Practice Essentials
Chronic kidney disease (CKD)—or chronic renal failure (CRF), as it was historically termed—is a term that encompasses all degrees of decreased kidney function, from damaged–at risk through mild, moderate, and severe chronic kidney failure. [1] CKD is a worldwide public health problem. In the United States, there is a rising incidence and prevalence of kidney failure, with poor outcomes and high cost (see Epidemiology).
CKD is more prevalent in the elderly population Almost half of the patients with CKD are older than 70 years. However, while younger patients with CKD typically experience progressive loss of kidney function, 30% of patients over 65 years of age with CKD have stable disease. [2]
CKD is associated with an increased risk of cardiovascular disease and end-stage kidney disease (ESKD). Kidney disease is the 10th leading cause of death in the United States. [3]
The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation (NKF) established a definition and classification of CKD in 2002. [4] The KDOQI and the international guideline group Kidney Disease Improving Global Outcomes (KDIGO) subsequently updated these guidelines. [5] These guidelines have allowed better communication among physicians and have facilitated intervention at the different stages of the disease.
The guidelines define CKD as either kidney damage or a decreased glomerular filtration rate (GFR) of less than 60 mL/min/1.73 m2 for at least 3 months. Whatever the underlying etiology, once the loss of nephrons and reduction of functional renal mass reaches a certain point, the remaining nephrons begin a process of irreversible sclerosis that leads to a progressive decline in the GFR. [6]
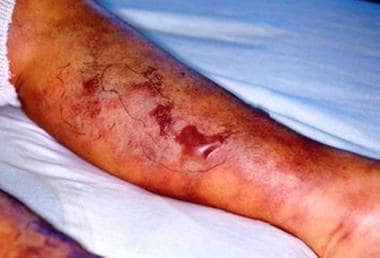
Hyperparathyroidism is one of the pathologic manifestations of CKD. See the image below.
Staging
The different stages of CKD form a continuum. The stages of CKD are classified as follows [5] :
-
Stage 1: Kidney damage with normal or increased GFR (>90 mL/min/1.73 m 2)
-
Stage 2: Mild reduction in GFR (60-89 mL/min/1.73 m 2)
-
Stage 3a: Moderate reduction in GFR (45-59 mL/min/1.73 m 2)
-
Stage 3b: Moderate reduction in GFR (30-44 mL/min/1.73 m 2)
-
Stage 4: Severe reduction in GFR (15-29 mL/min/1.73 m 2)
-
Stage 5: Kidney failure (GFR < 15 mL/min/1.73 m 2 or dialysis)
By itself, measurement of GFR may not be sufficient for identifying stage 1 and stage 2 CKD, because in those patients the GFR may in fact be normal or borderline normal. In such cases, the presence of one or more of the following markers of kidney damage can establish the diagnosis [5] :
-
Albuminuria (albumin excretion > 30 mg/24 hr or albumin:creatinine ratio > 30 mg/g [> 3 mg/mmol])
-
Urine sediment abnormalities
-
Electrolyte and other abnormalities due to tubular disorders
-
Histologic abnormalities
-
Structural abnormalities detected by imaging
-
History of kidney transplantation
Hypertension is a frequent sign of CKD but should not by itself be considered a marker of it, because elevated blood pressure is also common among people without CKD.
In an update of its CKD classification system, Kidney Disease: Improving Global Outcomes (KDIGO) advised that GFR and albuminuria levels be used together, rather than separately, to improve prognostic accuracy in the assessment of CKD. [5] More specifically, the guidelines recommended the inclusion of estimated GFR and albuminuria levels when evaluating risks for overall mortality, cardiovascular disease, ESKD, acute kidney injury, and the progression of CKD.
KDIGO guidelines recommend referral to a kidney specialist for patients with any of the following5:
-
GFR < 30 mL/min/1.73 m 2
-
A consistent finding of significant albuminuria (albumin-to-creatinine ratio ≥ 300 mg/g [≥ 30 mg/mmol] or albumin excretion rate ≥ 300 mg/24 hours, approximately equivalent to urine protein-to-creatinine ratio ≥ 500 mg/g [≥50 mg/mmol] or protein excretion rate ≥ 500 mg/24 hours)
-
Progression of CKD
Signs and symptoms
Patients with CKD stages 1-3 are generally asymptomatic. Typically, it is not until stages 4-5 (GFR < 30 mL/min/1.73 m²) that endocrine/metabolic derangements or disturbances in water or electrolyte balance become clinically manifest.
Signs of metabolic acidosis in stage 5 CKD include the following:
-
Loss of lean body mass
-
Muscle weakness
Signs of alterations in the way the kidneys are handling salt and water in stage 5 include the following:
-
Peripheral edema
-
Pulmonary edema
-
Hypertension
Anemia in CKD is associated with the following:
-
Fatigue
-
Reduced exercise capacity
-
Impaired cognitive and immune function
-
Reduced quality of life
-
Development of cardiovascular disease
-
New onset of heart failure or the development of more severe heart failure
-
Increased cardiovascular mortality
Other manifestations of uremia in ESKD, many of which are more likely in patients who are being inadequately dialyzed, include the following:
-
Pericarditis: Can be complicated by cardiac tamponade, possibly resulting in death if unrecognized
-
Encephalopathy: Can progress to coma and death
-
Peripheral neuropathy, usually asymptomatic
-
Restless leg syndrome
-
Gastrointestinal symptoms: Anorexia, nausea, vomiting, diarrhea
-
Skin manifestations: Dry skin, pruritus, ecchymosis
-
Fatigue, increased somnolence, failure to thrive
-
Malnutrition
-
Erectile dysfunction, decreased libido, amenorrhea
-
Platelet dysfunction with tendency to bleed
Screen adult patients with CKD for depressive symptoms; self-report scales at initiation of dialysis therapy reveal that 45% of these patients have such symptoms, albeit with a somatic emphasis.
