Background
Eisenmenger syndrome refers to any untreated congenital cardiac defect with intracardiac communication that leads to pulmonary hypertension, reversal of flow, and cyanosis. [1, 2, 3] The previous left-to-right shunt is converted into a right-to-left shunt secondary to elevated pulmonary artery pressures and associated pulmonary vascular disease. (See Etiology, Treatment, and Medication.)
Lesions in Eisenmenger syndrome, such as large septal defects, are characterized by high pulmonary pressure and/or a high pulmonary flow state. Development of the syndrome represents a point at which pulmonary hypertension is irreversible and is an indication that the cardiac lesion is likely inoperable (see the image below). (See Etiology, Treatment, and Medication.) Cardiac arrhythmias and sudden cardiac death are important late complications of this syndrome. Conservative management with medications and/or lung and cardiac transplantation are therapeutic approaches that can offer quality-of-life improvement.
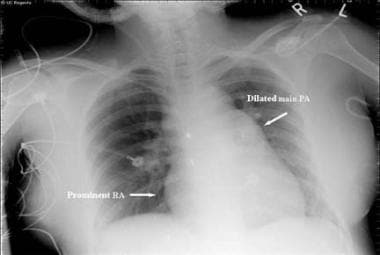 Eisenmenger Syndrome. This radiograph reveals an enlarged right heart and pulmonary artery dilatation in a 24-year-old woman with an unrestricted patent ductus arteriosus (PDA) and Eisenmenger syndrome. RA = right atrium.
Eisenmenger Syndrome. This radiograph reveals an enlarged right heart and pulmonary artery dilatation in a 24-year-old woman with an unrestricted patent ductus arteriosus (PDA) and Eisenmenger syndrome. RA = right atrium.
Eisenmenger syndrome was initially described in 1897, when Victor Eisenmenger reported on a patient with symptoms of dyspnea and cyanosis from infancy who subsequently developed heart failure and succumbed to massive hemoptysis. [4] An autopsy revealed a large ventricular septal defect (VSD) and an overriding aorta. This was the first description of a link between a large congenital cardiac shunt defect and the development of pulmonary hypertension. (See Presentation and Workup.)
Advances in the medical treatment of patients with severe pulmonary hypertension may improve survival in patients with Eisenmenger syndrome and may potentially reverse the process in selected patients to a point at which they again become candidates for surgical repair. (See Treatment and Medication.) [5]
Pulmonary hypertension
Pulmonary hypertension is defined as a mean pulmonary artery pressure above 25 mm Hg at rest or over 30 mm Hg during exercise. The World Health Organization (WHO) published a classification system of various etiologies of pulmonary hypertension; the most recent update was published in 2013 during the Fifth World Symposium on Pulmonary Hypertension in Nice, France. [6] Eisenmenger syndrome is considered part of the group 1 causes of pulmonary hypertension.
Intracardiac communication
Any intracardiac communication that allows high pulmonary blood flow will lead, over time, to irreversible pulmonary vascular injury, increased pulmonary artery pressures and, ultimately, to right-to-left intracardiac blood flow. Originally described in association with a large VSD, Eisenmenger syndrome can also manifest with a patent ductus arteriosus (PDA) or, less frequently, with other congenital cardiac anomalies, such as atrioventricular septal defects (AVSDs) and atrial septal defects (ASDs). (See the images below.)
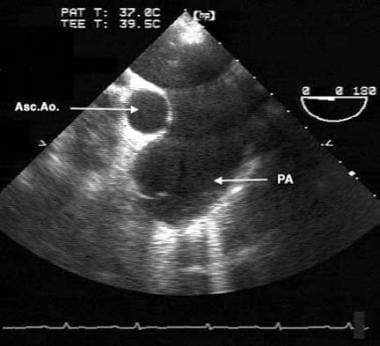 Eisenmenger Syndrome. This transesophageal echocardiographic image is from the midesophagus of a patient with Eisenmenger syndrome secondary to an unrestricted patent ductus arteriosus (PDA). It shows a severely dilated pulmonary artery (PA). Asc. Ao. = ascending aorta.
Eisenmenger Syndrome. This transesophageal echocardiographic image is from the midesophagus of a patient with Eisenmenger syndrome secondary to an unrestricted patent ductus arteriosus (PDA). It shows a severely dilated pulmonary artery (PA). Asc. Ao. = ascending aorta.
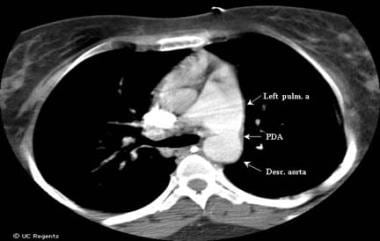 Eisenmenger Syndrome. This computed tomography (CT) chest scan shows a large, unrestricted patent ductus arteriosus (PDA) in a 24-year-old woman with Eisenmenger syndrome. Desc. aorta = descending aorta.
