Background
Over the past two decades, how trauma surgeons approach a patient with multiple severe injuries has undergone an evolution. Trauma surgeons no longer attempt to fix everything during the initial operation. The literature reflects their cumulative experience, which confirms that conservative operative techniques and short operating times, even when all organ repairs have not been completed, increase survival in civilian and military patients with multiple trauma. These principles hold for all affected regions of the body, including the abdominal cavity and its contents, which constitute the focus of this article. [1, 2, 3]
Damage-control principles are typically applied to patients who have multiple severe injuries. These patients are commonly hypothermic, acidotic, and coagulopathic. Under these circumstances, a deliberate, staged, reoperative approach is optimal.
The three commonly recognized stages of damage-control celiotomy are as follows:
-
Limited operative intervention for control of hemorrhage and contamination and prevention of further injury
-
Continuation of resuscitation in the surgical intensive care unit (ICU) setting
-
Reoperation for definitive repair, a second look for a possible missed injury or delayed manifestation of an organ injury, and formal closure of the incision if possible
Those who may require damage-control celiotomy are patients who are hypotensive (blood pressure [BP] < 90 mm Hg) with the following abdominal or pelvic traumatic injuries:
-
Blunt abdominal injury with intraperitoneal fluid noted on surgeon-performed focused assessment with sonography for trauma (FAST) or gross blood on surgeon-performed diagnostic peritoneal paracentesis (also known as DP tap)
-
Closed pelvic fracture with intraperitoneal fluid noted on surgeon-performed FAST or gross blood on surgeon-performed DP tap
-
Open pelvic fracture
-
Penetrating or blunt abdominal injury with bowel edema, hypothermia, or acidosis
-
Delayed injury with established severe intra-abdominal infection
Although the organ-specific operative techniques are beyond the scope of this article, patients who undergo damage-control celiotomy are at risk for the development of multiple life-threatening complications in the early postoperative period. The chief complication in the postoperative period is abdominal compartment syndrome (ACS).
Abdominal Compartment Syndrome: Etiology and Pathophysiology
ACS is a condition that elevates intra-abdominal pressure (IAP), adversely affects end-organ physiology, and disrupts patient homeostasis. ACS was described as early as the 1800s; however, only in the past couple of decades has it been consistently recognized in the surgical and medical patient population. The reported incidence of ACS is 10-15%, and if it is left untreated, it is uniformly fatal. With diagnosis and treatment, the mortality is 46-66%.
Causes
ACS is most often encountered during the early postoperative course and is commonly discovered in patients who have undergone damage-control celiotomy with primary fascial closure and intra-abdominal packing for coagulopathy. ACS may develop in people with the following conditions:
-
Massive ascites/massive intraperitoneal hemorrhage
-
Hemorrhagic pancreatitis
-
Pregnancy
-
Ruptured abdominal aortic aneurysm
-
Abdominal burns
-
Polytrauma
The aforementioned conditions may lead to decreased blood flow to the abdominal wall and organs. This derangement of cellular perfusion initiates cytokine release, destabilizing cell membranes and ultimately leading to cellular edema and cell death if not reversed. This process is clinically manifested by organ swelling, leading to secondary pressure effects on the respiratory, cardiovascular, and central nervous systems when the IAP rises above a critical level. (See the images below.)
Additional causes of elevated IAP include the following:
-
Free intraperitoneal blood
-
Capillary leak syndrome
-
Visceral edema
-
Ischemia-reperfusion injury
-
Mesenteric venous ligation/thrombosis
-
Positive-pressure ventilation (PPV)/positive end-expiratory pressure (PEEP)
-
Application of military antishock trousers (MAST)
The aforementioned conditions either directly or indirectly increase IAP in patients who are critically ill with ACS.
Pathophysiologic changes
Cerebral changes
Elevated IAP results in elevated intrathoracic pressure, leading to elevated central venous pressure (CVP) and causing an increase in intracerebral pressure. The Monroe-Kellie doctrine states that this increase in intracranial blood volume results in elevation of intracranial pressure (ICP).
During resuscitation and vascular volume expansion, intracerebral pressure and cerebral perfusion pressure (CPP) may increase transiently; however, these pressures will ultimately fall if abdominal pressure continues to increase. Because of a concomitant decrease in caval venous return, this will ultimately cause a fall in cardiac output that will negatively impact intracerebral perfusion pressure.
This fall in ICP may be transient as well if intrathoracic pressure increases as a consequence of increased IAP. This can cause increased intracerebral pressure resulting from increased internal jugular/superior caval venous pressure.
In a porcine model, Bloomfield et al demonstrated significant effects of elevated IAP on the central nervous system (CNS); elevated IAP resulted in increased ICP and decreased CPP. [4] The mechanism is a functional obstruction of jugular venous drainage due to the elevated pleural pressures and CVP. As previously mentioned, the increase in intracranial blood volume results in elevation of the ICP (the Monroe-Kellie doctrine).
Abdominal decompression has resulted in a return toward baseline for ICP and an improvement in the CPP. Head injury and concomitant abdominal injury is a frequently encountered clinical scenario. This observation (confirmed clinically) is important. Decompressive celiotomy in patients such as these has resulted in a dramatic reduction in ICP. Abdominal decompression in these patients has resulted in a return toward baseline for ICP and an improvement in CPP.
Ophthalmologic changes
Increased IAP can cause the rupture of retinal capillaries, resulting in the sudden onset of decreased central vision (Valsalva retinopathy). Valsalva retinopathy has been described in several settings where a sudden increase in IAP or intrathoracic pressure has occurred. The retinal hemorrhage usually resolves within days to months, and no specific treatment is necessary. If a patient with ACS develops visual changes, Valsalva retinopathy should be considered and an appropriate ophthalmic examination performed.
Cardiovascular changes
Increased IAP may cause the following problems:
-
Compression of splanchnic capacitance vessels - When IAP is 10-15 mm Hg, compression of splanchnic capacitance vessels may occur, causing temporary autotransfusion (~250-500 mL)
-
Decreased cardiac output
-
Increased heart rate (HR)
-
Unchanged mean arterial pressure (MAP)
-
The artificial elevation of capillary wedge pressures and CVP
An increase of IAP to greater than 15 mm Hg results in the following:
-
Increased systemic vascular resistance
-
Decreased venous return
-
Decreased stroke volume
-
Decreased cardiac output
-
Decreased BP
-
Compensatory tachycardia
Pulmonary changes
ACS may lead to pulmonary complications, as follows:
-
Diaphragm is forced cephalad
-
Chest-wall expansion is restricted
-
Dynamic and static compliance decrease
-
Peak and plateau pressures increase
-
Physiologic dead space and intrapulmonary shunting increase
-
Combined respiratory-metabolic acidosis is a common finding
Renal changes
ACS can lead to acute renal failure:
-
Oliguria occurs when IAP is greater than 20 mm Hg
-
Anuria occurs when IAP is greater than 30 mm Hg
Increased IAP causes the following:
-
Increased renal parenchymal pressure
-
The direct pressure on renal veins
-
Shunting of blood from the renal cortex to the medulla
-
Decreased renal blood flow/glomerular filtration rate (GFR)
Ureteral obstruction does not occur with increased IAP.
Increased IAP upregulates the juxtaglomerular apparatus, causing the following:
-
Renin, angiotensin (I and II)
-
Aldosterone, vasopressin
Changes involving the abdominal wall and viscera
Increased IAP results in the following:
-
Markedly reduced blood flow to the abdominal wall (IAP >40 mm Hg = blood flow 20% of normal; IAP of 30-40 mm Hg causes blood flow in celiac, hepatic, and mesenteric arteries and in portal and mesenteric veins)
-
Decreased perfusion of every intra-abdominal organ, except the adrenal gland
-
Severely decreased splanchnic flow with concomitant decreased cardiac output
-
Ischemia in the gastric, duodenal, and intestinal mucosa
Monitoring and measuring IAP
The most direct and accurate measurements of IAP are obtained via a cannula placed percutaneously into the peritoneum.
Indirect IAP is monitored through transfemorally placed inferior vena cava lines, nasogastric tubes, rectal tubes, and, most commonly, Foley catheters. The most accurate and simple way to determine the IAP is indirectly by measurement of the bladder pressure via a Foley catheter. The bladder pressure is essentially equivalent to the IAP.
