Practice Essentials
Membranoproliferative glomerulonephritis (MPGN) is an uncommon cause of chronic nephritis that occurs primarily in children and young adults. This entity refers to a pattern of glomerular injury based on the following three characteristic histopathologic findings:
- Proliferation of mesangial and endothelial cells and expansion of the mesangial matrix (see the first image below)
- Thickening of the peripheral capillary walls by subendothelial immune deposits and/or intramembranous dense deposits (see the second image below)
- Mesangial interposition into the capillary wall, giving rise to a double-contour or tram-track appearance on light microscopy (see the third image below)
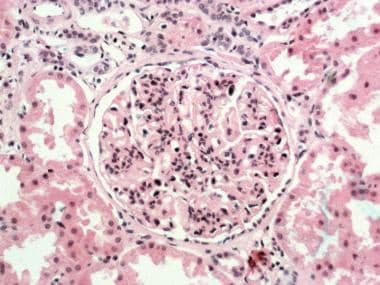 Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with lobular accentuation from increased mesangial cellularity. A segmental increase occurs in the mesangial matrix, and the peripheral capillary walls are thickened (hematoxylin and eosin stained section; original magnification × 250). Courtesy of John A. Minielly, MD.
Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with lobular accentuation from increased mesangial cellularity. A segmental increase occurs in the mesangial matrix, and the peripheral capillary walls are thickened (hematoxylin and eosin stained section; original magnification × 250). Courtesy of John A. Minielly, MD.
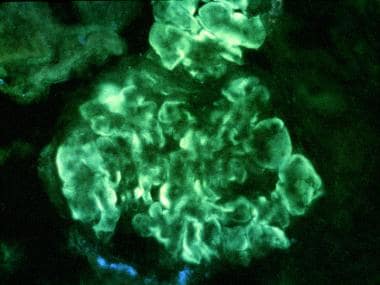 Membranoproliferative glomerulonephritis (MPGN) type I. Immunofluorescent stained section. Intense, peripheral, glomerular, capillary loop deposition of immunoglobulin G (IgG) in an interrupted linear pattern corresponding to extensive subendothelial immune deposits (original magnification × 400). Courtesy of John A. Minielly, MD.
Membranoproliferative glomerulonephritis (MPGN) type I. Immunofluorescent stained section. Intense, peripheral, glomerular, capillary loop deposition of immunoglobulin G (IgG) in an interrupted linear pattern corresponding to extensive subendothelial immune deposits (original magnification × 400). Courtesy of John A. Minielly, MD.
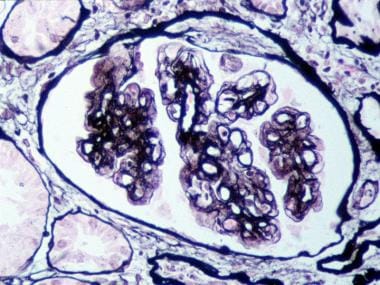 Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with mesangial interposition producing a double contouring of basement membranes, which, in areas, appear to surround subendothelial deposits (Jones silver methenamine–stained section; original magnification × 400). Courtesy of John A. Minielly, MD.
Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with mesangial interposition producing a double contouring of basement membranes, which, in areas, appear to surround subendothelial deposits (Jones silver methenamine–stained section; original magnification × 400). Courtesy of John A. Minielly, MD.
MPGN may be idiopathic or secondary in etiology. [1] The secondary types are more common than the idiopathic types and are diagnosed by carefully reviewing clinical features, laboratory data, and renal histopathology. Idiopathic MPGNs are subdivided into types I, II, and III based on ultrastructural appearance. The types are characterized by the following:
-
Type I – Subendothelial deposits
-
Type 2 – Dense deposits in the glomerular basement membrane
-
Type 3 – Subepithelial and subendothelial deposits
The light microscopy features are mostly indistinguishable among the three types.