See Presentation for more detail.
Diagnosis
Screening
American College of Physicians guidelines on screening for CKD include the following recommendations (weak recommendation, low-quality evidence):
-
Do not screen for CKD in asymptomatic adults without risk factors for CKD
-
Do not test for proteinuria in adults with or without diabetes who are currently taking an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin II-receptor blocker (ARB)
Laboratory studies
Laboratory studies used in the diagnosis of CKD can include the following:
-
Complete blood count (CBC)
-
Basic metabolic panel
-
Urinalysis
-
Serum albumin levels (but note that patients may have hypoalbuminemia due to malnutrition, urinary protein loss, or chronic inflammation)
-
Lipid profile: Patients with CKD have an increased risk of cardiovascular disease
Evidence of renal bone disease can be derived from the following tests:
-
Serum calcium and phosphate
-
25-hydroxyvitamin D
-
Alkaline phosphatase
-
Intact parathyroid hormone (PTH) levels
In certain cases, the following tests may also be ordered as part of the evaluation of patients with CKD:
-
Serum and urine protein electrophoresis and free light chains: Screen for a monoclonal protein possibly representing multiple myeloma
-
Antinuclear antibodies (ANA), double-stranded DNA antibody levels: Screen for systemic lupus erythematosus
-
Serum complement levels: Results may be depressed with some glomerulonephritides
-
Cytoplasmic and perinuclear pattern antineutrophil cytoplasmic antibody (C-ANCA and P-ANCA) levels: Positive findings are helpful in the diagnosis of granulomatosis with polyangiitis (Wegener granulomatosis); P-ANCA is also helpful in the diagnosis of microscopic polyangiitis
-
Anti–glomerular basement membrane (anti-GBM) antibodies: Presence is highly suggestive of underlying Goodpasture syndrome
-
Hepatitis B and C, human immunodeficiency virus (HIV), Venereal Disease Research Laboratory (VDRL) serology: Conditions associated with some glomerulonephritides
Imaging studies
Imaging studies that can be used in the diagnosis of CKD include the following:
-
Renal ultrasonography: Useful to screen for hydronephrosis, which may not be observed in early obstruction or dehydrated patients; or for involvement of the retroperitoneum with fibrosis, tumor, or diffuse adenopathy; small, echogenic kidneys are observed in advanced kidney failure
-
Retrograde pyelography: Useful in cases with high suspicion for obstruction despite negative renal ultrasonograms, as well as for diagnosing renal stones
-
Computed tomography (CT) scanning: Useful to better define renal masses and cysts usually noted on ultrasonograms; also the most sensitive test for identifying kidney stones
-
Magnetic resonance imaging (MRI): Useful in patients who require a CT scan but who cannot receive intravenous contrast; reliable in the diagnosis of renal vein thrombosis
-
Renal radionuclide scanning: Useful to screen for renal artery stenosis when performed with captopril administration; also quantitates the renal contribution to the GFR
Biopsy
Percutaneous kidney biopsy is generally indicated when kidney impairment and/or proteinuria approaching the nephrotic range are present and the diagnosis is unclear after appropriate workup.
See Workup for more detail.
Management
Early diagnosis and treatment of the underlying cause and/or institution of secondary preventive measures is imperative in patients with CKD. These may slow, or possibly halt, progression of the disease. The medical care of patients with CKD should focus on the following:
-
Delaying or halting the progression of CKD: Treatment of the underlying condition, if possible, is indicated
-
Diagnosing and treating the pathologic manifestations of CKD
-
Timely planning for long-term renal replacement therapy
The pathologic manifestations of CKD should be treated as follows:
-
Anemia: When the hemoglobin level is below 10 g/dL, treat with erythropoiesis-stimulating agents (ESAs), which include epoetin alfa and darbepoetin alfa after iron saturation and ferritin levels are at acceptable levels; patients on dialysis for more than 4 months may be treated with the hypoxia-inducible factor inhibitor daprodustat
-
Hyperphosphatemia: Treat with dietary phosphate binders and dietary phosphate restriction
-
Hypocalcemia: Treat with calcium supplements with or without calcitriol
-
Hyperparathyroidism: Treat with calcitriol or vitamin D analogues or calcimimetics
-
Volume overload: Treat with loop diuretics or ultrafiltration
-
Metabolic acidosis: Treat with oral alkali supplementation
-
Uremic manifestations: Treat with long-term renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplantation)
Indications for renal replacement therapy include the following:
-
Severe metabolic acidosis
-
Hyperkalemia
-
Pericarditis
-
Encephalopathy
-
Intractable volume overload
-
Failure to thrive and malnutrition
-
Peripheral neuropathy
-
Intractable gastrointestinal symptoms
-
In asymptomatic patients, a GFR of 5-9 mL/min/1.73 m², [7] irrespective of the cause of the CKD or the presence or absence of other comorbidities
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) issued a Clinical Practice Guideline for Nutrition in Chronic Renal Failure, as well as a revision of recommendations for Nutrition in Children with Chronic Kidney Disease.
