Practice Essentials
Euthyroid sick syndrome (also known as nonthyroidal illness syndrome) can be described as abnormal findings on thyroid function tests that occur in the setting of a nonthyroidal illness (NTI), without preexisting hypothalamic-pituitary and thyroid gland dysfunction. After recovery from an NTI, these thyroid function test result abnormalities should be completely reversible. [1, 2]
Multiple alterations in serum thyroid function test findings have been recognized in patients with a wide variety of NTIs without evidence of preexisting thyroid or hypothalamic-pituitary disease. The most prominent alterations are low serum triiodothyronine (T3) and elevated reverse T3 (rT3), leading to the general term "low T3 syndrome." Thyroid-stimulating hormone (TSH), thyroxine (T4), free T4 (FT4), and free T4 index (FTI) also are affected in variable degrees based on the severity and duration of the NTI. As the severity of the NTI increases, both serum T3 and T4 levels drop, but they gradually normalize as the patient recovers, as shown in the image below.
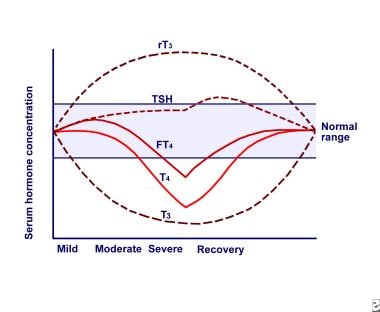 Euthyroid sick syndrome. Relationship between serum thyroid hormone concentrations and severity of nonthyroidal illness (NTI). Abbreviations: reverse triiodothyronine (rT3), thyroid-stimulating hormone (TSH), free thyroxine (FT4), thyroxine (T4), triiodothyronine (T3).
Euthyroid sick syndrome. Relationship between serum thyroid hormone concentrations and severity of nonthyroidal illness (NTI). Abbreviations: reverse triiodothyronine (rT3), thyroid-stimulating hormone (TSH), free thyroxine (FT4), thyroxine (T4), triiodothyronine (T3).
TSH is affected in variable degrees, but, in the overwhelming majority of patients, TSH is above 0.05 μ IU/mL. In severe, critical illness, most patients have reduced T4 levels. In patients hospitalized for NTI, about 10% have abnormally low TSH values; the highest incidence occurs in the most severely ill group. In the sickest patients who manifest low T4, TSH elevates to hypothyroid levels at the recovery phase, returning to reference range levels with complete recovery, as shown in the image below.
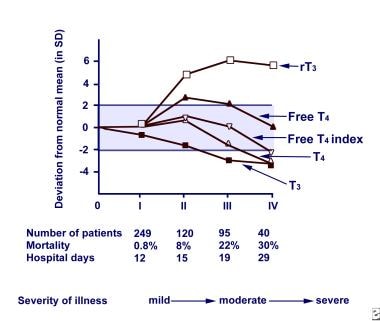 Euthyroid sick syndrome. Relationship between severity and duration of nonthyroidal illness (NTI) and thyroid hormone levels. Abbreviations: reverse triiodothyronine (rT3), free thyroxine (free T4), thyroxine (T4), triiodothyronine (T3).
Euthyroid sick syndrome. Relationship between severity and duration of nonthyroidal illness (NTI) and thyroid hormone levels. Abbreviations: reverse triiodothyronine (rT3), free thyroxine (free T4), thyroxine (T4), triiodothyronine (T3).
These changes in thyroid function test results are observed in most of the acute and chronic illnesses. Examples of illness include the following:
-
Inflammatory conditions
-
Starvation
-
Trauma
-
Surgery
-
Malignancy
Alterations in thyroid function test findings may reflect changes in production of thyroid hormone by effects on the thyroid itself, on the hypothalamic-pituitary-thyroid axis, on peripheral tissue metabolism of the hormones, or by a combination of these effects.