An alternative classification system has been proposed based on pathogenetic mechanism, as follows [2, 3] :
-
Immune complex–mediated GN (ICGN) with an MPGN pattern
-
GN with monoclonal immunoglobulin deposits
-
Glomerulonephritis with C3- and C4-dominant deposits
Clinical presentations are similar for the three types of MPGN, but they manifest somewhat different mechanisms of complement activation and predisposition to recur in kidney transplants. [4]
Conversion from one type to another has not been reported. Familial forms of all 3 types of MPGN have been described.
See also Pediatric Nephritis, Tubulointerstitial Nephritis, and Radiation Nephritis.
Pathophysiology
The normal complement system consists of the classic and alternative pathways. The classic pathway is activated by the interaction of C1 with an antigen-antibody complex. This interaction results in the formation of C4b2a, which is the classic pathway C3b convertase. The alternative pathway utilizes C3 and factors B and D to form the alternative pathway convertase C3b,Bb.
Small amounts of C3b are constantly being formed in the circulation, which are inactivated by factors H and I. The binding of C3b to a foreign antigen decreases its affinity for factor H and allows for the formation of increasing amounts of the alternate pathway convertase. The classic and alternate pathway convertases cause C3 activation, forming C3a and C3b. C3b is an opsonin itself, and C3 convertase facilitates the activation of the terminal pathway and the formation of the membrane attack complex C5b-9.
Hypocomplementemia in MPGN
Hypocomplementemia is a characteristic finding with all types of membranoproliferative glomerulonephritis (MPGN). Low C3 levels are present in approximately 75% of patients with this condition. Although hypocomplementemia bears no relation to the clinical course or prognosis of MPGN, it plays a role in initiating glomerular inflammation and injury. Hypocomplementemia results from increased catabolism and decreased C3 synthesis. The decreased C3 synthesis is likely caused by the negative feedback by C3 breakdown products.
Three nephritic antibodies are described in MPGN that play a role in the development of hypocomplementemia [5, 6] : (1) nephritic factor of the classic pathway (NFc or C4NeF), (2) nephritic factor of the amplification loop (NFa or C3NeF), and (3) nephritic factor of the terminal pathway (NFt).
The reason for genesis of nephritic antibodies is not known. These autoantibodies are not specific for MPGN and are also seen in poststreptococcal and lupus glomerulonephritis. NFc stabilizes the classic pathway C3 convertase C4b,2a. This nephritic factor does not cause C3 conversion unless C4b,2a production is ongoing. NFa (C3NEF) is an autoantibody to C3b,Bb. The binding of NFa to C3b,Bb stabilizes the complex, preventing degradation by its normal inactivators, resulting in complement activation and chronic consumption of C3.
NFt stabilizes the alternative pathway properdin-dependent C3/C5 convertase (C3Bb2,Bb,P) and leads to C3 activation and consumption. The consumption of C3 caused by NFt is much slower than that caused by NFa. NFt also activates the terminal complement components forming C5b-C9, the membrane attack complex.
MPGN type I
Circulating immune complexes are present in approximately 33% of patients with MPGN type I. In all patients with type I disease, immune complexes are found in the mesangium and subendothelial spaces, and they trigger complement activation and the release of cytokines and chemokines. The release of inflammatory mediators causes an influx of inflammatory cells and leads to mesangial and endothelial cell proliferation. Most patients with circulating immune complexes do not develop MPGN; thus, additional pathogenic factors (eg, nature of the antigen, size of complexes, type and charge on antibodies, local glomerular factors) must play a role.
In addition to circulating immune complexes becoming entrapped in the glomerular basement membrane (GBM), experimental evidence indicates that complexes may be formed in situ when antigens adhere to the GBM, and antibodies subsequently bind to these antigens. Formation of such immune complexes triggers the same cascade as described above.