See Treatment and Medication for more detail.
For a discussion of CKD in children, click here.
Pathophysiology
A normal kidney contains approximately 1 million nephrons, each of which contributes to the total glomerular filtration rate (GFR). In the face of renal injury (regardless of the etiology), the kidney has an innate ability to maintain GFR, despite progressive destruction of nephrons, as the remaining healthy nephrons manifest hyperfiltration and compensatory hypertrophy. This nephron adaptability allows for continued normal clearance of plasma solutes. Plasma levels of substances such as urea and creatinine start to show measurable increases only after total GFR has decreased 50%.
The plasma creatinine value will approximately double with a 50% reduction in GFR. For example, a rise in plasma creatinine from a baseline value of 0.6 mg/dL to 1.2 mg/dL in a patient, although still within the adult reference range, actually represents a loss of 50% of functioning nephron mass.
The hyperfiltration and hypertrophy of residual nephrons, although beneficial for the reasons noted, has been hypothesized to represent a major cause of progressive renal dysfunction. The increased glomerular capillary pressure may damage the capillaries, leading initially to secondary focal and segmental glomerulosclerosis (FSGS) and eventually to global glomerulosclerosis. This hypothesis is supported by studies of five-sixths nephrectomized rats, which develop lesions identical to those observed in humans with chronic kidney disease (CKD).
Factors other than the underlying disease process and glomerular hypertension that may cause progressive renal injury include the following:
-
Systemic hypertension
-
Nephrotoxins (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], intravenous contrast media)
-
Decreased perfusion (eg, from severe dehydration or episodes of shock)
-
Proteinuria (in addition to being a marker of CKD)
-
Hyperlipidemia
-
Hyperphosphatemia with calcium phosphate deposition
-
Smoking
-
Uncontrolled diabetes
Thaker et al found a strong association between episodes of acute kidney injury (AKI) and cumulative risk for the development of advanced CKD in patients with diabetes mellitus who experienced AKI in multiple hospitalizations. [8] Any AKI versus no AKI was a risk factor for stage 4 CKD, and each additional AKI episode doubled that risk. [8]
Findings from the Atherosclerosis Risk in Communities (ARIC) Study, a prospective observational cohort, suggest that inflammation and hemostasis are antecedent pathways for CKD. [9] This study used data from 1787 cases of CKD that developed between 1987 and 2004.
Childhood kidney function and CKD in children
In children, the GFR increases with age and is calculated with specific equations that are different than those for adults. Adjusted for body surface area, the GFR reaches adult levels by age 2-3 years.
Aspects of pediatric kidney function and the measure of creatinine are informative not only for children but also for adults. For example, it is important to realize that creatinine is derived from muscle and, therefore, that children and smaller individuals have lower creatinine levels independent of the GFR. Consequently, laboratory reports that do not supply appropriate pediatric normal ranges are misleading. The same is true for individuals who have low muscle mass for other reasons, such as malnutrition, cachexia, or amputation.
Another important note for childhood CKD is that physicians caring for children must be aware of normal blood pressure levels by age, sex, and height. Prompt recognition of hypertension at any age is important, because it may be caused by primary renal disease.
Fortunately, CKD during childhood is rare. Pediatric CKD is usually the result of congenital defects, such as posterior urethral valves or dysplastic kidney malformations. Another common cause is FSGS. Genetic kidney diseases are also frequently manifested in childhood CKD. Advances in pediatric nephrology have enabled great leaps in survival for pediatric CKD and end-stage renal disease (ESRD), including for children who need dialysis or transplantation.
Aging and kidney function
The biologic process of aging initiates various structural and functional changes within the kidney. [10, 11] Renal mass progressively declines with advancing age, and glomerulosclerosis leads to a decrease in renal weight. Histologic examination is notable for a decrease in glomerular number of as much as 30-50% by age 70 years. The GFR peaks during the third decade of life at approximately 120 mL/min/1.73 m2; it then undergoes an annual mean decline of approximately 1 mL/min/y/1.73 m2, reaching a mean value of 70 mL/min/1.73 m2 at age 70 years.
Ischemic obsolescence of cortical glomeruli is predominant, with relative sparing of the renal medulla. Juxtamedullary glomeruli see a shunting of blood from afferent to efferent arterioles, resulting in redistribution of blood flow favoring the renal medulla. These anatomic and functional changes in renal vasculature appear to contribute to an age-related decrease in renal blood flow.
Renal hemodynamic measurements in aged humans and animals suggest that altered functional response of the renal vasculature may be an underlying factor in diminished renal blood flow and increased filtration noted with progressive renal aging. The vasodilatory response is blunted in the elderly when compared with younger patients.
However, the vasoconstrictor response to intrarenal angiotensin is identical in young and older human subjects. A blunted vasodilatory capacity with appropriate vasoconstrictor response may indicate that the aged kidney is in a state of vasodilatation to compensate for the underlying sclerotic damage.
Given the histologic evidence for nephronal senescence with age, a decline in the GFR is expected. However, a wide variation in the rate of GFR decline is reported because of measurement methods, race, gender, genetic variance, and other risk factors for renal dysfunction.
