Practice Essentials
Increased levels of eosinophilic leukocytes in the blood can be idiopathic, or may result from a variety of conditions, including connective tissue diseases, helminthic infections, neoplasias, and allergic disorders. In this article, the term eosinophilia is defined as an increase in peripheral blood eosinophils to more than 600 cells per microliter (μL) of blood. Hypereosinophilia has generally been defined as a peripheral blood eosinophil count greater than 1500/μL. [1]
Although emphasis is placed on the number of eosinophils circulating in the peripheral blood, an increase in eosinophils can be observed in other body fluids (eg, cerebrospinal fluid [CSF], urine) and many body tissues (eg, skin, lung, heart, liver, intestine, bladder, bone marrow, muscle, nerve). See the images below.
Eosinophils are derived from hematopoietic stem cells initially committed to the myeloid line and then to the basophil-eosinophil granulocyte lineage. Nonpathologic functions of eosinophils and the cationic enzymes of their granules include mediating parasite defense reactions, allergic response, tissue inflammation, and immune modulation. [2, 3]
Tissues of the pulmonary and gastrointestinal systems are the normal residence for eosinophils, but peripheral, or blood, eosinophilia (absolute eosinophil count [AEC] >600 cells/µL) indicates an eosinophilic disorder. [4] Untreated, the eosinophilia can be categorized as mild (AEC 600-1500 cells/µL), moderate (AEC 1500-5000 cells/µL) or severe (AEC >5000 cells/µL). An increase in tissue eosinophilia may be seen with or without concurrent peripheral eosinophilia.
A secondary or reactive increase in blood eosinophils, tissue eosinophils, or both is associated with a wide variety of conditions, as follows [5, 6] :
-
Infections (especially helminthic parasites)
-
Allergic responses
-
Neoplasms
-
Connective tissue disorders
-
Medications
-
Endocrinopathies
Primary eosinophilia is not a reactive phenomenon and can be described as either clonal or idiopathic in nature. If an underlying molecular or cytogenetic abnormality can be identified, the eosinophilia can be designated as a clonal disorder. If reactive causes are ruled out and no underlying clonal origin is proven, the eosinophilia is described as idiopathic. [7]
Given the broad spectrum of conditions linked to eosinophilia, this article emphasizes the diagnostic considerations that clinicians may want to focus on in patients with eosinophilia. The individual disease manifestations and therapies for the dozens of diseases associated with eosinophilia are not described in detail; other Medscape articles specifically address these conditions, such as the following:
Pathophysiology
Over the past 2 decades, substantial progress has been made in understanding the mechanisms of eosinophil production, eosinophil programmed cell death (apoptosis), and how eosinophil immunology contributes to both host defenses against infections and to tissue damage within the host in cases of allergic and autoimmune diseases.
The primary stimuli for eosinophil production are interleukin (IL)-5, IL-3, and the granulocyte-macrophage colony-stimulating factor (GM-CSF). These cytokines are also the primary signals that inhibit eosinophil programmed cell death. Thus, eosinophilia can be triggered via these 3 eosinophilopoietic cytokines by increased eosinophil production, by eosinophil longevity, or by a combination of these. [2, 3]
In addition, an evolving number of chemotactic cytokines (ie, chemokines) have been established as causing eosinophils to migrate from their site of production in the bone marrow into the blood and then into peripheral tissues. These chemokines include eotaxin-1, eotaxin-2, and RANTES (regulated on activation normal T cell expressed and secreted).
Eosinophils are the source of a large number of cytokines, including the following:
-
IL-2, IL-3, IL-4, IL-5, IL-7, IL-13, and IL-16
-
Tumor necrosis factor–alpha (TNF-alpha),
-
Transforming growth factor–beta (TGF-beta)
-
RANTES
In addition to these cytokines, eosinophils are a source of several cationic proteins that also contribute to the immunologic responses against infectious disease agents and to tissue damage in allergic and autoimmune diseases. These cationic proteins include the following:
-
Eosinophil cationic protein (ECP)
-
Eosinophil peroxidase (EPO)
-
Charcot-Leyden crystal lysophospholipase
-
Major basic protein (MBP)
-
Eosinophil-derived neurotoxin (EDN)
Secondary eosinophilia is a reactive phenomenon driven by eosinophilopoietic cytokine release by nonmyeloid cells. Eosinophilic differentiation occurs in the bone marrow from myeloid progenitors through the actions of GM-CSF, IL-3, and IL-5. Mature eosinophils are released into the bloodstream where they migrate quickly to peripheral tissues of the bronchial and gastrointestinal mucosa and skin. Their survival is short, unless apoptosis is blocked by cytokines (GM-CSF, IL-3, and IL-5).
