Practice Essentials
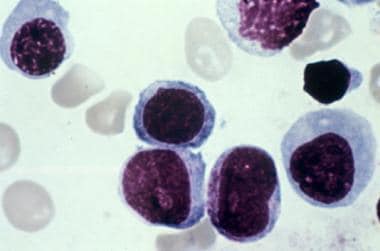
Erythroleukemia is a subtype of acute myeloid leukemia (AML) that is distinguished by erythroblastic proliferation. [1] Patients usuallly present with nonspecific signs and symptoms from the anemia, thrombocytopenia, and leukopenia resulting from the replacement of bone marrow by leukemic cells (see Presentation). Bone marrow aspiration and biopsy are critical in making the diagnosis of acute erythroleukemia (see the image below, and Workup). The treatment of acute erythroleukemia is similar to that used for other subtypes of AML (see Treatment and Medication).
Background
Giovanni Di Guglielmo first described erythroleukemia in the early twentieth century, and the disorder is often still referred to as acute Di Guglielmo syndrome. It is classified as an M6 subtype of AML in the French-American-British (FAB) classification system on the basis of morphologic and cytochemical criteria. [2]
Acute erythroleukemia has traditionally been recognized as having two subtypes: the more common erythroid/myeloid subtype, defined by the presence of increased erythroid cells and myeloid blasts; and the very uncommon pure erythroid subtype, characterized by expansion of immature erythroid cells only. [3] The 2016 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia continued to recognize pure erythroid leukemia but eliminated the erythroid/myeloid type of acute erythroleukemia. Cases previously classified as erythroid/myeloid subtype, based on the 2008 WHO classification, are currently categorized either as myelodysplastic syndrome with excess blasts or acute myeloid leukemia, not otherwise specified. [4]
Pathophysiology
Erythroleukemia is a neoplastic proliferation of erythroid and myeloid precursors of bone marrow hematopoietic stem cells. [5] In rare cases, however, a pure erythroid proliferation may occur. Erythroleukemia shares clinical and pathologic features with myelodysplastic syndromes, especially with erythroid-predominant myelodysplastic syndromes. [6]
The leukemic cells in erythroleukemia often carry complex karyotypes and mutations in oncogenes known to be associated with AML. However, on the basis of a study of the genetic and transcriptional landscape of 33 patients with acute erythroleukemia, Fagnan et al propose a transcriptome-based space that helps distinguish acute erythroleukemia from other myeloid leukemias. [7] These researchers describe a spectrum of genetic lesions that can be classified into three distinct molecular subgroups characterized by the following:
-
TP53 mutations
-
Mutations in epigenetic regulators (eg, DNMT3A, TET2, or IDH2), often accompanied by mutations in splicing factor genes
-
Undefined cases with low mutational burden
Further work by Fagnan et al in an animal model found that aberrant expression of oncogenic driver transcription factors results in erythroleukemia by downregulating the GATA1-regulated erythroid epigenome. In vivo work showed that the outcomes of disease (erythroid, myeloid, or both) depend on the driving oncogene and the hematopoietic target cell in which it is aberrantly expressed. [7, 8]
Iacobucci et al studied the genomic features of 159 childhood and adult cases of acute erythroleukemia and defined five age-related subgroups with distinct transcriptional profiles [9] :
-
TP53 mutated (mostly in adults)
-
NPM1 mutated
-
KMT2A mutated/rearranged
-
DDX41 mutated (adult)
-
NUP98 rearranged (pediatric)
Etiology
De novo cases of erythroleukemia are not associated with any identifiable risk factors. The most common predisposing factors in secondary acute erythroleukemia are as follows:
-
Ionizing radiation - Thorium dioxide suspension (Thorotrast), a radiographic contrast medium used in the 1940s, is associated with increased risk of erythroleukemia (latent period of 10-30 y after exposure).
-
Previous exposure to chemotherapy drugs (eg, alkylating agents) - These agents may be used in the treatment of Hodgkin lymphoma, multiple myeloma, bone marrow transplant, ovarian cancer, breast cancer, and nonneoplastic disorders (eg, collagen-vascular disease).
-
Rare cases of familial erythroleukemia (autosomal dominant with variable penetrance), manifesting in the sixth decade of life.