Eisenmenger Syndrome. This computed tomography (CT) chest scan shows a large, unrestricted patent ductus arteriosus (PDA) in a 24-year-old woman with Eisenmenger syndrome. Desc. aorta = descending aorta.
Examples of congenital heart disease subtypes that may cause pulmonary vascular disease and proceed to Eisenmenger syndrome include the following:
-
Increased pulmonary arterial flow: ASD, systemic arteriovenous fistulae, total anomalous pulmonary venous return
-
Increased pulmonary arterial pressure and flow: Large VSD, large PDA, truncus arteriosus, single ventricle with unobstructed pulmonary blood flow
-
Elevated pulmonary venous pressure: Mitral stenosis, cor triatriatum, obstructed pulmonary venous return
Pathophysiology
Over time, any communication that allows a left-to-right shunt causes increased pulmonary vascular flow and, eventually, irreversible vascular injury. Systemic-to-pulmonary communications are usually harmless prenatally because the high pulmonary vascular resistance of the fetus limits left-to-right shunting. After birth, the pulmonary vascular resistance normally decreases and there is an increase in right ventricular compliance, resulting in a left-to-right shunt and an increase in pulmonary blood flow. Moreover, the constant high pulmonary blood flow produces progressive structural abnormalities and histologic changes in the pulmonary vasculature. [7] The histologic changes are mediated by increased endothelin 1, elevated thromboxane, platelet activation, and production of intrinsic elastase and vascular endothelial growth factors. [8, 9] With years, pulmonary vascular resistance increases and right-to-left shunt develops at the chambers or great arteries. Cyanosis also develops over time, initially on exertion and eventually at rest.
Etiology
Eisenmenger syndrome occurs in patients with large, congenital cardiac or surgically-created extracardiac left-to-right shunts. These shunts initially cause increased pulmonary blood flow. If left unchecked, increased pulmonary blood flow and/or elevated pulmonary arterial pressure can result in remodeling of the pulmonary microvasculature, with subsequent obstruction to pulmonary blood flow. This is commonly referred to as pulmonary vascular obstructive disease (PVOD).
According to Ohms law, flow (Q) is inversely related to resistance (R) and is directly proportional to pressure (P), as represented by the equation Q = P/R. Any increase in flow, as is observed in patients with intracardiac defects and initial left-to-right shunts, results in increased pulmonary artery pressures. Additionally, any increase in resistance, as occurs in PVOD, results in a decrease in effective flow at the same pressure.
The progression to Eisenmenger physiology is represented by a spectrum of morphologic changes in the capillary bed that progress from reversible lesions to irreversible ones. Endothelial dysfunction and smooth muscle proliferation result from the changes in flow and pressure, increasing the PVR. [10]
The cellular and molecular mechanisms remain fully uncharacterized, representing pathways of inflammation, cell proliferation, increase in the extracellular matrix, vasoconstriction, fibrosis, and intravascular thrombosis. [2, 11] The mechanism of pulmonary hypertension in congenital heart disease may share characteristics with other mechanisms of pulmonary hypertension, but the pathways remain complex. They include derangements in the expression of vasoactive substances such as prostacyclin, thromboxane, nitric oxide, and endothelin 1. [11]
In 1958, Heath and Edwards proposed a histologic classification to describe the changes in Eisenmenger syndrome. [12] Stages I and II represent disease that is most likely reversible. Stage III disease may still be reversible, but in progressing to stages IV-VI, the disease is thought to become irreversible. In current practice, pulmonary biopsies are rarely performed for this condition.
Natural history
The size of the intracardiac shunt plays an important role in the likelihood of development of the syndrome. About 3% of patients with a small ventricular septal defect (VSD) (≤1.5 cm) and 50% of patients with a large VSD (>1.5cm) can develop Eisenmenger syndrome. [13] Onset of the syndrome is usually earlier (infancy) in patients with VSD or patent ductus arteriosus (PDA), whereas it tends to present more often during adulthood in patients with atrial septal defect (ASD).
Failure to reduce pulmonary pressures in the first 2 years of life may result in the irreversible progression of endothelial dysfunction and pulmonary vascular remodeling. This translates into the aforementioned progressive changes described by Heath and Edwards. [12] The condition then advances to irreversible pulmonary hypertension. Historically, death occurs between ages 30 and 35 years. [1] Death may occur from sudden cardiac death, hemoptysis, thromboembolic events, heart failure, pregnancy complications, noncardiac surgery complications, and central nervous system infections.