To measure the bladder pressure, the following steps must be completed:
-
Inject 50-100 mL of sterile saline into the Foley catheter via the aspiration port
-
Cross-clamp the sterile tubing of the urinary drainage bag just distal to the culture aspiration port
-
Connect the end of the drainage bag tubing to the indwelling Foley catheter
-
Release the clamp just enough to allow the tubing proximal to the clamp to fill with fluid from the bladder
-
Reapply the clamp
-
Y-connect a pressure transducer to the drainage bag via the culture aspiration port of the tubing using a 16-gauge needle
-
Determine the IAP from the transducer using the top of the symphysis pubis bone as the zero point with the patient in the supine position; a handheld manometer connected to the Foley catheter via the column of fluid in the tubing may be used instead of a transducer (see the image below)
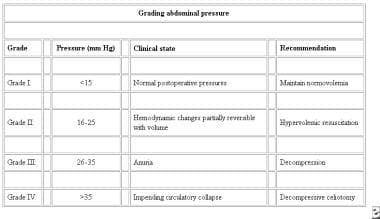 Grading abdominal pressure. Courtesy of Saggi BH, Sugerman HJ, Ivatury RR, et al. Abdominal compartment syndrome. J Trauma. 1998;45:597-609.
Grading abdominal pressure. Courtesy of Saggi BH, Sugerman HJ, Ivatury RR, et al. Abdominal compartment syndrome. J Trauma. 1998;45:597-609.
Release of abdominal compartment syndrome
Morris et al and other investigators have noted that the sudden release of ACS may lead to an ischemia-reperfusion injury, causing acidosis, vasodilatation, cardiac dysfunction, and cardiac arrest. [5] Morris et al have also recommended that before the abdominal cavity is released, the patient should be preloaded with 2 L of 0.45% normal saline, 50 g/L of mannitol, and 100 mEq of sodium bicarbonate crystalloid solution. [5] Vasodilators, such as dobutamine or phosphodiesterase inhibitors, may also be beneficial.
Abdominal Compartment Syndrome: Prevention and Treatment
Leaving the abdominal incision open during surgery prevents ACS. ACS more commonly presents in the early postoperative period (24-72 hr); however, it can present later than this time frame.
Temporary abdominal closure
The techniques of temporary abdominal closure (TAC) are varied, and each has its advantages and disadvantages. All of them face a similar challenge: management of the open abdomen (OA). To date, no prospective randomized studies have compared the effectiveness of these various techniques or validated the concept of the OA protocol. However, retrospective data in the form of case and cohort studies do exist, and they consistently show that maintaining the OA protocol in high-risk groups has been effective in reducing mortality in a clinical setting.
The benefits of TAC include the following:
-
It allows viscera to expand and thus prevents abdominal hypertension
-
It allows the patient to return to the critical care setting for further resuscitation; restoration of the physiologic reserve, tissue perfusion, and normothermia; correction of acid-base balance; and normalization of coagulation
-
It allows the trauma team to assess the patient further and to define other potential life- or limb-threatening injuries
Strategy
With a damage-control celiotomy, the trauma surgeon must decide to convert to a limited procedure within 5 minutes of starting the operative procedure. This decision is based on the initial physiologic state of the patient and a rapid initial assessment of the internal injuries. It is imperative not to wait for evidence of metabolic failure to manifest. This decision is vital for the patient's survival. Damage-control surgery (DCS) intends to accomplish the following:
-
Control of hemorrhage
-
Prevention of contamination
-
Avoidance of further injury
The trauma surgeon should be familiar with different TAC techniques, including their indications, their advantages, and their disadvantages.
The patient is reevaluated in 24-36 hours. The trauma surgeon must maintain a low index of suspicion for delayed or occult injuries, particularly in patients with blunt polytrauma.
Every effort is made to spare the fascia to the extent possible. Repeated attempts at TAC that use the aponeurotic fascia cause recurrent direct and indirect (ischemic) tissue damage. This damage degrades the native tissue, decreasing its tensile and elastic capacity, and increases the potential for delayed incisional hernia.
Definitive closure should be attempted within 7-10 days. After this time frame, loss of the abdominal domain and lateral retraction of the recti and aponeurotic edges tend to be maximal. [6]
Types of Temporary Abdominal Closure
Towel-clipping of skin edges
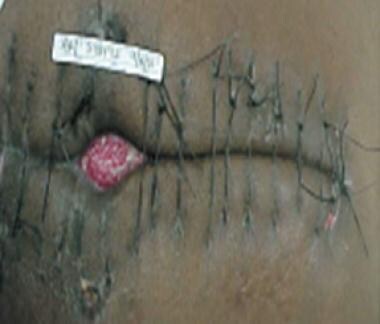
One of the simplest and fastest forms of TAC is the towel-clipping of the skin edges. Towel clips are placed 1 cm apart and 1 cm away from each side of the skin edge. As many as 30 standard perforating towel clips may be required to close an incision. The incision may then be covered with an adherent plastic drape (eg, Vi-Drape, Steri-Drape). Covering the incision decreases the manipulation of the towel clips while the patient is being transferred.
This technique may be used in the rapid temporary closure of thoracic or groin incisions in patients with trauma injuries who are in unstable condition and patients undergoing general surgery. (See the images below.)
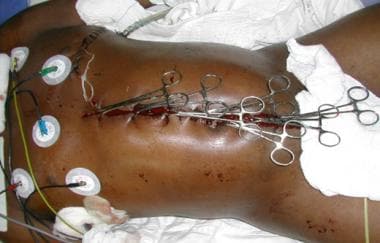 Left lateral thoracotomy with towel-clip closure of damage-control celiotomy. Courtesy of Pedro Gustavo R Teixeira, Trauma Surgeon, Brazil, The Trauma Imagebank.
Left lateral thoracotomy with towel-clip closure of damage-control celiotomy. Courtesy of Pedro Gustavo R Teixeira, Trauma Surgeon, Brazil, The Trauma Imagebank.
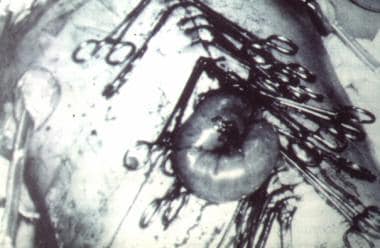 Evisceration of bowel (as illustrated) may occur if towel clips are not placed properly (1 cm from skin edge X 1 cm apart). This temporizing measure may not decompress abdomen adequately. Intra-abdominal pressures as high as 50 mm Hg have been obtained with this type of closure technique.
Evisceration of bowel (as illustrated) may occur if towel clips are not placed properly (1 cm from skin edge X 1 cm apart). This temporizing measure may not decompress abdomen adequately. Intra-abdominal pressures as high as 50 mm Hg have been obtained with this type of closure technique.
Open packing of the abdomen
Open packing of the abdomen is a form of TAC that has been used for more than two decades at the Detroit Receiving Hospital. The abdominal-wall defect and the exposed viscera are covered with rayon cloth. This rayon cloth is then covered with a gauze dressing. Widely spaced retention-type sutures are placed, encompassing all layers of the abdominal wall, and are tied above the gauze packing.
As bowel edema diminishes, the gauze dressing is removed, and the retention sutures are gradually tightened until the incision can be closed. Bender et al reported successful fascial closure in 15 of 17 patients who survived beyond the initial 24 hours. [7]
Zipper closure
First described by Leguit in 1982, [8] zipper closures (see the image below) were popularized by Stone et al in their OA approach to pancreatic abscesses. [9]
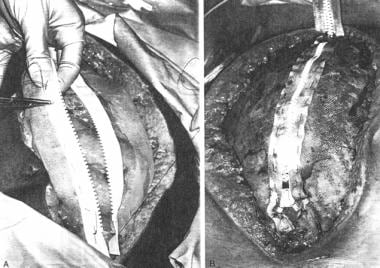 Either conventional zipper or commercial zipper is sewn to skin or fascia with continuous suture of 0 or 2-0 nylon or polypropylene. By use of skin, fascia is spared and incidence of postoperative fascial dehiscence may be diminished.
Either conventional zipper or commercial zipper is sewn to skin or fascia with continuous suture of 0 or 2-0 nylon or polypropylene. By use of skin, fascia is spared and incidence of postoperative fascial dehiscence may be diminished.