Genetics
Most cases of CKD are acquired rather than inherited, although CKD in a child is more likely to have a genetic or inherited cause. Well-described genetic syndromes associated with CKD include autosomal dominant polycystic kidney disease (ADPKD) and Alport syndrome. Other examples of specific single-gene or few-gene mutations associated with CKD include Dent disease, nephronophthisis, and atypical hemolytic uremic syndrome (HUS).
APOL1 gene
More recently, researchers have begun to identify genetic contributions to increased risk for development or progression of CKD. Friedman et al found that more than 3 million black persons with genetic variants in both copies of apolipoprotein L1 (APOL1) are at higher risk for hypertension-attributable ESRD and FSGS. In contrast, black individuals without the risk genotype and European Americans appear to have similar risk for developing nondiabetic CKD. [12]
FGF-23 gene
Circulating levels of the phosphate-regulating hormone fibroblast growth factor 23 (FGF-23) are affected by variants in the FGF23 gene. Isakova et al reported that elevated FGF-23 levels are an independent risk factor for ESRD in patients who have fairly well-preserved kidney function (stages 2-4) and for mortality across the scope of CKD. [13]
Single-nucleotide polymorphisms
A review of 16 single-nucleotide polymorphisms (SNPs) that had been associated with variation in GFR found that development of albuminuria was associated mostly with an SNP in the SHROOM3 gene. [14] Even accounting for this variant, however, there is evidence that some unknown genetic variant influences the development of albuminuria in CKD. This study also suggests a separate genetic influence on development of albuminuria versus reduction in GFR. [14]
A genome-wide association study (GWAS) that included over 130,000 patients found 6 SNPs associated with reduced GFR, located in or near MPPED2, DDX1, SLC47A1, CDK12, CASP9, and INO80. [15] The SNP in SLC47A1 was associated with decreased GFR in nondiabetic individuals, whereas SNPs located in the DNAJC16 and CDK12 genes were associated with decreased GFR in individuals younger than 65 years. [15]
Immune-system and RAS genes
A number of genes have been associated with the development of ESRD. Many of these genes involve aspects of the immune system (eg, CCR3, IL1RN, IL4). [16]
Unsurprisingly, polymorphisms in genes involving the renin-angiotensin system (RAS) have also been implicated in predisposition to CKD. One study found that patients with CKD were significantly more likely to have the A2350G polymorphism in the ACE gene, which encodes the angiotensin-converting enzyme (ACE). [17] They were also more likely to have the C573T polymorphism in the AGTR1 gene, which encodes the angiotensin II type 1 receptor. [17]
Hyperkalemia
The ability to maintain potassium excretion at near-normal levels is generally maintained in CKD, as long as aldosterone secretion and distal flow are maintained. Another defense against potassium retention in patients with CKD is increased potassium excretion in the gastrointestinal tract, which also is under control of aldosterone.
Hyperkalemia usually does not develop until the GFR falls to less than 20-25 mL/min/1.73 m², at which point the kidneys have decreased ability to excrete potassium. Hyperkalemia can be observed sooner in patients who ingest a potassium-rich diet or have low serum aldosterone levels. Common sources of low aldosterone levels are diabetes mellitus and the use of ACE inhibitors, NSAIDs, or beta-blockers.
Hyperkalemia in CKD can be aggravated by an extracellular shift of potassium, such as occurs in the setting of acidemia or from lack of insulin.
Hypokalemia
Hypokalemia is uncommon but can develop in patients with very poor intake of potassium, gastrointestinal or urinary loss of potassium, or diarrhea or in patients who use diuretics.
Metabolic acidosis
Metabolic acidosis often is a mixture of normal anion gap and increased anion gap; the latter is observed generally with stage 5 CKD but with the anion gap generally not higher than 20 mEq/L. In CKD, the kidneys are unable to produce enough ammonia in the proximal tubules to excrete the endogenous acid into the urine in the form of ammonium. In stage 5 CKD, accumulation of phosphates, sulfates, and other organic anions are the cause of the increase in anion gap.
Metabolic acidosis has been shown to have deleterious effects on protein balance, leading to the following:
-
Negative nitrogen balance
-
Increased protein degradation
-
Increased essential amino acid oxidation
-
Reduced albumin synthesis
-
Lack of adaptation to a low-protein diet
Hence, metabolic acidosis is associated with protein-energy malnutrition, loss of lean body mass, and muscle weakness. The mechanism for reducing protein may include effects on adenosine triphosphate (ATP)–dependent ubiquitin proteasomes and increased activity of branched-chain keto acid dehydrogenases.
Metabolic acidosis also leads to an increase in fibrosis and rapid progression of kidney disease, by causing an increase in ammoniagenesis to enhance hydrogen excretion.
In addition, metabolic acidosis is a factor in the development of renal osteodystrophy, because bone acts as a buffer for excess acid, with resultant loss of mineral. Acidosis may interfere with vitamin D metabolism, and patients who are persistently more acidotic are more likely to have osteomalacia or low-turnover bone disease.
Salt- and water-handling abnormalities
Salt and water handling by the kidney is altered in CKD. Extracellular volume expansion and total-body volume overload results from failure of sodium and free-water excretion. This generally becomes clinically manifested when the GFR falls to less than 10-15 mL/min/1.73 m², when compensatory mechanisms have become exhausted.
As kidney function declines further, sodium retention and extracellular volume expansion lead to peripheral edema and, not uncommonly, pulmonary edema and hypertension. At a higher GFR, excess sodium and water intake could result in a similar picture if the ingested amounts of sodium and water exceed the available potential for compensatory excretion.