Activation of complement and the resulting hypocomplementemia may cause defective clearance of circulating immune complexes. The nephritic factor of the classic pathway (ie, NFc or C4NeF) is found in approximately 15% of patients. This nephritic factor stabilizes the classic pathway C3 convertase C4b,2a and potentiates C3 activation and consumption. [7] The role of this nephritic factor in the pathogenesis of MPGN type I is unclear. Approximately 20% of patients have the nephritic factor of the terminal pathway.
MPGN type II (or dense deposit disease)
MPGN type II, or dense deposit disease, is a separate entity that has been conventionally classified with MPGN because of the similarities of light microscopic appearance. The pathogenesis of MPGN type II is not known.
This disease is systemic, as evidenced by dense deposits in the kidney, splenic sinusoids, Bruch membrane of the retina, as well as its association with acquired partial lipodystrophy. [8, 9] MPGN type II also has a high incidence of recurrence in renal allografts. The chemical composition and origin of the dense deposits are not known, although bright staining with thioflavine-T and wheat germ agglutinin suggests the presence of N-acetyl-glucosamine. No circulating immune complexes are observed in MPGN type II.
Dense deposit disease is associated with multiple complement abnormalities, including a persistent reduction of C3 levels. One hypothesis is that the dense deposits cause complement activation. [10] This hypothesis is supported by the tram-track distribution of C3 deposits along the basement membrane.
NFa is present in 80% of patients with dense deposit disease. NFa stabilizes the alternative pathway convertase and results in complement activation and chronic C3 consumption. Deficiency of factor H, functionally defective factor H, mutant factor H binding site of C3 (Marder disease), and presence of inhibitory or blocking factor H antibodies, described in MPGN, may lead to an accumulation of the alternative pathway convertase and chronic C3 consumption.
Partial lipoid dystrophy (PLD) is associated commonly with MPGN type II and the presence of NFa. Adipocytes produce adipsin, which is identical to complement factor D and is responsible for activating the preconvertase C3b,Bb. NFa causes a lysis of adipocytes that produce adipsin, and the distribution of fat atrophy in partial lipoid dystrophy follows variations in the amount of adipsin produced by adipocytes. By analogy, NFa may cause damage to glomerular cells that produce complement.
MPGN type III
The glomerular deposits contain C3, C5, and properdin, indicating activation of the alternative complement pathway. Signs of activation of the classic pathway are minimal, and circulating immune complexes do not appear to play a role in the genesis of this variant.
Changes in the capillary wall are hypothesized to be the primary event leading to activation of the complement pathway. This hypothesis is supported by the deposition of C3Bb2,Bbconvertase components in the basement membrane. The deposits of convertase and membrane attack complex may lyse the basement membrane and stimulate new membrane formation. NFt is present in 60-80% of patients with MPGN type III. NFt stabilizes the alternative pathway properdin-dependent C3/C5 convertase (C3Bb2,Bb,P) and also activates the terminal complement components, forming C5b-C9 (ie, the membrane attack complex).
A familial form of MPGN type III with an autosomal dominant pattern of inheritance has been identified with genetic linkage to band 1q31-32. [11] Genes in this area of chromosome 1 code for proteins that regulate the C3 convertase activity.
C3 glomerulonephritis [12]
C3 glomerulonephritis is a recently described entity with immunofluorescence findings of isolated glomerular C3 deposits. C3 glomerulonephritis is similar in etiology to dense deposit disease, arising as a result of alternate complement activation or mutations in the complement-regulating proteins. C3NeF is commonly present. C3 glomerulonephritis has been associated with antifactor H activity and monoclonal gammopathies. Serum C3 levels are usually low, but they can be normal along with normal C4 levels. [13] It is important to note that a normal serum C3 level does not exclude C3 glomerulonephritis. Dense deposit disease and C3GN both lack immunoglobulin staining on immunofluorescence and are the result of alternative pathway dysregulation. [14] These 2 entities are often referred to as C3 glomerulopathy in literature.
Etiology
Immune complex–mediated conditions, autoimmune diseases, chronic infections, chronic and recovered thrombotic microangiopathies, paraprotein deposition diseases, and malignant neoplasms associated with a membranoproliferative pattern of injury are summarized below.