Wittmann patch
The approach using the Wittmann Patch (STARSURGICAL, Inc, Burlington, WI) was first reported by Teichman et al, [10] Wittmann et al, [11] and Aprahamian et al. [12] As bowel edema resolves, the excess Velcro-biocompatible patch material is removed and the fascial edges approximated. Tension closure is accomplished by the adherence of the overlapping Velcro-like sheets.
The major advantage of this approach is the ease of access for repeated surgical interventions and the capacity for applying tension to the midline fascia, which helps prevent lateral retraction of the aponeurotic edges, permitting definitive delayed primary closure in most cases (see the images below).
 Artificial burr attaching loop sheet to right fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
Artificial burr attaching loop sheet to right fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
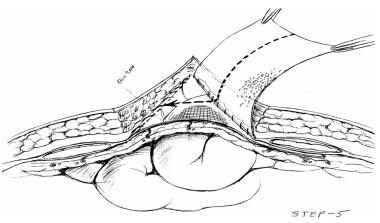 Artificial burr attaching hook sheet to left fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
Artificial burr attaching hook sheet to left fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
 Two sheets of Velcro-like biocompatible material are sewn to midline fascia. Velcro-like material can be adjusted to accommodate increased intra-abdominal pressure (IAP), or, as IAP decreases, it may be trimmed and incision approximated accordingly. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
Two sheets of Velcro-like biocompatible material are sewn to midline fascia. Velcro-like material can be adjusted to accommodate increased intra-abdominal pressure (IAP), or, as IAP decreases, it may be trimmed and incision approximated accordingly. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
Synthetic mesh closure
Polytetrafluoroethylene mesh
The polytetrafluoroethylene (PTFE) 2-mm biocompatible prosthetic abdominal-wall graft is strong and watertight and creates a bed for granulation tissue, which may be covered with a split-thickness skin graft when the prosthesis is removed. PTFE is expensive, and similar outcomes may be achieved with less costly absorbable mesh or Silastic (silo) dressing changes. (See the image below.)
 Gore-Tex 2-mm mesh is sewn to itself and to skin or fascia (as in this case) to achieve temporary closure.
Gore-Tex 2-mm mesh is sewn to itself and to skin or fascia (as in this case) to achieve temporary closure.
Polypropylene-polyethylene mesh
Several authors have reported the use of polypropylene-polyethylene mesh in the setting of a contaminated wound (eg, fasciitis, intra-abdominal sepsis). (See the image below.) Healing has been reported, even in wounds where frank purulent discharge is present.
Although short-term successes have occurred, numerous long-term complications have been reported with this mesh, including the following:
-
Increased incidence of postoperative wound sepsis
-
Increased incidence of enteric fistulas
-
Significantly decreased survivability of split-thickness skin grafts
The experience recorded by Voyles et al, [13] Stone et al, [9] and Jones et al [14] strongly suggests that permanent rigid-type prosthetic mesh should not be inserted in the setting of abdominal-wall defects with associated contamination from the gastrointestinal tract secondary to trauma, intra-abdominal sepsis, or necrotizing infections involving the abdominal wall. (See the image above.)
Absorbable mesh
Synthetic absorbable mesh has been used extensively in TAC. Polyglactin and polyglycolic acid have been in the surgical armamentarium for more than two decades. This type of prosthetic mesh implant has been used for the repair of traumatic liver, splenic, and renal injuries and for pelvic floor repair in the setting of abdominal peroneal resection of the rectum. Although early burst strength (at 8 wk) is comparable to that of permanent mesh, as the mesh is absorbed (at 10-12 wk), hernias inevitably develop in most patients.
As described by Bender et al, the mesh is applied loosely over the abdominal contents and then covered with fine-mesh gauze packing, with the bowel maintained below the absorbable mesh and within the abdominal contents. [7] This may decrease bowel-wall distention, thinning, and subsequent desiccation, thereby potentially reducing the incidence of enterocutaneous fistula.
The choice between polyglactin mesh and polyglycolic acid mesh is primarily determined by the surgeon's preference. However, Brasel et al reported some advantages to the use of polyglycolic acid mesh. [15] This mesh has wider interstices, which Brasel et al suggested may allow more efficient drainage of intra-abdominal fluid and thus may decrease potential delayed complications (eg, abdominal distention, ileus, and abscess). (See the images below.)
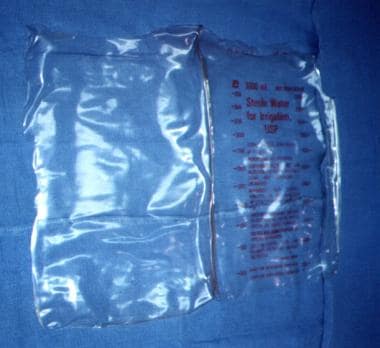
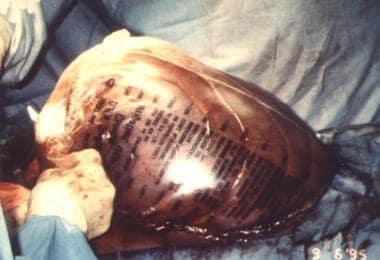
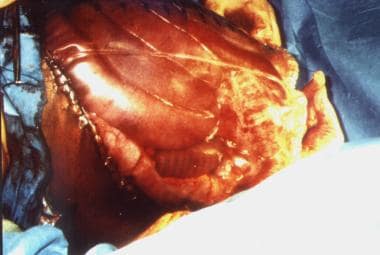
Silastic (plastic bag) closure
A presterilized (gas) soft 3-L plastic cystoscopy fluid irrigation bag is cut and shaped to cover the abdominal incision and extruded viscera. This bag is either stapled to the skin edges of the wound with a standard (wide) skin-stapling device or sutured with monofilament nonabsorbable suture material, thus preserving the fascia. Sterile antibiotic-soaked towels (using Kantrex) may be applied over the silo, which is then covered with an iodine-impregnated adhesive plastic drape.
An alternative is to apply sterile towels over the silo and then secure them with a Montgomery abdominal wound binder, being careful not to create increased abdominal pressure while securing the dressing. The wound is inspected and the dressing is changed every 24 hours (or as needed). Intravenous (IV)/cystoscopy bag silos may be replaced in the ICU setting using standard sterile surgical techniques and equipment.
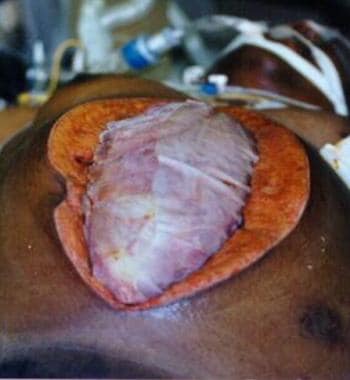
This technique is a variation of the silon (silo) closure used for the repair of gastroschisis and omphalocele. In hospitals in Colombia, South America, IV bag closure (also known as the Bogotá Bag) has been used extensively and successfully. (See the images below.)
 Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome.
Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome.
 Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome. Note the distended small-bowel loops.
Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome. Note the distended small-bowel loops.
The video below explains the benefits of using Silastic closures for TAC.
Silastic closures are fast and effective temporary closure modes and have some significant cost benefits, as reported by Fernandez et al. [16] (See the images below.)
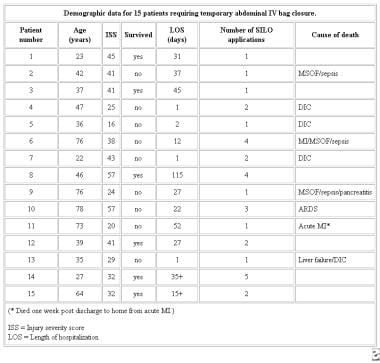 Demographic data for 15 patients requiring temporary abdominal IV bag closure. Courtesy of Fernandez L, Norwood S, Roettger R, et al. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40:258-60.
Demographic data for 15 patients requiring temporary abdominal IV bag closure. Courtesy of Fernandez L, Norwood S, Roettger R, et al. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40:258-60.
Methods of Definitive Abdominal-Wall Reconstruction
Primary delayed fascial closure
Primary delayed fascial closure (at 5-10 d) may be attempted if the abdominal cavity can be closed without significant elevation of IAP. A high index of suspicion for recurrent ACS must be maintained.
Elevated peak airway pressure or plateau pressures (>30 mm Hg), increased urinary catheter bladder pressures (>25 mm Hg), and accompanying deteriorating clinical parameters (eg, abdominal distention or decreased urine output) should prompt a careful reevaluation of the patient and consideration for decompressive celiotomy.