A general conviction exists that patients with thyroid function test result abnormalities do not have hypothyroidism despite the low serum hormone levels in blood and low T3 in most of the tissues. Many patients with NTI also receive drugs that affect thyroid hormone regulation and metabolism. This discussion does not consider pharmacologic interference an intrinsic part of the spectrum of changes in hypothalamic-pituitary-thyroid function that occur in NTI. Consider pharmacologic interferences as part of the evaluation of a patient who has thyroid function test result abnormalities.
Workup in and management of euthyroid sick syndrome
Recommended tests include the following:
-
Total T4
-
Total T3
-
TSH
-
Free T4
-
rT3
-
Free T3
Thyroid hormones have been used in the setting of NTI in various settings with T4 and T3 replacement and still remain controversial.
Pathophysiology
Proposed mechanisms explaining abnormalities in thyroid hormone levels
Accuracy of test assays in nonthyroidal illness
Abnormalities of thyroid function test results might represent test artifacts or true abnormalities. According to one proposition, the assays would indicate reference range thyroid hormone levels in the blood if appropriate tests were applied.
Inhibition of thyroid hormone binding to thyroid-binding proteins and tissues
Some authors propose that serum thyroid hormone abnormalities are due to inhibition of thyroid hormone binding to proteins, thus preventing tests from appropriately reflecting free hormone levels. This binding inhibitor can be present both in the serum and in body tissues and might inhibit uptake of thyroid hormones by cells or prevent binding to nuclear T3 receptors, thus inhibiting the action of the hormone. This inhibitor is associated with the nonesterified fatty acid (NEFA) fraction in the serum.
Contrary to this proposition, substantial evidence indicates that, in an in vivo state, the levels of binding inhibitors do not reach levels sufficient to influence the circulating levels of free T4, even in patients who are severely ill. Also, some studies have failed to demonstrate an existing binding inhibitor.
Cytokines
Cytokines are thought to play a role in NTI—particularly interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-alpha, and interferon-beta. Cytokines are thought to affect the hypothalamus, the pituitary, or other tissues, inhibiting production of TSH, thyroid-releasing hormone (TRH), thyroglobulin, T3, and thyroid-binding globulins. Cytokines are also thought to decrease the activity of type 1 deiodinase and to decrease the binding capacity of T3 nuclear receptors.
It has been proposed that several components of the thyroid hormone synthesis pathway are down-regulated by cytokines directly on the level of thyrocyte, eventually leading to decreased secretion of T4 and T3. [3]
Interferon-gamma has various effects on human thyrocytes in culture. Interferon-gamma was shown to inhibit TSH-induced thyroid hormone and thyroglobulin secretion, TSH-induced thyroglobulin mRNA expression, TSH-induced thyroid peroxidase expression, and TSH- and cAMP-induced up-regulation of TSH receptors on thyroid cells. Interferon-gamma was also demonstrated to inhibit the TSH-induced increase in sodium-iodide symporter (NIS) expression in rat FTRL-5 cells, leading to diminished iodide uptake. In addition, overexpression of interferon-gamma in thyroid cells in a transgenic mouse leads to primary hypothyroidism due to a significant decrease in NIS mRNA and protein expression.
TNF-alpha is known to inhibit TSH-induced cAMP response thyroglobulin production and release in cultured thyrocytes. TNF-alpha also inhibits NIS expression in rat FTRL-5 cells. Cytokines were also shown to inhibit type 1 deiodinase expression and activity in rat thyrocyte and FRTL-5 cells. [4]
Deiodination
Peripheral deiodination of T4 to T3 is impaired, largely secondary to decreased activity of type 1 deiodinase enzyme, which deiodinates T4 to T3. Diminished enzyme activity accounts for decreased deiodination of T4 to T3.