Immune complex–mediated disease
In the past, most patients were thought to have idiopathic membranoproliferative glomerulonephritis (MPGN). With the discovery of secondary causes, especially hepatitis C, the number of patients deemed to have idiopathic MPGN has declined.
Autoimmune diseases
Autoimmune diseases associated with a membranoproliferative pattern of renal injury include the following:
-
Systemic lupus erythematosus (SLE)
-
Sjögren syndrome
-
Rheumatoid arthritis
-
Inherited complement deficiencies (in particular, C2 deficiency)
-
Scleroderma
-
Celiac disease
-
Systemic sclerosis [15]
Chronic infections
Chronic viral, bacterial, protozoal, and mycoplasmal infections, as well as chronic liver disease (cirrhosis and alpha1-antitrypsin deficiency), are associated with a membranoproliferative pattern of renal injury, as follows:
-
Viral – Hepatitis B, hepatitis C, [16] cryoglobulinemia type II
-
Bacterial – Endocarditis, infected ventriculoatrial (or jugular) shunt, multiple visceral abscesses, leprosy
-
Protozoal – Malaria, schistosomiasis
A rare case of Haemophilus parainfluenzae endocarditis associated with MPGN has been reported. [17]
COVID-19 immunizations
Both relapse and de novo cases of MPGN have been reported following administration of Pfizer and AstraZeneca vaccines against COVID-19. [18, 19]
Chronic and recovered thrombotic microangiopathies
The following are chronic and recovered thrombotic microangiopathies associated with a membranoproliferative pattern of kidney injury [20] :
-
Healing phase of hemolytic uremic syndrome (HUS) and/or thrombotic thrombocytopenic purpura (TTP)
-
Syndromes of circulating antiphospholipid (anticardiolipin) antibodies
-
Radiation nephritis
-
Nephropathy associated with bone marrow transplantation
-
Sickle cell anemia and polycythemia
-
Transplant glomerulopathy
Paraprotein deposition diseases
Paraprotein deposition diseases that are associated with a membranoproliferative pattern of renal injury include the following:
-
Glomerulonephropathies associated with cryoglobulinemia type I
-
Waldenström macroglobulinemia
-
Immunotactoid glomerulopathy
-
Immunoglobulin light-chain or heavy-chain deposition diseases
-
Fibrillary glomerulonephritis
-
Monoclonal gammopathy of unknown significance
Malignant neoplasms
Lymphoma, leukemia, and carcinoma are associated with a membranoproliferative pattern of renal injury.
Epidemiology
United States statistics
Membranoproliferative glomerulonephritis (MPGN) is observed in 6-12% of US patients receiving renal biopsies to evaluate glomerular diseases. This entity accounts for 7% of children and 12% of adults with idiopathic nephrotic syndrome.
Globally, MPGN causes a significant proportion of the cases of nephritis among patients in nonindustrialized countries. For example, in Mexico, MPGN accounts for 40% of all patients with nephritis. Most of these patients have type I disease; MPGN type II is uncommon. However, the incidence of MPGN type I is decreasing progressively in developed countries, which may be explained by a change in environmental factors, especially a decline in infections.
In an investigation of the changing patterns of adult primary glomerular disease occurrence in a single region of the United Kingdom, Hanko et al analyzed the results of 1844 native renal biopsies taken between 1976 and 2005 (inclusive) and found the presence of primary glomerulonephritis was revealed in 49% of the biopsies, with the most common forms being immunoglobulin A (IgA) nephropathy (38.8%). [21] Other common forms were membranous nephropathy (29.4%), minimal-change disease (MCD) (9.8%), MPGN type 1 (9.6%), and focal segmental glomerulosclerosis (FSGS) (5.7%). The incidence of IgA nephropathy increased significantly over the study period, whereas the occurrence of membranous nephropathy decreased. [21]
Racial, sexual, and age differences in incidence
In the United States, MPGN predominantly affects the white population. Type I disease affects women more often than men, whereas a nearly equal sex distribution is seen in MPGN type II.