These types of patients are challenging from both a pathophysiologic and a surgical technical standpoint. Optimal care is best achieved through a multidisciplinary approach that is led by the surgeon in close collaboration with the anesthesia and ICU teams and conducted in a staged manner. Two published algorithms, by Fernandez [6] and by Coccolini et al, [17] have outlined the step-by-step surgical decision-making more commonly involved in the care of the OA patient. (See the images below.)
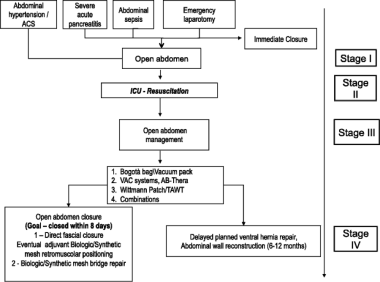 Schematic flowchart for treatment of open abdomen. Courtesy of Coccolini F, Biffl W, Catena F, et al. The open abdomen, indications, management and definitive closure. World J Emerg Surg. 2015. 10:32.
Schematic flowchart for treatment of open abdomen. Courtesy of Coccolini F, Biffl W, Catena F, et al. The open abdomen, indications, management and definitive closure. World J Emerg Surg. 2015. 10:32.
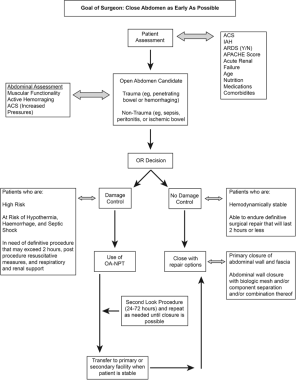 Clinical approach for using OA-NPT. Courtesy of John Wiley and Sons [Fernández LG. Management of the open abdomen: clinical recommendations for the trauma/acute care surgeon and general surgeon. Int Wound J. 2016 Sep 13;Suppl 3:25-34.].
Clinical approach for using OA-NPT. Courtesy of John Wiley and Sons [Fernández LG. Management of the open abdomen: clinical recommendations for the trauma/acute care surgeon and general surgeon. Int Wound J. 2016 Sep 13;Suppl 3:25-34.].
Fabian protocol
Fabian et al published their experience with their eponymous protocol, [18] after which the patients are subsequently brought back for definitive reconstruction (usually within 6-12 mo). The stages are as follows:
-
Stage I - Prosthesis placement (polyglactin or polyglycolic acid; see the first image below)
-
Stage II - Mesh removal or allowance of granulation tissue to cover the mesh
-
Stage III - Split-thickness skin graft applied when granulation tissue is adequate (see the second image below)
-
Stage IV - Definitive abdominal wall reconstruction (at 6-12 mo)
Closure of Abdominal Wall (Creation of Ventral Hernia and Definitive Closure)
Creation of ventral hernia
Sure-Closure skin-stretching system
The Sure-Closure skin-stretching system (MedChem, Woburn, MA) is a patented, disposable, molded device made of stainless steel and plastic parts and used to provide sufficient skin in advance of closures for fasciotomies and trauma repairs of various types, including the closure of the open abdomen. The use of the Sure-Closure system can minimize the need for more extensive secondary wound closure techniques.
The device is attached intraoperatively by first inserting needles parallel to the wound edges. These needles serve to distribute tension forces over the length of the incision. Gauges on the device monitor the applied forces, ensuring a safe and permanent skin stretching. The device allows the surgeon to take advantage of the inherent viscoelastic properties of the skin by mechanically stretching the skin and allowing it to relax under tension; the surgeon then has sufficient skin to affect a suitable closure.
The device comes in sizes of 50 mm and 75 mm. The 50-mm device is designed for smaller skin defects with uneven surfaces, whereas the 75-mm device is designed for larger skin defects with relatively flat, even surfaces.
The Sure-Closure skin-stretching system was first described by Hirshowitz et al and has been used extensively in the plastic, orthopedic, cancer, general surgical, and trauma patient populations. [19]
In a clinical study comparing the Sure-Closure system with more conventional wound closure techniques, Narayanan et al were able to demonstrate a cost-reduction trend in their study cohort. [20] Their cost analysis included the costs of the following: operating room time, operating room supplies, anesthesia, monitoring, recovery room time, wound care supplies, pharmacy charges, and hospital room and board. They also noted above-average healing of the wounds at 1 month and 3 months, with better cosmesis than was seen in comparable conventionally closed wounds. This experience has been confirmed by other reported clinical studies.
Using the Sure-Closure device potentially offers the following:
-
Relatively fast closure (usually within 30-60 min), with healthy skin and subcutaneous tissue
-
Cosmetically superior appearance in comparison with that of split-thickness skin grafts
-
Decreased need for future scar reduction
-
Shorter hospital stay
-
Bedside application
-
Reduced overall cost
By using the Sure-Closure skin-stretching system, the surgeon can close most cases of skin defects that would more commonly require secondary wound closure techniques, such as myocutaneous flaps or skin grafts.
The Sure-Closure device accomplishes skin stretching by using two intradermal needles in conjunction with a tension rod that connects two self-aligning U-arms. (See the images below.) The device contains a graduated tension indicator that registers after 1 kg of force is applied. There is a built-in safety clutch mechanism that prevents excessive tension by limiting the total force to 3 kg.
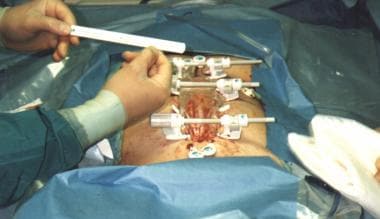 Approximation of abdominal skin edges using Sure-Closure device. Note that abdominal silo resides in partial intraperitoneal position.
Approximation of abdominal skin edges using Sure-Closure device. Note that abdominal silo resides in partial intraperitoneal position.
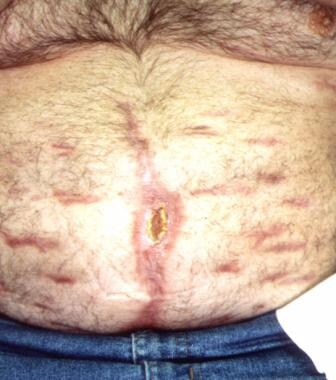 Patient with blunt trauma with nearly healed ventral hernia (subcutaneous flap advancement technique with Sure-Closure device).
Patient with blunt trauma with nearly healed ventral hernia (subcutaneous flap advancement technique with Sure-Closure device).
The Sure-Closure system is used in the following settings:
-
Fasciotomy closures
-
Skin cancer resections
-
Scar revisions
-
Closure of traumatic wounds (including open abdomen)
-
Delayed primary closures
Definitive closure
Vacuum-assisted closure therapy
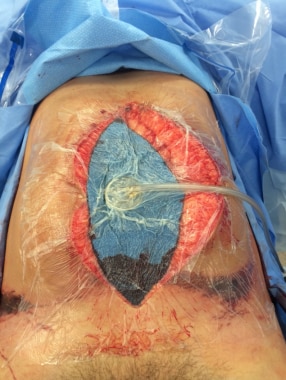
Fascial Vacuum-Assisted Closure® (V.A.C.®) Therapy (3M, St Paul, MN) is a more recently developed concept in the management of the open abdomen that enables fascial closure as long as 1 month after the initial laparotomy. This avoids the need for abdominal-wall reconstruction in the future, as well as the attendant operative risks incurred with such reconstruction.
The main functional component of V.A.C.® Therapy is the use of a nonadherent, polyethylene sheet to cover the exposed viscera and the placement of a polyurethane sponge under controlled negative pressure. The polyethylene sheet helps prevent visceral–abdominal wall adhesions that inhibit movement of the abdominal wall. The polyurethane sponge, when placed under negative pressure (suction), provides the counter traction required to inhibit abdominal-wall retraction and creates an environment where approximation of the abdominal wall may occur.
Miller et al reviewed 646 patients with trauma injuries who underwent laparotomies, of whom 148 required management of an OA over 5 years (1996-2001), and reported excellent results. [21] Of the 148 patients, 85 survived to closure. Patients treated with the OA technique who were unable to undergo fascial closure by the early postoperative period (postoperative day 9) were treated with fascial V.A.C.® Therapy. Patients treated with a planned hernia (HERNIA group, Fabian protocol) were compared with those undergoing fascial closure 9 or more days after the initial laparotomy (LATE group), all of whom underwent fascial V.A.C.® Therapy.