Dysregulated production of these cytokines by various cell populations account for secondary hypereosinophilia such as seen in nonmyeloid malignancies (eg, Hodgkin lymphoma; transitional cell carcinoma [TCC] of the bladder; adenocarcinomas of the stomach, colon, and uterus; large cell undifferentiated lung carcinomas; and large cell cervical tumors), allergic reactions, parasitic infections, and other conditions.
Eosinophilia is a feature of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, a rare delayed hypersensitivity reaction that typically develops 2 to 8 weeks after starting a drug. Many drugs have been linked to DRESS, including anticonvulsants, antibiotics, and allopurinol. The clinical features include skin rash (widely variable, but often maculopapular or erythematous), fever, lymphadenopathy, and inflammation of one or more organs (eg, liver, lung, brain, kidney, heart). Other hematologic abnormalities may include thrombocytopenia and atypical lymphocytes. DRESS may be fatal, with mortality rates depending on the severity of the organ involvement. [8, 9]
Primary eosinophilias include both clonal and idiopathic hypereosinophilic syndrome (HES). These disorders have very heterogeneous underlying pathophysiologies, not all of which are well-defined. They are by definition eosinophilia for longer than 6 months, without evidence of reactive cause and with signs and symptoms of organ involvement. [10]
In some neoplastic disorders, the hypereosinophilia is part of neoplastic clonal expansion affecting the myeloid lineage. This pathophysiology would describe the eosinophilia in the following disorders:
-
Chronic myelogenous leukemia (CML), Philadelphia chromosome positive (Ph+) or BCR-ABL positive
-
Acute myeloid leukemia (AML), including inv(16), t(16;66)(p13;q22)
A number of hypereosinophilic syndrome (HES) cases exhibit clonal expansion of abnormal lymphocytes. Immunophenotypically, they are characterized by aberrant and immature T cells, which exhibit abnormal cytokine production. T-cell receptor gene rearrangements are demonstrated in many. These T cells produce high levels of IL-5, thought to cause the hypereosinophilia.
Eosinophilia is further classified as clonal or idiopathic, both clinically and pathologically. The World Health Organization (WHO) proposed criteria to distinguish idiopathic hypereosinophilic syndrome (HES) from chronic eosinophilic leukemia–not otherwise specified (CEL-NOS). [1] WHO diagnostic criteria for CEL-NOS are as follows:
-
Absence of the Philadelphia chromosome or a rearrangement involving PDGFRA/B and FGFR1
-
Exclusion of other acute or chronic primary marrow neoplasms associated with eosinophilia (eg, AML, myelodysplastic syndrome, systemic mastocytosis)
-
Blasts of more than 2% in peripheral blood, or bone marrow blasts of more than 5% but less than 20% (the upper threshold is to exclude acute leukemia as a diagnosis)
-
Evidence for a clonal marker
The underlying chromosomal abnormalities leading to CEL have been described in some cases. A deletion on chromosome band 4q12 resulting in the FIP1L1-PDGFRA (FIR1- like-1–platelet-derived growth factor receptor–alpha) fusion gene causes an abnormal constitutively activated tyrosine kinase. These patients demonstrate CHIC2 gene deletion in peripheral blood mononuclear cells as a result of this fusion gene.
Another fusion gene involving BCR-PDGFRA has been seen in CML with marked eosinophilia. Mutations involving PDGFRB rearrangements have been described, as well as FGFR1 (fibroblast growth factor receptor–1) fusions. [3, 11, 12, 13] Clinical features of eosinophil leukemia result from accumulation of leukemic cells in bone marrow, liver, and spleen. [14] Inflammatory mediators from the eosinophils themselves cause tissue damage to the pericardium, myocardium, endocardium, and nervous system.