The idiopathic forms of MPGN are more common in children and young adults (range, 6-30 y). Isolated reports of involvement in patients as young as 2 years and as old as 80 years are noted in the literature. Secondary types of MPGN predominate among adults. [22]
Prognosis
The main predictors of an adverse outcome in membranoproliferative glomerulonephritis (MPGN) are nephrotic syndrome and hypertension at presentation, low glomerular filtration rate (GFR) at 1 year, and older age. [23, 24] Histologic characteristics of crescent formation, interstitial fibrosis, tubular atrophy, and multiple sclerotic glomeruli indicate a poor prognosis. However, hypocomplementemia is not a predictor of disease severity or prognosis.
MPGN type I with nephrotic syndrome is a progressive disease, with 50% of patients developing end-stage kidney disease (ESKD) after 10 years and 90% of patients developing ESKD after 20 years. MPGN type I without nephrotic proteinuria has a 10-year renal survival rate of 85%. [25]
MPGN type II is generally more aggressive than type I disease and has a median renal survival rate of 5-12 years. ESKD develops in 50% of the patients within 10 years of diagnosis. C3 glomerulonephritis, on the other hand, has been associated with preserved renal function in about 50% of patients, whereas approximately 15% of patients progress to ESKD. [2]
Data on outcomes with MPGN type III are very limited. Iitaka et al found that 7 patients who were followed for 9-17 years maintained their renal function over this period, [26] whereas Anders et al reported that 4 of 8 patients in their series developed ESKD. [27] In a study comparing therapy with alternate-day corticosteroids in 21 patients with type I disease and 25 patients with type III disease (followed for a minimum of 5 y), the investigators found that patients with MPGN type III had a greater decline in GFR, but there was no difference in the number of patients reaching ESKD in the 2 groups. [28]
In a study of all adult ESKD patients in Australia and New Zealand who commenced kidney replacement therapy from 1996 through 2016, rates of survival on dialysis and following kidney transplantation were comparable in the 456 patients with MPGN and the 12,660 patients with another form of glomerulonephritis. However, patients with MPGN had significantly higher rates of allograft loss due to disease recurrence. [29]
Kawasaki et al reported worse prognosis in pediatric patients with MPGN related to complement component C3 than in those with immune complex–mediated MPGN. In their study of 37 patients, those with C3-related MPGN were more likely to be nonresponsive to therapy or progress to ESKD. [30]
-
Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with lobular accentuation from increased mesangial cellularity. A segmental increase occurs in the mesangial matrix, and the peripheral capillary walls are thickened (hematoxylin and eosin stained section; original magnification × 250). Courtesy of John A. Minielly, MD.
-
Membranoproliferative glomerulonephritis (MPGN) type I. Electron microscopy of prominent, glomerular, subendothelial, immune-type electron deposits (original magnification × 11,400). Courtesy of John A. Minielly, MD.
-
Membranoproliferative glomerulonephritis (MPGN) type I. Glomerulus with mesangial interposition producing a double contouring of basement membranes, which, in areas, appear to surround subendothelial deposits (Jones silver methenamine–stained section; original magnification × 400). Courtesy of John A. Minielly, MD.
-
Membranoproliferative glomerulonephritis (MPGN) type II. Electron microscopy of glomerular basement membrane, intramembranous, somewhat linear, electron dense deposit (ie, dense deposit disease; original magnification × 11,400). Courtesy of John A. Minielly, MD.
-
Membranoproliferative glomerulonephritis (MPGN) type I. Immunofluorescent stained section. Intense, peripheral, glomerular, capillary loop deposition of immunoglobulin G (IgG) in an interrupted linear pattern corresponding to extensive subendothelial immune deposits (original magnification × 400). Courtesy of John A. Minielly, MD.