Fifty-nine patients underwent fascial closure, 37 of them before postoperative day 9 and the remaining 22 on or after postoperative day 9. [21] Mean time to fascial closure in the LATE group was 21 days (range, 9-49 d). Injury severity scores, admissions base deficit, number of fistulas, number of operations, and mortality were similar between the HERNIA group and the LATE group. The incidences of abscess, wound dehiscence, and fistula were nearly identical in the two groups. The differences between the groups were not significant with respect to time in the ICU, total hospital stay, incidence of acute respiratory distress syndrome (ARDS), multiple organ failure, and death.
The fascial closure rate (71.08%) reported by Miller et al [21] compared favorably with the results previously published by Barker et al in their large review of fascial closure rates using the standard vacuum pack technique; the fascial closure rate in the earlier study was 70%. [22]
Bruhin et al published evidence-based recommendations for the use of negative-pressure wound therapy (NPWT) in the OA. [23]
Case study
In the following case study, Dennis E Weiland, MD, and John M Stein, MD (Scottsdale Health Care-Osborn, Scottsdale, AZ), illustrate the capabilities of abdominal V.A.C.® Therapy.
A 25-year-old man was admitted with two gunshot wounds to the abdomen. Repair of liver laceration with abdominal washout was accomplished (see the image below).
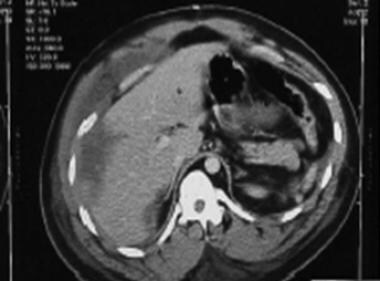 CT scan of 25-year-old man who presented with two gunshot wounds to abdomen. Scan shows liver injury and abdominal distention, 8/30/01.
CT scan of 25-year-old man who presented with two gunshot wounds to abdomen. Scan shows liver injury and abdominal distention, 8/30/01.
Postoperatively, the patient developed severe abdominal distention and respiratory distress. He required a decompression laparotomy for ACS. He was placed on suction drainage for 2 days. V.A.C.® Therapy was initiated on day 3. The wound was closed by delayed primary closure 12 days after the initial decompression laparotomy.
The diagnosis was ACS secondary to a gunshot wound to the abdomen. The prognosis was excellent once the skin was closed over the fascia.
V.A.C.® Therapy was provided as follows:
-
8/25/01 - Laparotomy, cholecystectomy, and repair of liver laceration with drainage
-
8/28/01 - Laparotomy, abdominal decompression with drainage tubes and plastic abdominal dressings, and release of abdominal adhesions
-
8/30/01 - Removal of decompressive dressings and application of V.A.C. ® Therapy (see the images below)
-
9/05/01 - Removal of V.A.C. ® system, partial closure of the wound, and reapplication of V.A.C. ® Therapy
-
9/09/01 - Removal of V.A.C. ® system and delayed primary closure of the abdominal wound
After discharge, the patient continued to have follow-up visits in the wound clinic.
The open skin over the fascia will be closed either by contraction or by secondary closure. The original wound measured 30 cm × 15 cm at the time of the decompression laparotomy. Subsequently, the wound measured 20 cm × 3-4 cm. (See the image below.)
Open-abdomen negative-pressure therapy
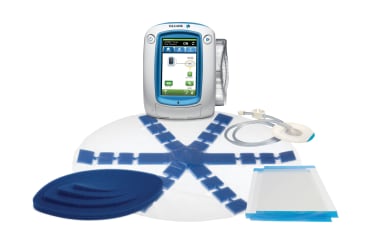
The ABThera™ Open Abdomen Negative Pressure Therapy System (3M, St Paul, MN) is a form of dynamic OA negative-pressure therapy (OA-NPT) management (see the images below). The laboratory and human clinical effectiveness of this method of TAC are well established in the literature. [24]
These devices provide subatmospheric pressure, which militates against bowel edema. They aid in decreasing bacterial counts and inflammatory cytokines commonly found in OA. These self-contained systems protect the abdominal wound from the environment as well as the environment from the wound. They reduce the need for frequent dressing changes is diminished while protecting the surrounding skin, as well as improving overall fluid management. [25, 26]
Kirkpatrick et al compared the ABThera™ with the Barker vacuum-packing technique and noted a survival difference between patients randomized to ABThera™ and those randomized to Barker vacuum packing; this difference was independent of improvement of peritoneal fluid drainage, fascial closure rates, or markers of systemic inflammation. [27] They recommended that further research will be required to determine and further define the observed potential mechanisms of improved outcomes noted in their study.
In a more recent communication by this author, the advantages of using a commercially available, standardized, dynamic TAC such as OA‐NPT were described, and a simplified clinical algorithm was provided for the management of patients with an OA. [6]
In a bench study evaluating pressure distribution, fluid extraction efficiency, and reproducibility of treatment in an in-vitro test model that reproduced the physical conditions of an OA, [28] Delgado and Sammons compared the ABThera™ Active Abdominal Therapy System, the V.A.C.® Abdominal Dressing System, and the Barker vacuum-packing technique. For the purposes of their study, they defined three zones, as follows:
-
Zone 1 (closest to negative-pressure source)
-
Zone 2 (immediately outside of the manifold material edge)
-
Zone 3 (most distal from the negative pressure source)
In all three zones, pressure distribution was significantly better with the V.A.C.® Abdominal Dressing System than with the Barker vacuum-packing technique. [28] In zone 1, there were no pressure distribution differences between the ABThera™ and V.A.C.® systems; however, in zones 2 and 3, ABThera™ Therapy was significantly superior to the V.A.C. Abdominal Dressing System. The authors concluded that ABThera™ Therapy was significantly superior to Barker vacuum packing in all three evaluated zones and significantly superior to the V.A.C.® Abdominal Dressing System in zones 2 and 3.
The data demonstrated superior fluid removal with the use of OA‐NPT; however, the study results suggested that not all OA-NPT methods are equivalent in terms of function and effectiveness.
Technical pearls for application of NPWT
In an excellent review article from 2012, Demetriades provided operating surgeons with some best-practice advice regarding the application of NPWT with the ABThera™ device. [26] This advice included the following:
-
No active intra-abdominal bleeding - Start with negative pressure at −125 mmHg
-
Suspicion of active bleeding due to coagulopathy and not amenable to surgical repair - Consider starting with low pressures, −25 to −50 mm Hg, and closely monitor output
-
Postoperatively, monitor for bleeding in the canister
-
Postoperatively, monitor bladder pressures for intra-abdominal hypertension
In 2009, a guideline statement regarding the use of the ABThera™ Open Abdomen Negative Pressure Therapy System and its application in the OA patient was developed by an international consensus group of expert surgeons. [29] The main outcomes from this meeting included the development of a new classification system, which could be used as a model of care in the management of the OA. [30] This system was further refined in 2016 (see Table 1 below). [31]
Table 1. Amended 2016 Classification of Open Abdomen [31] (Open Table in a new window)
| Grade | Description | Subdivisions |
|---|---|---|
| 1 | No adherence between bowel and abdominal wall or fixity of abdominal wall (lateralization) | 1A - Clean 1B - Contaminated 1C - With enteric leak; enteric leak controlled by closure, exteriorization into the stoma, or permanent enterocutaneous fistula is considered clean |
| 2 | Developing fixation | 2A - Clean 2B - Contaminated 2C - With an enteric leak |
| 3 | Frozen abdomen | 3A - Clean 3B - Contaminated |
| 4 | Established enteroatmospheric fistula, defined as permanent enteric leak into the open abdomen, associated with granulation tissue | — |
Further development of open abdomen negative-pressure therapy
The ABThera Advance™ Open Abdomen Dressing is a next-generation OA-NPT device based on the ABThera™ Open Abdomen Dressing. It features a uniquely configured perforated foam that is designed to collapse medially when negative pressure is applied, actively drawing wound edges together (see the images below).