Epidemiology
Acute erythroleukemia accounts for 3-5% of all de novo AMLs and 20-30% of secondary leukemias. The incidence of erythroleukemia increases in people older than 50 years. Mazzella et al described 2 peaks, one in the seventh decade of life and a second, smaller peak in the fourth decade. [2, 10] Although very rare in children, acute erythroleukemia has been reported in children from the newborn period through age 7 years. Occurrence has a slight male predominance. No racial predilection is known.
Prognosis
Patients with acute erythroleukemia have a poor prognosis. Problems encountered in the treatment of acute erythroleukemia include primary induction failure, relapse, and the toxicity of chemotherapeutic agents.
Many factors influence patients’ responses to chemotherapy and their duration of remission, including the following [11] :
-
Findings from cytogenetic evaluation affect the prognosis.
-
No specific chromosome abnormalities are associated with this subtype.
-
Multidrug resistant phenotype (positive Pgp expression) is associated with a poor prognosis.
-
Determining the myeloblast-to-erythroblast ratio at diagnosis helps to predict prognosis; a higher ratio is associated with a favorable prognosis.
A study by Santos et al compared the prognosis in 91 patients with newly diagnosed erythroleukemia with that of patients in a control group suffering from other subtypes of AML. [11] A history of the predisposing factor myelodysplastic syndrome was present in 50% of the patients in the erythroleukemia group and 41% of the patients in the control group. Poor-risk cytogenetics were present in 61% of the erythroleukemia patients and 38% of the control patients. Complete remission rates were 62% in the erythroleukemia group and 58% in the control group. The median period of disease-free survival was 32 weeks for erythroleukemia patients and 49 weeks for control subjects. The median period of overall survival was 36 weeks for erythroleukemia patients and 43 weeks for control subjects.
After carrying out a multivariate analysis, these authors concluded that erythroleukemia is not an independent risk factor in disease-free and overall survival, and that well-known AML prognostic factors should guide treatment decisions. [11]
Remission can be achieved in many patients when treated with the standard myeloid protocol (ie, cytarabine [cytosine arabinoside; ara-C] with an anthracycline). Kowal-Vern et al reported that subtypes characterized by predominance of proerythroblasts are not targeted by conventional AML protocols and suggested that this might be related to the poor outcome observed in these patients. [12]
Multidrug resistance gene (ie, MDR1) expression correlates with unfavorable cytogenetic aberrations and is responsible for poor response to chemotherapy and short survival time. Patients with refractory or relapsed erythroleukemia may be tested for Pgp (ie, MDR1 product). MDR modulators (eg, cyclosporin A, quinidine, verapamil, PSC 833) are being used in a clinical trial setting to overcome this resistance. [10]
A less favorable outcome may be observed in elderly patients, in patients with secondary erythroleukemia (usually after treatment with alkylating agents), and in patients with unfavorable cytogenetics.
Furthermore, patients with the distinct entity of pure erythroid leukemia (PEL) may have an unusually poor prognosis. While the International Consensus Classification of Myeloid Neoplasms and Acute Leukemias includes PEL under a broader category of acute myeloid leukemia with mutated TP53, [13] the 2016 assigned World Health Organization (WHO) categories fail to capture the distinct features of PEL. [4]
PEL is characterized as a neoplastic erythroid hyperproliferation with maturation arrest. E-cadherin is the most sensitive and specific marker for immature erythroblasts and is helpful in distinguishing PEL from other erythroid proliferations. The phenotype of PEL correlates with a very complex karyotype and an extremely aggressive clinical course. Median survival has been reported between 1.8 and 3 months with ranges of 0.2-9.3 months. [14, 15]
Iacobucci et al reported an association between genomic features and outcome in acute eyrthroleukemia, with NPM1 mutations and HOXB9 overexpression being associated with a favorable prognosis and TP53, FLT3 or RB1 alterations associated with poor survival. Patients with NPM1-mutated cases had a 5-year survival of 87.5 %, while those with TP53-mutated cases had median survival of 13 months, with no patients surviving at 5 years. [9]
Patient Education
Patients should be educated about the signs of febrile neutropenia and thrombocytopenia. The long-term adverse effects of chemotherapeutic agents must be clearly explained, and issues related to chemotherapy-associated infertility (eg, sperm banking) must be presented and discussed. Procedure-related adverse effects and failure to obtain informed consent should also be addressed.
For patient education information, see the Leukemia Directory.
-
Bone marrow aspirate showing erythroblasts in a patient with erythroleukemia. Courtesy of Maurice Barcos, MD, PhD, Department of Pathology, Roswell Park Cancer Institute, Buffalo, NY.