Type 1 deiodinase enzyme deiodinates T4 to T3. Diminished enzyme activity results in decreased deiodination of T4 to T3. The role of type 1 deiodinase in the pathogenesis of NTIs has been extensively studied, as type 1 deiodinase is involved in the production of serum T3 (which is decreased during illness) via outer-ring deiodination and in the clearance of rT3 (leading to increased rT3 concentrations during illness in humans) via inner-ring deiodination. Type 1 deiodinase is localized in the plasma membrane and largely expressed in liver, kidney, thyroid, and pituitary. It is positively regulated by T3. Nonthyroidal illness induces a marked decrease in liver type 1 deiodinase mRNA expression and its activity in critically ill patients and in various NTI animal models. [4]
Type 2 deiodinase enzyme is localized in the endoplasmic reticulum of the cells and deiodinates T4 into biologically active T3. Type 2 deiodinase is the main enzyme involved in the production of tissue T3 and is largely involved in local thyroid hormone metabolism. Type 2 deiodinase is negatively regulated by thyroid hormone, both pretranscription and posttranscription, as T3 down-regulates type 2 deiodinase mRNA expression, while T4 and rT3 (which are both substrates for type 2 deiodinase) affect type 2 deiodinase activity via increasing type 2 deiodinase ubiquitination and subsequent proteasomal degradation. [5]
Multiple studies have demonstrated the major role of type 2 deiodinase in the central part of hypothalamic-pituitary-thyroid axis that is altered during illness. The unresponsiveness of the hypothalamic-pituitary-thyroid axis to low serum thyroid hormone levels has been suggested to be mediated by increased production of T3 via elevated type 2 deiodinase activity in tanycytes (specialized cells that the wall of the third ventricle), as mice lacking the TR-beta do not show an illness-induced hypothalamic TRH decrease . In addition, global type 2 deiodinase knockout mice do not show a suppression of TRH upon lipopolysaccharide stimulation. [4]
Inflammation-induced type 2 deiodinase up-regulation in the hypothalamus was found to be independent of the fall in serum thyroid hormone concentrations, in contrast to type 2 deiodinase expression in other brain areas, such as the cortex and pituitary. [6]
A role for inflammatory cytokines was suggested, as lipopolysaccharide administration results in a rapid increase of pro-inflammatory cytokines, including TNF-alpha, IL-1, and IL-6. The type 2 deiodinase promoter contains nuclear factor (NF)–kappa-B–responsive elements and is thus sensitive to inflammatory signal transduction pathways. [7, 8] NF-kappa-B is therefore seen as a possible mediator of the inflammation-induced increase in type 2 deiodinase expression in the hypothalamus.
Type 3 deiodinase is localized in the plasma membrane of cells and can be considered as the major thyroid hormone–inactivating enzyme, as it catalyzes inner-ring deiodination of both T4 and T3, resulting in the production of biologically inactive rT3 and rT2 . Type 3 deiodinase is highly expressed in the placenta during fetal development and protects the fetus from overexposure of T3. In the adult, type 3 deiodinase is expressed in brain neurons, liver, and some parts of the immune system, although physiological levels are considerably low. [9]
Illness influences type 3 deiodinase expression and activity in the liver, but the results from animal studies vary. Although liver type 3 deiodinase mRNA expression and activity levels are decreased during acute and chronic inflammation and sepsis, hepatic type 3 deiodinase expression and activity are increased in rabbits with prolonged critical illness. Slightly increased type 3 deiodinase activity is also observed in the livers of severely ill patients.