In a comparative study of four healthy pigs with an open abdominal wound that was treated with the ABThera SensaT.R.A.C.™ or the ABThera Advance™ at –125 mm Hg for 5 minutes, the latter showed a 31% increase in overall tissue approximation and a 39% increase in the skin. More important, a 20% increase in medial fascia movement was noted, with no increase in IAP. [32]
At present, peer-reviewed clinical evidence regarding the ABThera Advance™ is limited to case presentations and case series. Huang et al reported a rare case of large-bowel obstruction (LBO) caused by adhesions in a burn patient with ACS who was treated with the OA technique and the ABThera Advance™.The authors were able to close the abdomen using primary fascial closure within 24 hours of the application of the device. [33]
In a 2021 case series describing their initial clinical experience, Fernández and Matthews evaluated the ABThera Advance™ foam dressing for providing TAC in four patients who required OA management. [34] The two surgeons exercised independent surgical discretion in selecting the patients who would receive the foam dressing. The surgical procedures used to resolve the underlying pathology were completed by using the OA technique. A standard method of application, designed to maximize the biomechanical properties of the next-generation OA-NPT foam dressing, was used in the study cohort (see the image below).
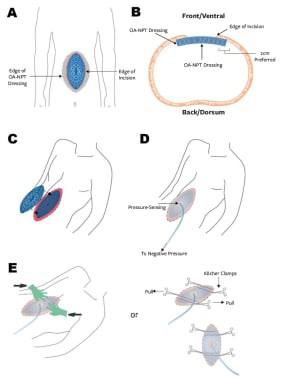 (A) Schematic of Fernández Tuck Technique using open abdomen negative pressure therapy (OA-NPT); (B) cross-sectional view of Fernández Tuck Technique; (C) second layer of blue foam is placed on top of initial layer, so that foam is flush with skin level; (D) application of OA-NPT; and (E) medial force is applied while on negative pressure either with hands or clamps. Courtesy of HMP Global, Wounds, Copyright 2021 [Fernandez L, Matthews. MR. Clinical Observations in Patients With Open Abdomens Managed With Negative Pressure Therapy Using a Perforated Foam Dressing: A Limited Case Series With Brief Literature Review. Wounds. ePub ahead of print: 2021 Jan 15. Online at: https://www.hmpgloballearningnetwork.com/node/5335.].
(A) Schematic of Fernández Tuck Technique using open abdomen negative pressure therapy (OA-NPT); (B) cross-sectional view of Fernández Tuck Technique; (C) second layer of blue foam is placed on top of initial layer, so that foam is flush with skin level; (D) application of OA-NPT; and (E) medial force is applied while on negative pressure either with hands or clamps. Courtesy of HMP Global, Wounds, Copyright 2021 [Fernandez L, Matthews. MR. Clinical Observations in Patients With Open Abdomens Managed With Negative Pressure Therapy Using a Perforated Foam Dressing: A Limited Case Series With Brief Literature Review. Wounds. ePub ahead of print: 2021 Jan 15. Online at: https://www.hmpgloballearningnetwork.com/node/5335.].
All four patients had intermittent urinary bladder pressures recorded as a method for determining IAP and monitoring for clinical evidence of intra-abdominal hypertension (IAH) or ACS. [34] After 12-22 days, all four patients underwent closure with native fascia and were discharged from the hospital. The next-generation OA-NPT dressing application was found to be similar to the standard OA-NPT dressing while providing clinically improved increased medial tension. No gastrointestinal complications related to the next-generation OA-NPT developed, indicating a similar safety profile to that of the standard OA-NPT.
Similar in function to its predecessor, the ABThera Advance™ helps protect the abdominal contents from the external environment. The visceral protective layer provides separation between the abdominal wall and the viscera, mitigating adhesions (see the figure below).
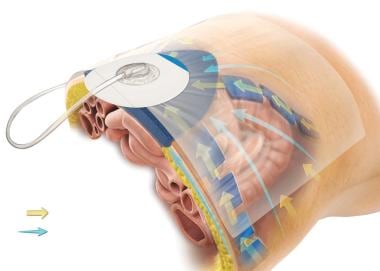 Method of action illustration for 3M™ AbThera™ Open Abdomen Negative Pressure Therapy. Courtesy of 3M. © 2021, 3M. All rights reserved.
Method of action illustration for 3M™ AbThera™ Open Abdomen Negative Pressure Therapy. Courtesy of 3M. © 2021, 3M. All rights reserved.
The technique for applying the ABThera Advance™ Dressing is, for the most part, the same as a technique for applying the ABThera™ Open Abdomen Negative Pressure Therapy Dressing (see the image below). The visceral protective layer is placed between the viscera and the abdominal wall, tucked into the pericolic gutters. The dressing is then cut to size and placed over the visceral protective layer, followed by the application of the drape over the skin around the wound skin and the ABThera Advance™ Dressing. A 2.5-cm hole is cut in the drape, and the SensaT.R.A.C.™ pad is placed directly over the hole.
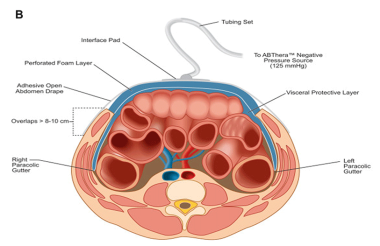 ABThera™ Open Abdomen Negative Pressure Therapy (NPT) System. Courtesy of BioMed Central Ltd, Springer Nature [Roberts DJ, Jenne CN, Ball CG, et al. Efficacy and safety of active negative pressure peritoneal therapy for reducing the systemic inflammatory response after damage control laparotomy (the Intra-peritoneal Vacuum Trial): study protocol for a randomized controlled trial. Trials. 2013;14:141. Online at: https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-14-141.].
ABThera™ Open Abdomen Negative Pressure Therapy (NPT) System. Courtesy of BioMed Central Ltd, Springer Nature [Roberts DJ, Jenne CN, Ball CG, et al. Efficacy and safety of active negative pressure peritoneal therapy for reducing the systemic inflammatory response after damage control laparotomy (the Intra-peritoneal Vacuum Trial): study protocol for a randomized controlled trial. Trials. 2013;14:141. Online at: https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-14-141.].
The available data, though preliminary, appear to suggest that the ABThera Advance™ Open Abdomen Dressing is effective in the removal of intraperitoneal fluid, [24, 35, 36] helps reduce edema, [33, 34] may improve medial tension [33, 34] (thereby minimizing fascial retraction and loss of domain [6, 24, 33, 34, 35, 36, 37] ), and allows rapid access for reentry into the abdominal cavity without sutures being needed for placement. Further comprehensive studies will be required to validate these initial findings.
Open abdomen technique in trauma and nontrauma patients
The OA technique is an important component of DCS in the management of trauma patients. [38] DCS and the OAT are most often indicated for uncontrolled bleeding noted during surgery. [1] The duration of OA for trauma patients has been reported to be between 2 and 6 days, with more than 80% of these patients achieving primary fascial closure (PFC). [38] Although often lifesaving, the OA technique can have serious complications, and TAC devices are used to facilitate a more controlled delayed abdominal wall closure. [6]
In 10% of nontrauma patients requiring emergency laparotomies who are treated with the OA technique, PFC cannot be achieved; however, abdominal-wall closure with a noncrosslinked biologic mesh may be of benefit. [6, 39]
Depending on the underlying pathology, the rate of incisional hernia development after primary fascial closure in the OA technique may be higher than that seen after elective laparotomy (incidence, 5-20%). [40]
Fascial closure techniques in open abdomen patients
Primary fascial closure
Primary closure of the fascia (ie, direct approximation of the fascial edges) has the lowest rate of short-term complications after definitive closure of the OA. The incidence of ventral hernia after primary fascial closure for the OA has been reported to be as high as 30%. [41] A study (N = 71) by Tuveri et al found that with reconstruction of the midline fascia by primary closure, placement of bilateral rectus abdominis muscle sheath relaxing incisions, and application of a biologic mesh in an onlay technique, a ventral hernia rate of 1.4% was observed. [42]
Functional closures
A functional closure occurs when the residual fascial defect is closed with a mesh (inlay technique). In the OA , this is more commonly done with a biologic mesh, which provides a scaffold for host-cell migration, creating a neofascial tissue. [42]
Published evidence on the use of biologic mesh grafts in functional abdominal closure has indicated an overall short-term success rate of greater than 90% over more than 2000 years of cumulative implant time. [43] If the skin cannot be closed over the biologic implant, functional closure of the fascial defect may not be successful. Exposed biologic mesh, if not properly hydrated, may rapidly deteriorate and become more susceptible to infection as a consequence of the lack of granulation tissue formation over it. [43, 44]
The incidence of late ventral hernia after functional biologic mesh closure to manage OA is variable and may depend on the type of biologic mesh used and the technique employed in its implantation. [43, 44, 45, 46] The length of time for which the abdomen remains open appears to affest the rate of successful closure and the incidence of late hernia. Scott et al suggested that the earlier the closure, the better the outcome is likely to be. [46]
Management of the OA is complex. Definitive closure surgical approaches are still debated, and important issues remain to be addressed through more rigorously conducted studies. The OA technique should be carefully applied to carefully and appropriately selected patients. The author recommends closure at the earliest physiologically appropriate time in order to minimize potential complications. [6, 44, 45, 46]
Abdominal Compartment Syndrome and Abdominal Hypertension Not Present, Patient Requires Reoperation
Intraperitoneal silo approach
Among the trauma patient population, the more common indications for reoperations include the following:
-
Bleeding
-
Infection
-
Presence of ACS
-
Reassessment of the abdomen for bowel viability or possible missed or delayed injury
The management of the severely contaminated abdomen, severe peritonitis, and intra-abdominal sepsis by an open approach has been discussed in the literature. First proposed by Steinberg, the intraperitoneal silo approach has been applied in several settings and surgical patient groups. [47]
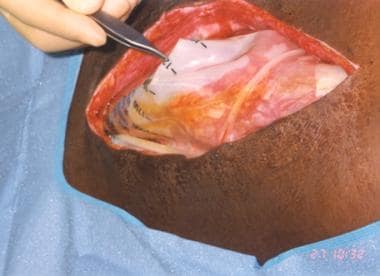
Fernandez et al described a technique that evolved from their experience with the use of the Silastic silo closure for patients with ACS. [48] They used the extraperitoneal silo in the intraperitoneal (IP) position in selected patients who did not have ACS and whose injuries would benefit from a second-look procedure (see the image below).