In 38 patients with chronic eosinophilia studied by array comparative genomic hybridization (aCGH), Arefi et al found that aCGH revealed clonality in eosinophils in most patients with myeloproliferative neoplasias. These authors suggested that aCGH could be a useful technique for defining clonality in these diseases. [15]
Finally, idiopathic hypereosinophilic syndrome (HES) is the diagnosis of exclusion in patients with marked prolonged (> 6 mo) eosinophilia with multiple organ involvement but without identifiable cytogenetic or molecular abnormalities. Organ damage occurs from release of the contents of eosinophilic granules. Some of these cases transform into identifiable entities. Because of the increasing ability to identify clonal markers, few cases now meet the classical definition of idiopathic HES; WHO considers HES to be a provisional diagnosis until a primary or secondary cause of eosinophilia is established. [16]
Etiology
The mnemonic CHINA (ie, Connective tissue diseases, Helminthic infections, Idiopathic hypereosinophilic syndrome [HES], Neoplasia, Allergies) describes the categories of diseases that sometimes are associated with blood eosinophilia.
Connective tissue diseases include the following:
-
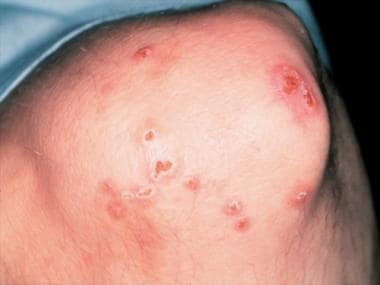
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss Syndrome) (See the images below.)
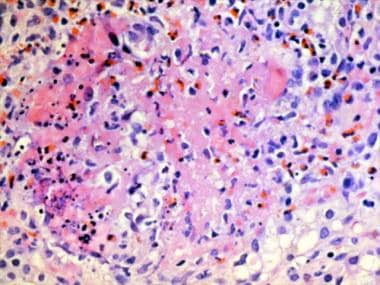 Granuloma with a central core of eosinophilic debris surrounded by a peripheral palisade of epithelioid histiocytes and eosinophils from a patient with Churg-Strauss syndrome (allergic granulomatosis).
Granuloma with a central core of eosinophilic debris surrounded by a peripheral palisade of epithelioid histiocytes and eosinophils from a patient with Churg-Strauss syndrome (allergic granulomatosis).
-
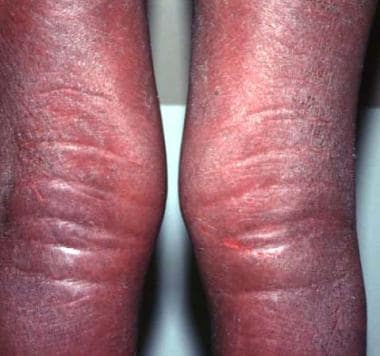
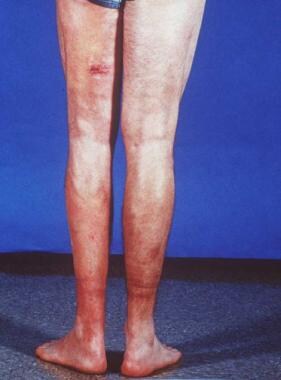
Eosinophilic fasciitis (See the images below.)
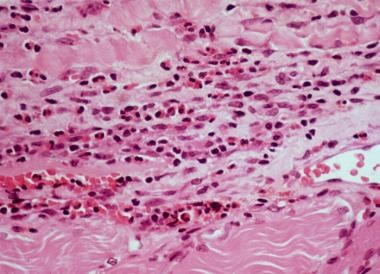 High-power photomicrograph of fascia shows heavy inflammatory infiltration with numerous eosinophils, lymphocytes, and occasional plasma cells in a patient with eosinophilic fasciitis.