Clinically, patients gradually develop the following complications of advanced pulmonary vascular disease:
-
Dyspnea upon exertion
-
Syncope due to low cardiac output
-
Chest pain
-
Cyanosis
-
Congestive right heart failure
-
Pulmonary hemorrhage/hemoptysis
-
Dysrhythmia
-
Stroke
-
Brain abscess
-
Erythrocytosis and hyperviscosity complications
-
Endocarditis
The time frame for this process depends on the anatomic nature of the lesion and whether conditions, such as trisomy 21 (Down syndrome), that are known to accelerate the development of PVOD are present. [14] Without intervention, reversal of flow may happen in early childhood or around puberty, and progression of symptoms may lead to death by the second or third decade of life. [13, 15] Interestingly, adult patients with Eisenmenger syndrome may have a better prognosis compared to those with other causes of idiopathic pulmonary hypertension. [16]
Causes
Causes of Eisenmenger syndrome include the following:
-
Large, uncorrected cardiac shunt
-
Large, nonrestrictive VSD
-
Nonrestrictive PDA
-
Atrioventricular septal defect, including a large ostium primum ASD without a ventricular component
-
Aortopulmonary window
-
Palliative, surgically created systemic-to-pulmonary anastomosis for treatment of congenital heart disease
Epidemiology
Eisenmenger syndrome usually develops before puberty, but it may also start manifesting in adolescence and early adulthood. Approximately 8% of patients with congenital heart disease develop Eisenmenger syndrome. [17] Quality of life is limited by symptoms, but patients can survive until the third and fourth decades of life.
The prevalence of Eisenmenger syndrome is difficult to quantify, but it is declining in the developed world with the identification and surgical correction of congenital heart conditions. [3] Patients from underdeveloped countries are more likely to have late presentations of Eisenmenger syndrome due to uncorrected congenital cardiac lesions.
The frequency of pulmonary hypertension and the subsequent development of reversed shunting vary depending on the heart defect and operative intervention. Such variations include the following [18] :
-
Large, nonrestrictive ventricular septal defect (VSD) or patent ductus arteriosus (PDA): Approximately 50% of infants with one of these defects develop pulmonary hypertension by early childhood
-
VSD or PDA and transposition of the great arteries: About 40% of patients develop pulmonary hypertension within the first year of life
-
Large secundum atrial septal defect (ASD): The natural history of a large secundum ASD differs in that the 10% of cases that progress to pulmonary hypertension do so more slowly and usually not until after the third decade of life
-
Persistent truncus arteriosus and unrestricted pulmonary blood flow: All patients develop severe pulmonary hypertension by the second year of life
-
Common atrioventricular canal: Almost all patients develop severe pulmonary hypertension by the second year of life
-
Surgically-created systemic-to-pulmonary shunt: The frequency of pulmonary hypertension varies depending on size and anatomy
-
Blalock-Taussig anastomosis (surgical procedure communicating the subclavian artery to the pulmonary artery): About 10% of patients develop pulmonary hypertension
-
Waterston (another palliative procedure to communicate the ascending aorta to pulmonary artery) or Potts (descending aorta to pulmonary artery) shunt: About 30% of patients develop pulmonary hypertension
Prognosis
Eisenmenger syndrome is uniformly fatal; however, some patients survive into the sixth decade of life. The usual life expectancy of a patient with Eisenmenger syndrome is 20-40 years if the syndrome is diagnosed promptly and treated with vigilance. The onset of pulmonary hemorrhage is usually the hallmark of rapid progression of the disease. [19]
A systematic review by Diller et al was able to estimate 10-year mortality rates of 30-40%. [20] In a study of 153 German patients with Eisenmenger syndrome, 10-year mortality approached 60-70% in those who did not receive disease-directed therapy. [21]
The complications of chronic cyanotic heart disease affect multiple organ systems, including the hematologic, skeletal, renal, and neurologic systems, causing significant morbidity and mortality.
The quality of life is poor in patients with Eisenmenger syndrome, because exercise tolerance is extremely limited (due to limited oxygen uptake resulting from an inability to increase pulmonary blood flow) and complications are profound. Signs of poor prognosis are syncope, elevated right-sided pressures, and hypoxemia.
A study by Salehian et al reported that left ventricular (LV) dysfunction (defined as LV ejection fraction [LVEF] < 50%), right ventricular (RV) hypertrophy, arrhythmias, low serum albumin, and signs and symptoms of heart failure predict mortality in patients with Eisenmenger syndrome. [22] A simple echocardiographic score that relies on RV and right atrial characteristics was found to predict adverse outcomes in patients with Eisenmenger syndrome that is not associated with complex congenital heart disease. [23]
Uncorrected congenital heart disease with development of the Eisenmenger complex portends an insidious progression to near-complete physical disability.
Mortality
Patients with Eisenmenger syndrome usually do not survive beyond the second or third decade. Long-term survival depends on the patient’s age at the onset of pulmonary hypertension and the coexistence of additional adverse features, such as Down syndrome. Survival predominantly depends on RV function. In other conditions, such as pregnancy, mortality from Eisenmenger syndrome is reported to be approximately 50%, although it may be higher.