During prolonged critical illness, decreased food intake might be an important factor in regulating liver deiodinases. Fasting for 36 hours or reducing food intake by 50% for 3 weeks results in pronounced increase of type 3 deiodinase expression and activity in the liver. As prolonged illness is associated with persistently diminished food intake, the differences in type 3 deiodinase activity between the several illness models might be explained by the dominant role of reduced food intake. One of the major hormones that are sensitive to food intake is leptin. In the setting of acute and chronic inflammation, serum leptin levels are higher via IL-1 beta, whereas serum leptin levels are diminished in prolonged critical illness. The reduction in leptin levels is known to be important for the increase in type 3 deiodinase activity during fasting in mice and thus might also be important for the regulation of type 3 deiodinase during illness. [4]
An alternative explanation is that reduced tissue uptake of T4 secondary to deficiency of cytosolic cofactors (eg, nicotinamide adenine dinucleotide phosphate [NADPH], glutathione) results in decreased substrate for type 1 deiodinase enzyme. Type 1 deiodinase is a selenoprotein; because selenium deficiency is common in critically ill patients, some propose that selenium deficiency may contribute to type 1 deiodinase malfunction. Cytokines (eg, IL-1 beta, TNF-alpha, interferon-gamma) decrease type 1 deiodinase messenger RNA (mRNA) in vitro. Type 1 deiodinase does not exist in the pituitary, where T3 levels are within the reference range, because of enhanced local deiodination. This indicates that an enhancement of intrapituitary T4 to T3 conversion exists due to pituitary-specific and brain-specific type 2 deiodinase.
Inhibition of thyroid-releasing hormone and thyroid-stimulating hormone secretion
Cytokines, cortisol, and leptin, as well as changes in brain thyroid hormone metabolism, affect inhibition and secretion of TRH and TSH.
Inhibition of plasma membrane transport of iodothyronines
Serum factors, such as bilirubin, NEFA, furanoic acid, hippuric acid, and indoxyl sulphate, which are present in various NTIs, have been shown to inhibit transport of thyroid hormones.
Thyroxine-binding globulin decrease and desialation
T4-binding globulin (TBG) is a member of the serine protease inhibitors. Diminished T4 in NTI has been proposed to be due to low TBG caused by protease cleavage at inflammatory sites in acute inflammatory conditions. One other hypothesis for the cause of disproportionately low serum T4 concentrations in patients with NTI is the presence of abnormal serum binding due to desialation of TBG.
The effects of nonthyroidal illness
Triiodothyronine and reverse triiodothyronine
In healthy people, 20% of T3 production comes from thyroidal secretion and 80% from peripheral deiodination from T4. In NTI, thyroidal production of T3 is normal, but the peripheral production of T3 is decreased. The fractional rate of transport of T3 to tissues is unaltered. Production of T3 is decreased, but its clearance is unchanged. Production of rT3 is unchanged, while its clearance is diminished.
In rat hepatocytes, rT3 and T4 have been demonstrated to be transported in the same mechanism, which implies that a diminished transport of rT3 to the liver would accompany inhibition of transport of T4 to the liver (eg, as in during calorie deprivation). Because the liver is the main site of disposal of T3, this leads to a diminished metabolic clearance rate of rT3 and T4.
Another explanation could be reduced 5'-deiodinase tissue activity, resulting in decreased T3 production from T4 and reduced breakdown of rT3. The decreased production of T3 during early and late starvation has been explained as either a diminished activity of the enzyme (deiodinase) itself or a deficiency of cytosolic cofactors, such as NADPH or glutathione. Specific deiodinative enzymes, 3 of which have been identified, affect deiodination of iodothyronines. Type 1 deiodinase is present in the liver, kidney, and thyroid and affects both 5 and 5' deiodination of T3. Type 2 deiodinase is present in the brain, pituitary, and brown adipose tissue and is active only in 5' deiodination. Type 3 deiodinase is found particularly in the brain, skin, and placenta, and it deiodinates iodothyronines at the 5 locations.
Both type II and type III enzymes are insensitive to 6-propylthiouracil (PTU). Alterations of serum thyroid hormone parameters in cases of calorie deprivation exhibit similarities to the changes observed in NTI. Fasted animals had decreased 5'-deiodinase activity. The activity of type 1 deiodinase is inhibited by 6-PTU. Because it is a selenoprotein and selenium deficiency is common in critically ill patients, selenium deficiency also may contribute to its malfunction.