Tubulointerstitial renal diseases represent the minority of cases of CKD. However, it is important to note that such diseases often cause fluid loss rather than overload. Thus, despite moderate or severe reductions in GFR, tubulointerstitial renal diseases may manifest first as polyuria and volume depletion, with inability to concentrate the urine. These symptoms may be subtle and require close attention to be recognized. Volume overload occurs only when GFR reduction becomes very severe.
Anemia
Normochromic normocytic anemia principally develops from decreased renal synthesis of erythropoietin, the hormone responsible for bone marrow stimulation for red blood cell (RBC) production. The anemia starts early in the course of the disease and becomes more severe as viable renal mass shrinks and the GFR progressively decreases.
Using data from the National Health and Nutrition Examination Survey (NHANES), Stauffer and Fan found that anemia was twice as prevalent in people with CKD (15.4%) as in the general population (7.6%). The prevalence of anemia increased with stage of CKD, from 8.4% at stage 1 to 53.4% at stage 5. [18]
No reticulocyte response occurs. RBC survival is decreased, and bleeding tendency is increased from the uremia-induced platelet dysfunction. Other causes of anemia in CKD include the following:
-
Chronic blood loss: Uremia-induced platelet dysfunction enhances bleeding tendency
-
Secondary hyperparathyroidism
-
Inflammation
-
Nutritional deficiency
-
Accumulation of inhibitors of erythropoiesis
Bone disease
Renal bone disease is a common complication of CKD. It results in skeletal complications (eg, abnormality of bone turnover, mineralization, linear growth) and extraskeletal complications (eg, vascular or soft-tissue calcification).
Different types of bone disease occur with CKD, as follows:
-
High-turnover bone disease from high parathyroid hormone (PTH) levels
-
Low-turnover bone disease (adynamic bone disease)
-
Defective mineralization (osteomalacia)
-
Mixed disease
-
Beta-2-microglobulin–associated bone disease
Bone disease in children is similar but occurs during growth. Therefore, children with CKD are at risk for short stature, bone curvature, and poor mineralization (“renal rickets” is the equivalent term for adult osteomalacia).
CKD–mineral and bone disorder (CKD-MBD) involves biochemical abnormalities related to bone metabolism. CKD-MBD may result from alteration in levels of serum phosphorus, PTH, vitamin D, and alkaline phosphatase.
Secondary hyperparathyroidism develops in CKD because of the following factors:
-
Hyperphosphatemia
-
Hypocalcemia
-
Decreased renal synthesis of 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D, or calcitriol)
-
Intrinsic alteration in the parathyroid glands, which gives rise to increased PTH secretion and increased parathyroid growth
-
Skeletal resistance to PTH
Calcium and calcitriol are primary feedback inhibitors; hyperphosphatemia is a stimulus to PTH synthesis and secretion.
Hyperphosphatemia and hypocalcemia
Phosphate retention begins in early CKD; when the GFR falls, less phosphate is filtered and excreted, but because of increased PTH secretion, which increases renal excretion, serum levels do not rise initially. As the GFR falls toward CKD stages 4-5, hyperphosphatemia develops from the inability of the kidneys to excrete the excess dietary intake.
Hyperphosphatemia suppresses the renal hydroxylation of inactive 25-hydroxyvitamin D to calcitriol, so serum calcitriol levels are low when the GFR is less than 30 mL/min/1.73 m². Increased phosphate concentration also affects PTH concentration by its direct effect on the parathyroid glands (posttranscriptional effect).
Hypocalcemia develops primarily from decreased intestinal calcium absorption because of low plasma calcitriol levels. It also possibly results from increased calcium-phosphate binding, caused by elevated serum phosphate levels.
Increased PTH secretion
Low serum calcitriol levels, hypocalcemia, and hyperphosphatemia have all been demonstrated to independently trigger PTH synthesis and secretion. As these stimuli persist in CKD, particularly in the more advanced stages, PTH secretion becomes maladaptive, and the parathyroid glands, which initially hypertrophy, become hyperplastic. The persistently elevated PTH levels exacerbate hyperphosphatemia from bone resorption of phosphate.
Skeletal manifestations
If serum levels of PTH remain elevated, a high ̶ bone turnover lesion, known as osteitis fibrosa, develops. This is one of several bone lesions, which as a group are commonly known as renal osteodystrophy and which develop in patients with severe CKD. Osteitis fibrosa is common in patients with ESRD.
The prevalence of adynamic bone disease in the United States has increased, and its onset before the initiation of dialysis has been reported in some cases. The pathogenesis of adynamic bone disease is not well defined, but possible contributing factors include the following:
-
High calcium load
-
Use of vitamin D sterols
-
Increasing age
-
Previous corticosteroid therapy
-
Peritoneal dialysis
-
Increased level of N-terminally truncated PTH fragments
Low-turnover osteomalacia in the setting of CKD is associated with aluminum accumulation. It is markedly less common than high-turnover bone disease.
Another form of bone disease is dialysis-related amyloidosis, which is now uncommon in the era of improved dialysis membranes. This condition occurs from beta-2-microglobulin accumulation in patients who have required chronic dialysis for at least 8-10 years. It manifests with cysts at the ends of long bones.
