both European white and Asian populations. It is responsible for 1-5% of all types of glomerulonephritis and for 10-20% of crescentic glomerulonephritis. [19, 20]
Racial, sexual, and age-related differences in incidence
Anti-GBM disease occurs more commonly in white people than in black people, but it also may be more common in certain ethnic groups, such as the Maoris of New Zealand. The age distribution is bimodal, 20-30 years and 60-70 years. The prevalence of the disease is higher in men in the younger age group and women in the older age subgroup.
A subgroup of patients is double-positive for anti-GBM antibodies and antineutrophilic cytoplasmic antibodies. The peak age incidence for this subgroup is 60-70 years, with a male predominance. [8]
Prognosis
In the past, Goodpasture syndrome was usually fatal. Aggressive therapy with plasmapheresis, corticosteroids, and immunosuppressive agents has dramatically improved prognosis. [21] With this approach, the 5-year survival rate exceeds 80% and fewer than 30% of patients require long-term dialysis. In a study of patients patients admitted to intensive care units for acute manifestation of small-vessel vasculitis, including anti-GBM disease, delayed administration of cyclophosphamide was associated with a higher mortality rate. [22]
Patients presenting with serum creatinine levels greater than 4 mg/dL, oliguria, and more than 50% crescents on renal biopsy rarely recover. They usually progress to end-stage renal failure that requires long-term dialysis. In a retrospective analysis of patients with anti-GBM disease who started renal replacement therapy for end-stage renal disease (ESRD) in Australia and New Zealand (ANZDATA Registry), the median survival rate was 5.93 years with death predicted by older age and history of pulmonary hemorrhage. [20]
-
Goodpasture syndrome. A 45-year-old man was admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
-
Goodpasture syndrome. Close-up view of gross pathology in a 45-year-old man admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
-
Cytoplasmic antineutrophilic cytoplasmic antibodies (c-ANCA), which can appear in Goodpasture syndrome, are also commonly observed in Wegener granulomatosis and other vasculitides.
-
Perinuclear antineutrophilic cytoplasmic antibodies (p-ANCA), which can appear in Goodpasture syndrome, are also observed in Churg-Strauss vasculitis and occasionally in Wegener granulomatosis.
-
This is a renal biopsy slide of a patient who presented with hemoptysis and hematuria. The renal biopsy revealed crescentic glomerulonephritis, which may be caused by systemic lupus erythematosus, vasculitis, or Goodpasture syndrome.
-
Goodpasture syndrome. A 35-year-old man who previously smoked cigarettes heavily, developed massive hemoptysis. The blood work showed positive anti–glomerular basement membrane antibodies.
-
Immunofluorescence staining for immunoglobulin (IgG) reveals diffuse, high-intensity, linear staining of the glomerular basement membrane in a patient with anti–glomerular basement membrane (GBM) disease. Courtesy of Glen Markowitz, MD, Department of Pathology, Columbia University.