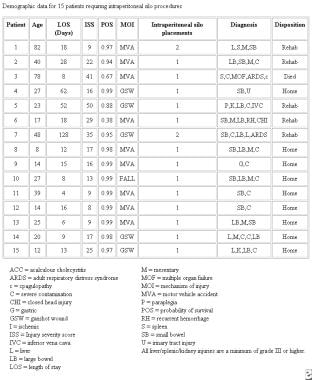 Demographic data for 15 patients requiring intraperitoneal silo procedures. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Demographic data for 15 patients requiring intraperitoneal silo procedures. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
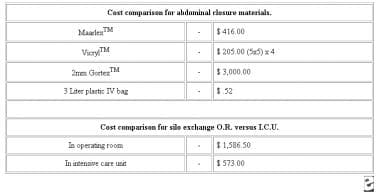
In the study by Fernandez et al, the approximate total hospital cost of the silo was $15.94, with an approximate patient cost of $57 (see the image below). [48] There was one death in the group. In addition, there was one IP silo failure in a patient who developed small-bowel dehiscence; this patient underwent IP silo replacement in the ICU.
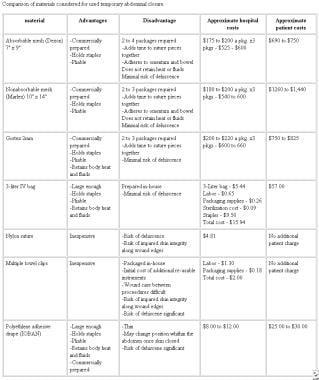 Comparison of materials considered for use in temporary abdominal closure. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Comparison of materials considered for use in temporary abdominal closure. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Placement of intraperitoneal silo
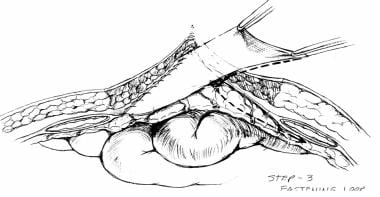
The technique of IP silo placement (see the images below) is simple and straightforward, as follows (see the images below):
-
A presterilized (gas) soft 3-L plastic cystoscopy fluid irrigation bag is commonly used
-
A small epigastric slit incision is made at the cephalad portion of the silo; this incision allows placement of the IP silo around the falciform ligament and round ligament of the liver (if still present after initial damage-control celiotomy) without causing injury
-
The inferior corners and lateral edges of the silo are gently tucked into the right and left lower abdominal quadrants and the lateral pericolic gutters, respectively
-
Finally, the skin is approximated with skin clips, and a sterile dressing is applied
 Presterilized 3-L cystoscopy irrigation bag. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds 1999 Sept;467-78.
Presterilized 3-L cystoscopy irrigation bag. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds 1999 Sept;467-78.
 Epigastric slit. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Epigastric slit. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
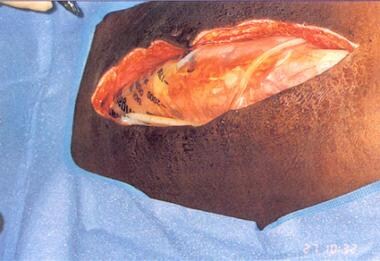 Silo in place within peritoneal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Silo in place within peritoneal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
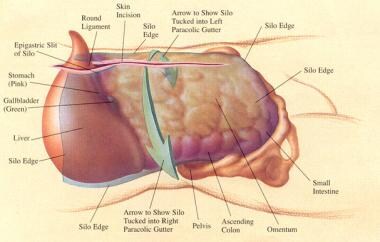 Diagram of silo placement within abdominal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Diagram of silo placement within abdominal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
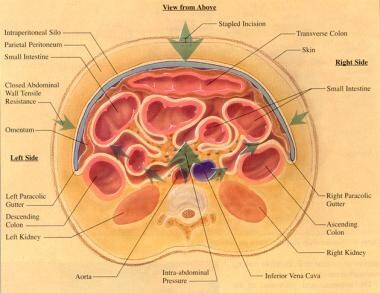 Cross-section of abdomen with intraperitoneal silo in place. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Cross-section of abdomen with intraperitoneal silo in place. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
Conclusion
Several techniques in the surgical armamentarium are available to effect temporary closure of the OA. One of the least expensive and most rapid is the use of the gas-sterilized 3-L plastic cystoscopy irrigation bag. This bag is commonly available, and its application is straightforward. In the author's opinion, it is the preferred initial method of temporary closure, particularly in patients who may require multiple reoperative interventions in an austere setting.
Of the techniques described within this article, the Sure-Closure skin-stretching system has the potential to obviate split-thickness skin grafting in the setting of the OA, particularly if approximation of the skin can be achieved within the first 7-10 days. The Sure-Closure device facilitates the creation of a ventral hernia that may be repaired at a later date in an elective fashion.
The Wittmann patch, V.A.C.® Therapy, and the ABThera™ Open Abdomen Negative Pressure Therapy System are inherently designed to effect not only a temporary closure but also a permanent fascial closure in most patients. Their cost is small in comparison with the substantial cost and morbidity associated with a second, planned abdominal-wall reconstructive procedure, which would commonly be required in this patient population. These devices represent major advances in surgical technique and are welcome additions to the extant surgical doctrine.
After an extensive review of the current literature, it is the author's opinion that the ABThera™ Open Abdomen Negative Pressure Therapy System represents the state of the art in the management of the OA patient, in that it provides a quick, efficient, effective, and safe form of temporary abdominal closure and, in many cases, enhances primary fascial closure as well.
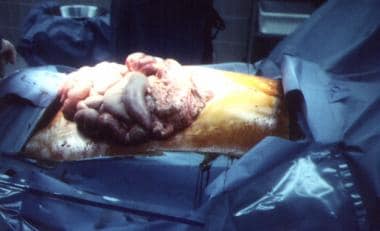
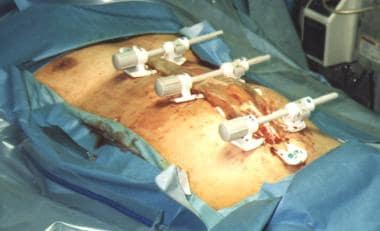
-
Damage-control celiotomy in progress.