Calciphylaxis
Calciphylaxis, or calcific uremic arteriolopathy, results from the deposition of calcium in the arteriolar microvasculature of the deep dermis and subcutaneous adipose tissue. It is is classically associated with CKD, and particularly with ESRD, in patients receiving maintenance dialysis. Although CKD can result in secondary hyperparathyroidism, with increasing serum calcium levels and arteriolar microcalcification, calciphylaxis may develop in individuals with CKD who have normal serum calcium and phosphate concentrations. [19]
Patients with calciphylaxis typically present with exquisitely painful retiform purpura or tender nodules, most often on the abdomen and proximal lower extremities, which can progress to cutaneous necrosis. Cutaneous infection may lead to sepsis, which is the most common cause of death in these patients. Reported 1-year mortality rates range from 45% to 80%. [19]
Etiology
Causes of chronic kidney disease (CKD) include the following:
-
Diabetic kidney disease
-
Hypertension
-
Vascular disease
-
Glomerular disease (primary or secondary)
-
Cystic kidney diseases
-
Tubulointerstitial disease
-
Urinary tract obstruction or dysfunction
-
Recurrent kidney stone disease
-
Congenital (birth) defects of the kidney or bladder
-
Unrecovered acute kidney injury
Vascular diseases that can cause CKD include the following:
-
Renal artery stenosis
-
Cytoplasmic pattern antineutrophil cytoplasmic antibody (C-ANCA)–positive and perinuclear pattern antineutrophil cytoplasmic antibody (P-ANCA)–positive vasculitides
-
ANCA-negative vasculitides
-
Atheroemboli
-
Hypertensive nephrosclerosis
-
Renal vein thrombosis
Primary glomerular diseases include the following:
-
Membranous nephropathy
-
Alport syndrome
-
Immunoglobulin A (IgA) nephropathy
-
Focal and segmental glomerulosclerosis (FSGS)
-
Minimal change disease
-
Membranoproliferative glomerulonephritis (MPGN)
-
Complement-related diseases (eg, atypical hemolytic-uremic syndrome [HUS], dense deposit disease)
-
Rapidly progressive (crescentic) glomerulonephritis
Secondary causes of glomerular disease include the following:
-
Diabetes mellitus
-
Systemic lupus erythematosus
-
Rheumatoid arthritis
-
Mixed connective tissue disease
-
Scleroderma
-
Granulomatosis with polyangiitis (formerly known as Wegener granulomatosis)
-
Mixed cryoglobulinemia
-
Endocarditis
-
Hepatitis B and C
-
Syphilis
-
Human immunodeficiency virus (HIV) infection
-
Parasitic infection
-
Heroin use
-
Gold
-
Penicillamine
-
Amyloidosis
-
Light-chain deposition disease
-
Neoplasia
-
Thrombotic thrombocytopenic purpura (TTP)
-
Shiga-toxin or Streptococcus pneumoniae – related HUS
-
Henoch-Schönlein purpura
-
Reflux nephropathy
Causes of tubulointerstitial disease include the following:
-
Drugs (eg, sulfonamides, allopurinol)
-
Infection (viral, bacterial, parasitic)
-
Sjögren syndrome
-
Tubulointerstitial nephritis and uveitis (TINU) syndrome
-
Chronic hypokalemia
-
Chronic hypercalcemia
-
Sarcoidosis
-
Multiple myeloma cast nephropathy
-
Heavy metals
-
Radiation nephritis
-
Polycystic kidneys
-
Cystinosis and other inherited diseases
Urinary tract obstruction may result from any of the following:
-
Benign prostatic hypertrophy
-
Urolithiasis (kidney stones
-
Urethral stricture
-
Tumors
-
Neurogenic bladder
-
Congenital (birth) defects of the kidney or bladder
-
Retroperitoneal fibrosis
Epidemiology
In the United States, more than 1 in 7 adults—15% of the adult population, or 37 million people—are estimated to have chronic kidney disease (CKD). [20] Kidney disease is the 10th leading cause of death in the United States. [3]
According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the overall prevalence of CKD in the US has remained relatively stable since 2004. The largest increase occurred in people with stage 3 CKD, from 4.5% to 6.0%. [21]
The US prevalence of CKD increases dramatically with age: it is 6% in persons 18 to 44 years, 12% in those 45 to 64 years, and 38% in those 65 or older. [21] In the National Health and Nutrition Examination Survey (NHANES) study, the prevalence of stage 3 CKD decreased among individuals younger than 65 years, from 1.6% during the years 2013-2016 to 1.3% during the years 2017–March 2020 (the survey was terminated prematurely due to the COVID-19 pandemic), but was virtually unchanged among those age 65 years and older over that period. [21]
According to 2017–March 2020 NHANES data, the estimated prevalence of CKD in adults by stage was as follows [22] :
-
Stage 3: 5.1%
-
Stage 4: 0.3%
-
Stage 5: 0.2%
The adjusted incidence of end-stage renal disease (ESRD) in the US fell by 7.6% from 2000 to 2019. Over that period, however, the number of patients with newly registered ESRD rose from 94,466 to 134,862, an increase of 42.8%. [21] In 2021, nearly 786,000 people in the US (2 per 1,000 population) were currently living with ESRD. [20]
The US Surgeon General’s latest report on 10-year national objectives for improving the health of all Americans, Healthy People 2030, contains a chapter focused on CKD. For 2030, Healthy People lays out 14 objectives concerning reduction of the US incidence, morbidity, mortality, and health costs of CKD. Reducing kidney failure will require additional public health efforts, including effective preventive strategies and early detection and treatment of CKD.