Cytokines, such as IL-1 beta, TNF-alpha, and interferon-gamma, decrease type 1 deiodinase mRNA in vitro. Infusion of TNF-alpha decreases serum T3 and increases rT3. Soluble TNF-alpha, soluble TNF-alpha receptor, soluble IL-2 receptor antagonist, and IL-6 are inversely correlated with serum T3 levels. The elevations of soluble TNF-alpha receptor and IL-6 were independent determinants of serum T3 and accounted for 35% and 14%, respectively, of the change in T3. These cytokine changes can be concluded to occur concomitantly with changes in T3 and may play a pathogenic role through mechanisms that are not clearly defined. The increase of endogenous cortisol during illness apparently is not involved in inhibition of type I deiodinase.
Using an adenovirus model in mice hepatocyte primary cultures, it was demonstrated that forced expression of steroid receptor co-activator 1 (SRC-1) prevented the cytokine induced inhibition of type 1 deiodinase activity, suggesting the involvement of receptor co-activators in the nonthyroidal illness. [10]
FT3
Most studies have found FT3 hormones to be depressed.
Thyroxine
The decrease in the T4 binding of TBG has been used as an explanation for the low plasma T4 concentration in patients with NTI. The existence of a binding inhibitor could explain the observed alterations in T4 and free T4 fraction. TBG levels usually are within the reference range in patients with NTI and are somewhat lower in critically ill patients with low serum T4. Low TBG levels can be explained, according to some proposals, by rapid protease cleavage at inflammatory sites, particularly in acute inflammatory states (in which the decrease in TBG is too rapid to be accounted for by inhibition of synthesis).
In patients with NTI, serum T4 concentration has been demonstrated to be low because much of the circulating TBG in these patients is desialated. In NTI, the fractional rate of T4 transport from serum to tissues is reduced to 50% of the reference range value. This decrement in fractional rate of T4 transport is not related to the serum levels of total or free T4. Because in illness the reduction in the fractional rate of T4 transport from serum to tissues cannot be attributed to alterations in serum T4 binding, consider other causes such as an impairment of transport into tissues. In nonuremic critical illness, it has been demonstrated that elevated bilirubin or elevated NEFA and low albumin concentration may be at least partially responsible for the T4 transport inhibition in T3-producing tissues (eg, the liver).
A correlation exists between the probability of death and the levels of total T4. When serum T4 levels drop below 4 mcg/dL, the probability of death is about 50%; with serum T4 levels below 2 mcg/dL, the probability of death reaches 80%.
Free thyroxine
Evaluating thyroid function in patients with NTI has considerable challenges. No consensus exists as to whether free T4 levels are within the reference range, low, or high. Free T4 is believed to represent the hormone available to tissues. Measurement of total serum T4 has only limited value because nearly all (99.97%) of the circulating T4 is bound to TBG, T4-binding prealbumin (TBPA), and albumin. The rest of the circulating T4 (0.2-0.03%) is free T4. The circulating concentration of these binding proteins is understood to affect the total T4 concentration without necessarily changing the amount of free T4. Usually, TBG levels are within the reference range in patients with NTI and somewhat lower in critically ill patients with low serum T4. Decreased concentrations of one or more of the binding proteins would explain low levels of total T4 but does not explain a significant increase in free T4 fraction, which some patients with NTI exhibit.
Various explanations for the existence of inhibitors of T4 binding have been reported. Although low levels of TBPA and albumin may occur in patients with NTI, even complete inhibition of T4 binding to these proteins has been demonstrated to produce only about a 30% increase in free T4 fraction. Because free T4 fraction is increased above this level in many patients, other factors must be present. The observations of reduced total T4 and free T4 have been explained alternatively as either a fall in TBG levels or an inhibition of thyroid hormone binding to TBG. Some studies have shown a decrease in the T4 binding of TBG, which has been used as an explanation for the low plasma T4 concentration and, perhaps, the high free T4 fractions, in patients with NTI. Other studies postulate the existence of a binding inhibitor that could explain the observed alterations in free T4 fraction.