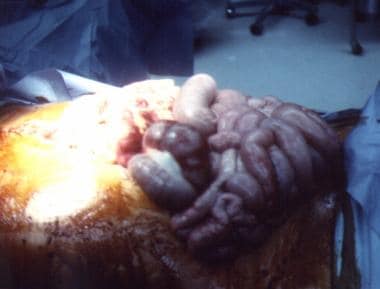
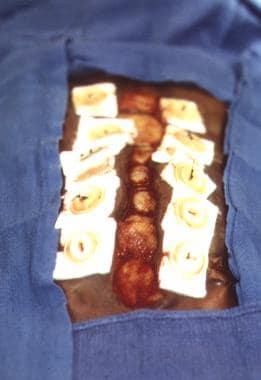
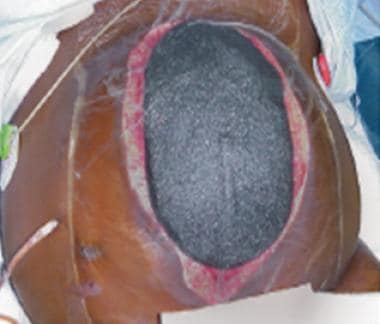
-
Edematous eviscerated bowel in patient with blunt trauma.
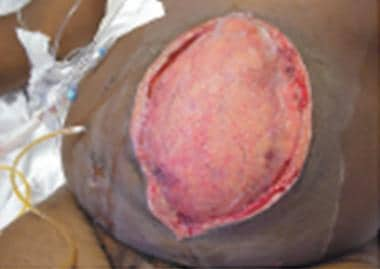
-
Close-up view of edematous bowel.
-
Grading abdominal pressure. Courtesy of Saggi BH, Sugerman HJ, Ivatury RR, et al. Abdominal compartment syndrome. J Trauma. 1998;45:597-609.
-
Left lateral thoracotomy with towel-clip closure of damage-control celiotomy. Courtesy of Pedro Gustavo R Teixeira, Trauma Surgeon, Brazil, The Trauma Imagebank.
-
Evisceration of bowel (as illustrated) may occur if towel clips are not placed properly (1 cm from skin edge X 1 cm apart). This temporizing measure may not decompress abdomen adequately. Intra-abdominal pressures as high as 50 mm Hg have been obtained with this type of closure technique.
-
Either conventional zipper or commercial zipper is sewn to skin or fascia with continuous suture of 0 or 2-0 nylon or polypropylene. By use of skin, fascia is spared and incidence of postoperative fascial dehiscence may be diminished.
-
Artificial burr attaching loop sheet to right fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
-
Artificial burr attaching hook sheet to left fascia. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
-
Two sheets of Velcro-like biocompatible material are sewn to midline fascia. Velcro-like material can be adjusted to accommodate increased intra-abdominal pressure (IAP), or, as IAP decreases, it may be trimmed and incision approximated accordingly. Courtesy of Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990 Mar-Apr. 14(2):218-26.
-
Gore-Tex 2-mm mesh is sewn to itself and to skin or fascia (as in this case) to achieve temporary closure.
-
Marlex mesh is sewn to itself and to fascia.
-
Dexon absorbable mesh is sewn to fascia.
-
Close-up view of Dexon mesh.
-
Presterilized (gas), 3-L cystoscopy irrigation bag.
-
Opened, gas-sterilized 3-L cystoscopy irrigation bag.
-
Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome.
-
Temporary IV bag silo closure in severe abdominal trauma. Video courtesy of Luis G Fernandez, MD.
-
Example of massive edema of bowel and liver in patient who experienced blunt trauma and developed abdominal compartment syndrome. Note the distended small-bowel loops.
-
Demographic data for 15 patients requiring temporary abdominal IV bag closure. Courtesy of Fernandez L, Norwood S, Roettger R, et al. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40:258-60.
-
Cost comparison for abdominal closure materials. Courtesy of Fernandez L, Norwood S, Roettger R, et al. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40:258-60.
-
Comparison of materials considered for use in temporary abdominal closure. Courtesy of Fox V, Miller J, Nix M. Temporary abdominal closure using an IV bag silo for severe trauma. AORN J. 1999 Mar;69(3):530-5, 537, 539-41.
-
Placement of absorbable mesh.
-
Split-thickness skin graft on patient with multiple gunshot wounds and enteric fistulae.
-
Approximation of abdominal skin edges using Sure-Closure device. Note that abdominal silo resides in partial intraperitoneal position.
-
Sure-Closure device in place.
-
Approximation of abdominal skin, creating ventral hernia.
-
Patient with blunt trauma with nearly healed ventral hernia (subcutaneous flap advancement technique with Sure-Closure device).
-
CT scan of 25-year-old man who presented with two gunshot wounds to abdomen. Scan shows liver injury and abdominal distention, 8/30/01.
-
Open abdominal wound before placement of Vacuum Assisted Closure® (V.A.C.®) system, 8/30/01.
-
Vacuum-assisted closure (V.A.C.®) dressing applied, 8/30/01.
-
First wound clinic follow-up, 9/18/01.
-
Demographic data for 15 patients requiring intraperitoneal silo procedures. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Comparison of materials considered for use in temporary abdominal closure. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Presterilized 3-L cystoscopy irrigation bag. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds 1999 Sept;467-78.
-
Epigastric slit. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Silo in place within peritoneal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Surgeon removes intraperitoneal silo.
-
Appearance of intraperitoneal silo after removal from peritoneal cavity.
-
Appearance of bowel after removal of intraperitoneal silo.
-
Diagram of silo placement within abdominal cavity. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Cross-section of abdomen with intraperitoneal silo in place. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Beginning skin closure. Courtesy of Fernandez L, Norwood S, Wilkins H, et al. Intraperitoneal silo: a form of temporary abdominal closure. Surg Rounds. 1999 Sept;467-78.
-
Schematic flowchart for treatment of open abdomen. Courtesy of Coccolini F, Biffl W, Catena F, et al. The open abdomen, indications, management and definitive closure. World J Emerg Surg. 2015. 10:32.
-
Clinical approach for using OA-NPT. Courtesy of John Wiley and Sons [Fernández LG. Management of the open abdomen: clinical recommendations for the trauma/acute care surgeon and general surgeon. Int Wound J. 2016 Sep 13;Suppl 3:25-34.].
-
AbThera™ SENSAT.R.A.C.™ Open Abdomen Dressing. Courtesy of 3M; used with permission.
-
AbThera™ Open Abdomen Dressing. Courtesy of 3M; used with permission.
-
Photo of an AbThera™ Open Abdomen Negative Pressure Therapy device (3M) placed on an OA patient.
-
(A) Schematic of Fernández Tuck Technique using open abdomen negative pressure therapy (OA-NPT); (B) cross-sectional view of Fernández Tuck Technique; (C) second layer of blue foam is placed on top of initial layer, so that foam is flush with skin level; (D) application of OA-NPT; and (E) medial force is applied while on negative pressure either with hands or clamps. Courtesy of HMP Global, Wounds, Copyright 2021 [Fernandez L, Matthews. MR. Clinical Observations in Patients With Open Abdomens Managed With Negative Pressure Therapy Using a Perforated Foam Dressing: A Limited Case Series With Brief Literature Review. Wounds. ePub ahead of print: 2021 Jan 15. Online at: https://www.hmpgloballearningnetwork.com/node/5335.].
-
ABThera™ Open Abdomen Negative Pressure Therapy (NPT) System. Courtesy of BioMed Central Ltd, Springer Nature [Roberts DJ, Jenne CN, Ball CG, et al. Efficacy and safety of active negative pressure peritoneal therapy for reducing the systemic inflammatory response after damage control laparotomy (the Intra-peritoneal Vacuum Trial): study protocol for a randomized controlled trial. Trials. 2013;14:141. Online at: https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-14-141.].
-
3M™ AbThera™ Advance Open Abdomen Dressing. Courtesy of 3M. © 2021, 3M. All rights reserved.
-
Method of action illustration for 3M™ AbThera™ Open Abdomen Negative Pressure Therapy. Courtesy of 3M. © 2021, 3M. All rights reserved.
Tables
What would you like to print?
- Background
- Abdominal Compartment Syndrome: Etiology and Pathophysiology
- Abdominal Compartment Syndrome: Prevention and Treatment
- Types of Temporary Abdominal Closure
- Methods of Definitive Abdominal-Wall Reconstruction
- Closure of Abdominal Wall (Creation of Ventral Hernia and Definitive Closure)
- Abdominal Compartment Syndrome and Abdominal Hypertension Not Present, Patient Requires Reoperation
- Conclusion
- Show All
- Media Gallery
- Tables
- References