High-power photomicrograph of fascia shows heavy inflammatory infiltration with numerous eosinophils, lymphocytes, and occasional plasma cells in a patient with eosinophilic fasciitis.
-
Eosinophilia-myalgia syndrome (due to tryptophan in the United States in 1989)
-
Toxic-oil syndrome (due to contaminated rapeseed oil in Spain in 1981)
Helminthic (ie, worm) parasitic infections include the following:
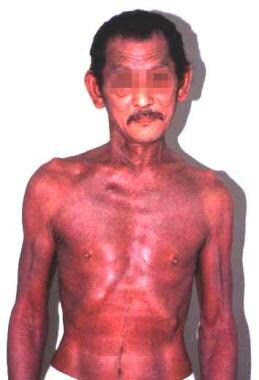
Idiopathic HES is shown in the images below:
Neoplasias include the following:
-
Lymphoma (eg, Hodgkin lymphoma, non-Hodgkin lymphoma)
-
Human T-cell lymphotropic virus I (HTLV-I) infection
-
Adult T-cell leukemia/lymphoma (ATLL)
-
Eosinophilic leukemia (very rare)
-
Gastric or lung carcinoma (ie, paraneoplastic eosinophilia)
Allergic/atopic diseases include the following:
In a retrospective review by Leverone et al of 2227 adults with peripheral blood eosinophilia, hematologic disorders comprised the most common cause (21.9%) of eosinophilia, followed by active bacterial infection (16.8%). The three most common specific causes of eosinophilia were asthma (6.4%), chronic lymphocytic leukemia (5.8%), and multiple myeloma (4.1%). [17]
Epidemiology
In the United States, compared with developing countries, eosinophilia occurs most commonly due to allergic conditions, including drug reactions and atopic asthma. Parasitic infections are rare.
Idiopathic hypereosinophilic syndrome (HES) is rare condition. The World Health Organization estimates an age‐adjusted incidence rate of approximately 0.036 per 100,000. [16]
Helminthic infections are the most common cause of eosinophilia worldwide due to the high prevalence of helminthic parasite infections, several of which are estimated to involve hundreds of millions of people.
No racial predilection exists for eosinophilia, although the occurrence of eosinophilia-associated helminthic parasitic infections is more common in certain geographic areas of the world.
No male or female predilection exists in most subtypes of eosinophilia. However, there is a marked male predominance in clonal disorders involving the PDGFRB fusion gene and a small male predominance in clonal disorders of the FGFR1 gene.
People of all ages can be affected by eosinophilia.
Prognosis
The prognosis in patients with secondary eosinophilia depends on the underlying disease. Many helminthic infections develop into chronic diseases that cause morbidity but not mortality. Similarly, allergic reactions and conditions associated with eosinophilia usually do not cause mortality.
Eosinophilia associated with nonmyeloid malignancies does not affect their individual prognosis, including mortality rates. The mortality and morbidity associated with clonal and idiopathic causes depends on the degree of tissue involvement, damage, or both at diagnosis; how quickly therapy is implemented; and treatment responsiveness.
The prognosis in patients with primary eosinophilias is determined by the following:
-
Degree of organ involvement at diagnosis
-
The timeliness of treatment
-
Responsiveness to treatment
-
Underlying cytogenetic and molecular pathophysiology
-
Indurated edematous plaques of hypereosinophilic syndrome on a patient's legs.
-
Erythroderma in a patient with hypereosinophilic syndrome.
-
Granuloma with a central core of eosinophilic debris surrounded by a peripheral palisade of epithelioid histiocytes and eosinophils from a patient with Churg-Strauss syndrome (allergic granulomatosis).
-
Magnified view of papules and nodules with central necrosis in a patient with Churg-Strauss syndrome (allergic granulomatosis).
-
High-power photomicrograph of fascia shows heavy inflammatory infiltration with numerous eosinophils, lymphocytes, and occasional plasma cells in a patient with eosinophilic fasciitis.
-
Lower back part of the legs in a patient with eosinophilic fasciitis shows hypopigmentation, induration, biopsy site, and asymmetric involvement.
-
Egg of Schistosoma hematobium, with its typical terminal spine.