The inhibitor also has been demonstrated to interfere with the binding of iodothyronines to solid matrices, thus interfering with the T3 resin uptake and explaining the low FTI found in patients with NTI. The inhibitor appears to be extractable with ether and was associated with the NEFA fraction in the serum. Furthermore, the extracted inhibitor from sera of patients with NTI reduced conversion of T4 to T3 in rat liver homogenates. The inhibitor could be extracted from extrathyroidal tissues as well.
The addition of NEFA to normal serum is able to raise the free T4 fraction only if total NEFA concentration is higher than 3 millimoles in normal serum, representing a NEFA-to-albumin molar ratio greater than 5:1. Because this high NEFA-to-albumin ratio is not reached even in severely ill patients, NEFA is unlikely to influence the circulating free T4 concentration in vivo. Inhibitors of binding were also observed during equilibrium dialysis assay in patients treated with heparin. This is due to an in vitro artifact that is not present in vivo.
Cytokines also can elevate free T4. When TNF-alpha was infused, it was observed that free T4 could elevate transiently in association with a significant rise in free fatty acids. However, other studies question the role of NEFA inhibition or whether any thyroid hormone–binding inhibitor exists at all.
Thyroid hormone receptor expression and DNA binding
In experimental mouse liver models, infection decreased thyroid hormone receptor (TR) expression as well as retinoid X receptor (RXR)–TR DNA binding. TR-alpha and TR-beta protein levels were both decreased when lipopolysaccharide was administered, particularly at 16 hours. Lipopolysaccharide exposure was also shown to reduce RXR protein levels in the liver. [11]
Methods used to measure free thyroxine and their comparison
An ongoing controversy concerns true free T4 levels in NTI. Various studies use different techniques to measure free T4 in NTI, but all methods have been challenged. Using these methods, free T4 has been found to be within the reference range, low, and high. The results of free T4 assays in NTI are method dependent and may be influenced by many variables.
Several methods can be used to measure free T4 directly, including equilibrium dialysis, a 2-step immunoextraction technique, a 1-step (analog) method, FTI (T3 resin-binding ratio), and ultrafiltration. Equilibrium dialysis usually is the reference method. In equilibrium dialysis, a small amount of radioactive tracer T4 and the unknown sample are placed in a dialysis membrane, which limits the diffusion of bound T4. The proportion of the hormone that is dialyzable (ie, free) is determined. The dialyzable hormone–to–total hormone ratio is used, with the concentration of T4 determined in a standard assay and then used to calculate the concentration of free T4.
A second type of assay is the 2-step radioimmunoassay (RIA). The patient's serum is equilibrated with a solid phase antibody to T4. The unoccupied antibody binding sites are quantified in a second step in which labeled hormone is added to the solid phase system. The 2-step assay appears to have the best correlation with equilibrium dialysis results.
The 1-step (analog) assay uses an analog, usually an alanine substitution for T4. The analog does not bind to proteins in the serum but does compete for binding with antibody to T4. The analog also binds to albumin, which has a low affinity but high capacity; therefore, if albumin concentration changes, then free T4 measurements change (ie, if albumin increases, free T4 decreases and vice versa). Such changes can produce spurious results. This technique is not used widely.
An FTI is calculated by multiplying the total T4 concentration by the T3 uptake (T3U). The T3U is an indirect estimate free T4 fraction, which is obtained by adding labeled T3 to serum and estimating how much of it remains free for binding to a secondary binder (eg, charcoal, talc, ion-exchange resin, anti-T3 antibody, immobilized albumin) added to the serum. In this way, the FTI reflects the actual free T4 concentration, although this appears to be less accurate in cases of very low or high TBG concentrations. The use of FTI had poor reliability in patients with NTI; both artificially low and high FTI values were encountered frequently. This discrepancy in reported results probably is attributable to differences in patient selection (eg, the severity of illness and drugs used that interfere with serum T4 binding). These findings seriously limit the usefulness of the FTI tests in patients with NTI.