International statistics
In 2017, 697.5 million cases of CKD (all stages) were recorded worldwide, for a global prevalence of 9.1%. From 1990 to 2017, the global all-age prevalence of CKD increased 29.3%, whereas the age-standardized prevalence remained stable. Globally, 1.2 million people died from CKD in 2017. The global all-age mortality rate from CKD increased 41.5% from 1990 to 2017. Diabetic nephropathy accounted for almost a third of disability-adjusted life years (DALYs) from CKD. Most of the burden of CKD was concentrated in the three lowest quintiles of the Socio-demographic index (SDI). [23]
Studies in Europe have reported a range of CKD prevalence, from 3.3% in Norway to 17.3% in Northeast Germany. A study of stages 3-5 CKD using Danish databases found a prevalence of 4.83-4.98% in 2006 to 2013; patients were predominantly women. [24] A study using data from 10 major metropolitan areas in China in 2021 reported a CKD crude prevalence of 10.1%. [25]
Race/ethnic-related demographics
In the US, the percentage of adults with CKD is as follows [20] :
-
Non-Hispanic whites: 13%
-
Non-Hispanic Blacks: 16%
-
Non-Hispanic Asians: 13%
-
Hispanics: 14%
In Mexican Americans, the prevalence of CKD in Mexican Americans had been lower than in other racial/ethnic groups, but nearly doubled between 2003-2004 and 2015-2016, from 1.6% to 3.5%. [26] According to NHANES data, from 2013-2016 to 2017–March 2020, the prevalence of stages 3 and 4 CKD increased in non-Hispanic Blacks, was unchanged in non-Hispanic Whites, and decreased slightly in Hispanics; in contrast, the prevalence of stage 5 CKD decreased in non-Hispanic Blacks but increased slightly in Hispanics. [22]
The incidence rate of ESRD among Blacks in the United States is nearly 4 times that for Whites. [21] Choi et al found that rates of ESRD among Black patients exceeded those among white patients at all levels of baseline estimated glomerular filtration rate (GFR). [27] Risk of ESRD among Black patients was highest at an estimated GFR of 45-59 mL/min/1.73 m2, as was the risk of mortality.
Schold et al found that among Black kidney transplant recipients, rates of graft loss and acute rejection were higher than in White recipients, especially among younger patients. [28] Hicks et al looked at the connection between Black patients with the sickle cell trait and their increased risk for kidney disease; the study found that sickle cell trait was not associated with diabetic or nondiabetic ESRD in a large sample of Black patients. [29]
Important differences also exist in the frequency of specific causes of CKD among different races. In the Chronic Kidney Disease in Children (CKiD) Study, for example, glomerular disease was much more common among nonwhite persons. [30] Overall, FSGS in particular is more common among Hispanic Americans and Black persons, as is the risk of nephropathy with diabetes or with hypertension; in contrast, IgA nephropathy is rare in Black individuals and more common among those with Asian ancestry. [31]
Sex-related demographics
In NHANES, the distribution of estimated GFRs for the stages of CKD was similar in both sexes. The United States Renal Data System (USRDS) 2022 Annual Data Report, however, notes that for all races, the incidence of ESRD in 2020 was 461 cases per million population in males and 280 cases per million population in females. [22]
CKD in children is somewhat more common in boys, because posterior urethral valves, the most common birth defect leading to CKD, occur only in boys. Importantly, many individuals with congenital kidney disease such as dysplasia or hypoplasia do not clinically manifest CKD or ESRD until adulthood.
Prognosis
Patients with chronic kidney disease (CKD) generally experience progressive loss of kidney function and are at risk for end-stage renal disease (ESRD). The rate of progression depends on age, the underlying diagnosis, the implementation and success of secondary preventive measures, and the individual patient. Timely initiation of chronic renal replacement therapy is imperative to prevent the uremic complications of CKD that can lead to significant morbidity and death.
Tangri et al developed and validated a model in adult patients that uses routine laboratory results to predict progression from CKD (stages 3-5) to kidney failure. [32] They reported that lower estimated glomerular filtration rate (GFR), higher albuminuria, younger age, and male sex pointed to a faster progression of kidney failure. Also, a lower serum albumin, calcium, and bicarbonate level and a higher serum phosphate level were found to predict an elevated risk of kidney failure. [32]
Hospitalization
Unadjusted rates of hospitalization in the CKD population, reflecting its total disease burden, are 3-5 times higher than those of patients without CKD. [33] After adjustment for gender, prior hospitalizations, and comorbidity, rates for patients with CKD are 1.4 times higher. Rates of hospitalization for cardiovascular disease and bacterial infection are particularly elevated. [33]
In the United States in 2018, hospital admissions among patients with ESRD declined, to an average of 1.7 per patient per year. However, emergency department visits rose, to an average of 3 per patient per year. [34]
Dialysis
Hemodialysis performed 6 times per week significantly increased the risk of vascular access complications compared with a conventional 3-day regimen in one study. [35, 36] Of 125 patients who received hemodialysis 6 days per week, 48 experienced the composite primary endpoint event of vascular repair, loss, or related hospitalization, compared with only 29 of the 120 patients undergoing conventional treatment. Results indicated that overall risk for a first access event was 76% higher with daily hemodialysis than with the conventional regimen. [35, 36]
Mortality
The mortality rates associated with CKD are striking. After adjustment for age, gender, race, comorbidity, and prior hospitalizations, mortality in patients with CKD in 2009 was 56% greater than that in patients without CKD. [33] For patients with stages 4-5 CKD, the adjusted mortality rate is 76% greater.