The most frequent terminal event in this syndrome is a combination of hypoxemia and arrhythmia in the setting of rapid increases in pulmonary vascular resistance or decreases in systemic vascular resistance (SVR). Death also commonly results from congestive heart failure, massive hemoptysis, or thromboembolism. [15]
Diller et al indicated that survival rates for untreated patients with Eisenmenger syndrome may have been overestimated in previous studies and that these rates have not improved since the 1970s. [20] Their report involved a literature review of 12 studies published between 1971 and 2013 (1131 patients total), in conjunction with an analysis of 219 contemporary, treatment-naïve patients at the investigators’ own institution.
The investigators stated that almost none of the studies appropriately accounted for immortal time bias and therefore overestimated patient’s survival chances by as much as 20 years. [20] As noted earlier, when they took immortal time bias into account, Diller et al determined that the 10-year mortality rate among untreated patients approached 30-40%. The investigators also found no indication that the chances of survival for these patients were better than they would have been in the 1970s, 1980s, or 1990s, although survival prospects were found to be better than for patients in the 1950s and 1960s. [20]
Complications
Complications in Eisenmenger syndrome include the following:
-
Hematologic complications: Include hyperviscosity syndromes related to secondary erythrocytosis and bleeding diatheses [24]
-
Nervous system complications: Include brain abscess, transient cerebral ischemia, paradoxical embolism, thrombotic stroke, and intracerebral hemorrhage
-
Hyperbilirubinemia: Increases the risk of gallstones
-
Hyperuricemia: Can cause nephrolithiasis and secondary gout
-
Hypertrophic osteoarthropathy: Causes bone pain and tenderness
-
Vision loss: Reports document transient vision loss related to peripheral retinal microvascular abnormalities
-
Congestive heart failure
-
Dysrhythmia
-
Pulmonary infarction and hemorrhage
-
Infective endocarditis
-
Syncope: The systemic vascular bed is prone to vasodilation and subsequent systemic arterial hypotension, which can cause syncope
-
Sudden death
Patient Education
Consider the following points in patient education:
-
Emphasize that diet and weight control are essential
-
Educate patients to avoid smoking
-
Provide an exercise prescription
-
Advise abstinence from or only moderate intake of alcohol
-
Stress the importance of bacterial endocarditis prophylaxis before surgical procedures
-
Educate patients about contraception options and pregnancy risk (there is about a 50% mortality in pregnant patients with Eisenmenger syndrome) [25]
-
Consider recommending contraception by means of tubal ligation
-
Note that oral or implantable contraceptives may promote pulmonary infarction through activation of the coagulation cascade
-
Educate patients about the signs and symptoms of polycythemia and hyperviscosity
-
Inform patients about the importance of dental hygiene
Additional resources for patients with pulmonary hypertension can be found at the Pulmonary Hypertension Association website.
-
Eisenmenger Syndrome. This radiograph reveals an enlarged right heart and pulmonary artery dilatation in a 24-year-old woman with an unrestricted patent ductus arteriosus (PDA) and Eisenmenger syndrome. RA = right atrium.
-
Eisenmenger Syndrome. Apical, 4-chamber, transthoracic echocardiographic view demonstrating an ostium primum atrial septal defect (ASD) with enlarged right-side chambers. RA = right atrium, RV = right ventricle, LA = left atrium, LV = left ventricle.
-
Eisenmenger Syndrome. This computed tomography (CT) chest scan shows a large, unrestricted patent ductus arteriosus (PDA) in a 24-year-old woman with Eisenmenger syndrome. Desc. aorta = descending aorta.
-
Eisenmenger Syndrome. This apical, 4-chamber, transthoracic echocardiographic segment shows color Doppler flow across the interatrial septum at the site of a large ostium primum atrial septal defect (ASD). RA = right atrium.
-
Eisenmenger Syndrome. This transesophageal echocardiographic image is from the midesophagus of a patient with Eisenmenger syndrome secondary to an unrestricted patent ductus arteriosus (PDA). It shows a severely dilated pulmonary artery (PA). Asc. Ao. = ascending aorta.
-
Eisenmenger Syndrome. This is a color Doppler interrogation of the tricuspid valve in a patient with Eisenmenger syndrome. It demonstrates an elevated estimated right ventricular systolic pressure of 106 mm Hg and right atrial pressure, reflecting pulmonary hypertension. TR = tricuspid regurgitation.
-
Eisenmenger Syndrome. This is a transthoracic Doppler examination of the pulmonic valve in a 24-year-old woman with Eisenmenger syndrome secondary to an uncorrected ostium primum atrial septal defect (ASD). It reveals an elevated estimated pulmonary artery diastolic pressure of 51 mm Hg and right atrial pressure. PR = pulmonic regurgitation.