The ultrafiltration method is a research assay in which ultrafiltrates of undiluted serum are used to measure free T4. In a study of 504 patients by Docter et al, free T4 was elevated in 54% of the patients with mild-to-severe NTI, according to measurements using equilibrium dialysis, and free T4 was elevated in about 24% of patients in the most severely ill group, as illustrated in the diagram below. [12] Another study by Melmed et al demonstrated that free T4 was reduced in ICU patients as measured by 6 different methods, including equilibrium dialysis. [13] Free T4 was found to be uniformly reduced as measured by all methods, but patients with liver disease and chronic renal failure exhibited more variable results. This study demonstrated that, overall, patients with NTI who have serum total T4 levels within the reference range typically do not have reduced free T4 by most assay methods.
See the image below.
 Euthyroid sick syndrome. Relationship between serum thyroid hormone concentrations and severity of nonthyroidal illness (NTI). Abbreviations: reverse triiodothyronine (rT3), thyroid-stimulating hormone (TSH), free thyroxine (FT4), thyroxine (T4), triiodothyronine (T3).
Euthyroid sick syndrome. Relationship between serum thyroid hormone concentrations and severity of nonthyroidal illness (NTI). Abbreviations: reverse triiodothyronine (rT3), thyroid-stimulating hormone (TSH), free thyroxine (FT4), thyroxine (T4), triiodothyronine (T3).
In an extensive comparison of 5 measurement methods, free T4 was extremely low in patients with NTI who had a serum level of total T4 less than 3 mcg/dL. Results obtained using ultrafiltration also are variable. Thus, although extensively studied, the question remains whether free T4 in patients with NTI actually is low, within the reference range, or even high.
Thyroid-stimulating hormone and thyroid-releasing hormone
Serum TSH is measured with immunometric assays. In describing a serum TSH assay, referring to its sensitivity in µU/L is preferable to using terms such as ultrasensitive or supersensitive.
Immunometric assays in general perform well, but the sensitivity of the same commercial kit assay in different laboratories can vary substantially. In this method, 2 monoclonal antibodies are used, between which TSH becomes "sandwiched." Usually, the antibody to which TSH first is bound is immobilized on a solid surface. After separation of the solid phase, the bound TSH is quantified with a second anti-TSH antibody labeled with iodine-125, an enzyme, a fluorescent probe, or a chemiluminescent tag. In general, the assays using a chemiluminescent principle seem to perform best. Serum TSH in NTI typically is within the reference range or reduced. Serum TSH may be markedly low, although it usually is not less than 0.05 µIU/mL. These low TSH levels are often observed without significant decrease in T4.
Some patients with NTI have slightly elevated serum TSH, which is thought to have reduced biological activity. After recovery from severe NTI, transient elevation of TSH to above-normal limits commonly occurs. Some authors interpret this TSH elevation as a sign of recovery from a hypothyroid state. Despite the distortion of TSH in some euthyroid patients with NTI, patients with NTI who have significant elevation of TSH usually have underlying primary hypothyroidism.
Inappropriately low levels of serum hormones T3 and T4 and low TSH response suggest central down-regulation of the hypothalamic-pituitary-thyroid axis. This is supported by the observation that TRH gene expression in the paraventricular nucleus of the hypothalamus in postmortem hypothalamic tissue of patients who died after prolonged illness was less than in patients who died of acute cardiac arrest. In addition, TRH mRNA expression in the paraventricular nucleus correlates positively with premortem serum TSH and T3 levels. [14]
Responsiveness of the pituitary to TRH during NTI varies; some patients respond normally, while many have a less-than-normal response. Normal responsiveness in the presence of low TSH may suggest that a hypothalamic abnormality is causing the low TSH and low T4. The down-regulation at the hypothalamus-pituitary level provides an explanation for the decreased sensitivity of TSH secretion to low serum T3 and T4 concentrations in patients with NTI. A diminution, or loss, of the diurnal rhythm of TSH also occurs, and some studies have produced evidence for a reduction of TSH glycosylation with lower TSH bioactivity.