Mortality rates are consistently higher for men than for women, and for black persons than for white individuals and patients of other races. For Medicare CKD patients aged 66 years and older, deaths per 1000 patient-years in 2009 were 75 for white patients and 83 for black patients. [33]
The highest mortality rate is within the first 6 months of initiating dialysis. Mortality then tends to improve over the next 6 months, before increasing gradually over the next 4 years. The 5-year survival rate for a patient undergoing long-term dialysis in the United States is approximately 35%, and approximately 25% in patients with diabetes.
A study by Sens found that the risk of mortality was elevated in patients with ESRD and congestive heart failure who received peritoneal dialysis compared with those who received hemodialysis. [37] Median survival time was 20.4 months in patients receiving peritoneal dialysis versus 36.7 months in the hemodialysis group.
At every age, patients with ESRD on dialysis have significantly increased mortality when compared with nondialysis patients and individuals without kidney disease. At age 60 years, a healthy person can expect to live for more than 20 years, whereas the life expectancy of a patient aged 60 years who is starting hemodialysis is closer to 4 years. Among patients aged 65 years or older who have ESRD, mortality rates are 6 times higher than in the general population. [33]
The most common cause of sudden death in patients with ESRD is hyperkalemia, which often follows missed dialysis or dietary indiscretion. The most common cause of death overall in the dialysis population is cardiovascular disease; cardiovascular mortality is 10-20 times higher in dialysis patients than in the general population. [38]
The morbidity and mortality of dialysis patients is much higher in the United States than in most other countries, which is probably a consequence of selection bias. Because of liberal criteria for receiving government-funded dialysis in the United States and the use of rationing (medical and economic) in most other countries, US patients receiving dialysis are on the average older and sicker than those in other countries.
In the National Health and Nutrition Examination Survey (NHANES) III prevalence study, hypoalbuminemia (a marker of protein-energy malnutrition and a powerful predictive marker of mortality in dialysis patients, as well as in the general population) was independently associated with low bicarbonate, as well as with the inflammatory marker C-reactive protein. A study by Raphael et al suggests that higher serum bicarbonate levels are associated with better survival and renal outcomes in African Americans. [39]
A study by Navaneethan et al found a connection between low levels of 25-hydroxyvitamin D (25[OH]D) and all-cause mortality in patients with nondialysis CKD. [40] Adjusted risk of mortality was 33% higher in patients whose 25(OH)D levels were below 15 ng/mL.
Morbidity and mortality among children with CKD and ESRD are much lower than among adults with these conditions, but they are strikingly higher than for healthy children. As with adults, the risk is highest among dialysis patients; consequently, transplantation is the preferred treatment for pediatric patients with ESRD.
Sexual and reproductive issues
Puberty is often delayed in boys and girls with significant CKD. Women with advanced CKD commonly develop menstrual irregularities, and those with ESRD are typically amenorrheic and infertile. However, pregnancy can occur and can be associated with accelerated renal decline, including in women with a kidney transplant. In advanced CKD and ESRD, pregnancy is associated with markedly decreased fetal survival.
Vitamin D
Many patients with CKD have low circulating levels of 25(OH)D. A study of 1099 patients (mostly men) with advanced CKD found that the lowest tertile of 1,25(OH)(2)D (< 15 pg/mL) was associated with death and initiation of long-term dialysis therapy compared with the highest tertile (> 22 pg/mL). [41] A retrospective cohort study in 12,763 non–dialysis-dependent patients with CKD found that 25(OH)D levels below 15 ng/mL were associated independently with all-cause mortality. [42]
Patient Education
Patients with chronic kidney disease (CKD) should be educated about the following:
-
Importance of avoiding factors leading to increased progression (see Etiology)
-
Natural disease progression
-
Prescribed medications (highlighting their potential benefits and adverse effects)
-
Avoidance of nephrotoxins
-
Diet (see Diet)
-
Renal replacement modalities, including peritoneal dialysis, hemodialysis, and transplantation
-
Timely placement of vascular access for hemodialysis
Women of childbearing age who have end-stage renal disease (ESRD) should be counseled that although their fertility is greatly reduced, pregnancy can occur and is associated with higher risk than in women who do not have renal disease. In addition, many medications used to treat CKD are potentially teratogenic; in particular, women taking angiotensin-converting enzyme (ACE) inhibitors and certain immunosuppressive treatments require clear counseling.
For patient education information, see Chronic Kidney Disease.
-
Chronic kidney disease: Calciphylaxis due to secondary hyperparathyroidism.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Guidelines
- Medication
- Medication Summary
- Calcium Salts
- Vitamin D Analogues
- PO4 Scavengers
- Hematopoietic Growth Factors
- Hypoxia-Inducible Factors Inhibitors
- Iron Products
- Calcimimetics
- Sodium-Glucose Transporter-2 (SGLT2) Inhibitors
- Mineralocorticoid Receptor Antagonists
- Sodium/Hydrogen Exchanger 3 (NHE3) Inhibitors
- Show All
- Questions & Answers
- References