That TSH is not elevated in the presence of low T4 indicates that the patients are not hypothyroid. Diminished release of TRH also is thought perhaps to result in low TSH and, thus, low output of thyroid hormones by the thyroid. Low TRH mRNA in hypothalamic paraventricular nuclei also has been demonstrated.
The role of cytokines, especially IL-1 beta, in the activity of the hypothalamic-pituitary-adrenal axis is well known. Cytokines also affect TRH in rats. IL-1 beta decreases the release of TSH in cultured rat anterior pituitary cells, but the role of TNF-alpha on TSH release is disputed. IL-6 decreases TSH secretion. In rodents, leptin has been demonstrated as a major mediator of changes in hypothalamic-pituitary-thyroid function during fasting. However, TSH secretion and thyroid gland function are less affected during NTI in humans than they are in animals. The role of leptin in patients with NTI is unclear. Leptin concentrations often are elevated during critical illness and increase acutely in response to administration of TNF-alpha or IL-1; however, the leptin increase is not related to changes in serum T3 and T4 concentrations.
Epidemiology
Frequency
United States
The frequency of thyroid function abnormalities is related to the magnitude of the illness. The most common abnormality is a T3 reduction, occurring in about 40-100% of cases of NTI, which parallels the increase of rT3. As the disease severity increases, T4 levels also decrease. Most patients who are critically ill have reduced T4 levels. In patients who are hospitalized with an NTI, about 10% have abnormally low TSH values. The highest incidence occurs among the most severely ill group.
International
International frequency is the same as in the United States.
Mortality/Morbidity
Mortality and morbidity depend on the underlying NTI, the severity, and, possibly, the duration of the illness. [15, 16] The magnitude of the thyroid function test result abnormalities seems to depend on the severity, rather than the type, of illness. T4 is believed to fall in proportion to severity of illness.
The probability of death correlates with the levels of T4. When serum total T4 levels drop below 4 mcg/dL, the probability of death is about 50%; with serum T4 levels below 2 mcg/dL, the probability of death reaches 80%.
A study by Świstek et al indicated that the prognosis for patients with coronavirus disease 2019 (COVID-19) is worse when euthyroid sick syndrome is present at the same time. The investigators reported that compared with patients with COVID-19 but without euthyroid sick syndrome, inflammatory biomarker levels (C-reactive protein and procalcitonin) were higher, hospitalizations were longer, the likelihood of needing high-flow nasal oxygen therapy or intubation was greater, and the mortality rate was higher, in those suffering from both conditions. For example, the mortality rate during hospitalization in patients with euthyroid sick syndrome was 34.1%, compared with 11.3% for individuals without the syndrome. [17]
A study by Wang et al indicated that in high-risk patients undergoing isolated coronary artery bypass grafting (CABG), the presence of associated euthyroid sick syndrome independently increases the risk of major adverse in-hospital cardiovascular and cerebral events, the odds ratio being 3.40. The investigators also found a correlation between CABG-associated euthyroid sick syndrome and a maximum Sequential Organ Failure Assessment score of over 11. [18]
Race
People of all races are affected equally in NTI.
Sex
Thyroid function test results in both sexes are affected equally in NTI.
Age
NTI can affect people at any age. The usual aging process appears to influence the responsiveness of various tissues to thyroid hormone. Because systemic chronic illnesses are common in individuals of an advanced age, altered metabolism might be responsible for abnormal findings on thyroid function tests in elderly patients experiencing chronic illnesses.
-
Euthyroid sick syndrome. Relationship between serum thyroid hormone concentrations and severity of nonthyroidal illness (NTI). Abbreviations: reverse triiodothyronine (rT3), thyroid-stimulating hormone (TSH), free thyroxine (FT4), thyroxine (T4), triiodothyronine (T3).
-
Euthyroid sick syndrome. Relationship between severity and duration of nonthyroidal illness (NTI) and thyroid hormone levels. Abbreviations: reverse triiodothyronine (rT3), free thyroxine (free T4), thyroxine (T4), triiodothyronine (T3).