Practice Essentials
Retroperitoneal fibrosis (RPF) is a form of chronic periaortitis characterized by the development of extensive fibrosis throughout the retroperitoneum, typically centered over the anterior surface of the fourth and fifth lumbar vertebrae and resulting in entrapment and obstruction of retroperitoneal structures, notably the ureters. [1] See the image below.
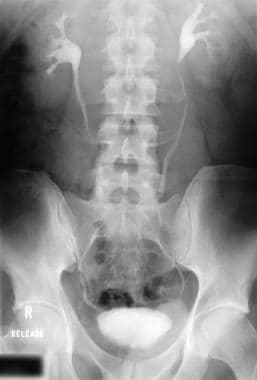 Intravenous urogram in a patient with retroperitoneal fibrosis shows medial deviation of the middle part of both ureters.
Intravenous urogram in a patient with retroperitoneal fibrosis shows medial deviation of the middle part of both ureters.
Approximately 70% of cases are idiopathic and can be immunoglobulin G4 (IgG4)–related. Secondary RPF is most commonly associated with drugs (eg, ergot alkaloids, methysergide, beta-blockers dopamine agonists), malignancies, radiotherapy, and infections (eg. tuberculosis, actinomycosis). [2]
Signs and symptoms
The symptoms and signs associated with RPF are nonspecific, including the following:
-
Fever
-
Lower-extremity edema
-
Phlebitis
-
Deep venous thrombosis
-
Weight loss, nausea, vomiting, anorexia, and malaise: Uncommon presentations
-
Raynaud phenomenon, ureteric colic, hematuria, claudication, and urinary frequency: Rare manifestations
RPF can also be associated with Crohn disease, ulcerative colitis, and sclerosing cholangitis.
See Presentation for more detail.
Diagnosis
The diagnosis of RPF requires a high degree of suspicion, and the evaluation of patients with presumed idiopathic RPF includes ruling out secondary RPF due to malignancy, infection, retroperitoneal injury, or drugs.
Examination findings in patients with RPF include the following:
-
Hypertension: Present in approximately 50% of patients
-
Abdominal mass (occasionally)
-
Obstruction of the ureters, with varying degree of abnormal kidney function: Earliest and most common organ involvement
Uncommon physical findings due to late complications include the following:
-
Ascites
-
Peripheral edema or thrombosis
-
Hydrocele
-
Jaundice
-
Small- or large-bowel obstruction
-
Spinal cord compression
Testing
Laboratory studies and potential results for patients with suspected RPF include the following:
-
Erythrocyte sedimentation rate: Elevated
-
C-reactive protein level: Elevated
-
Urea and creatinine levels: Elevated
-
Complete blood count: Normocytic normochromic anemia
-
Alkaline phosphatase levels (also reported as a marker [5] )
-
Antinuclear antibody levels (present in 60% of cases) [6]
-
Urinalysis: Usually normal; rarely, microscopic hematuria or pyuria is seen
-
Plasma electrophoresis: Polyclonal hypergammaglobulinemia
Imaging tests
The following imaging studies may be used to evaluate RPF:
-
Plain chest and abdominal radiography
-
Intravenous urography
-
Retrograde pyelography
-
Lymphangiography
-
Kidney ultrasonography
-
Abdominal CT scanning: Most frequently used imaging method for diagnosis and follow-up of RPF
-
Abdominal MRI
-
Positron emission tomography
Procedures
Biopsy and histologic/immunohistochemical examination of the affected tissue can aid in the diagnosis and determination of the extent of the disease, which in turn affect treatment management strategies. Open biopsy can ensure a definite histologic diagnosis, but it is associated with significant morbidity. Laparoscopic biopsy is safe, minimally invasive, cost-effective, and useful in making therapeutic decisions for retroperitoneal masses.
See Workup for more detail.
Management
There is currently no consensus on the appropriate management of patients with RPF, because no controlled therapeutic trials have been performed, and conservative therapy has occasionally yielded successful outcomes. [7, 8]
Optimal care in patients with RPF requires an integrated approach of surgical and nonsurgical therapies, and depends on the stage of the disease at diagnosis. The aims of management include the following:
-
Preserve kidney function
-
Prevent other organ involvement
-
Exclude malignancy
-
Relieve symptoms
Surgical treatment
Surgical ureterolysis has been the preferred primary mode of treatment for RPF, because it allows biopsy specimens to be obtained while ureteral obstruction is relieved. Thus, primary management of RPF consists of open biopsy, ureterolysis, and lateral/intraperitoneal transposition or omental wrapping of the involved ureter.
Other surgical interventions that may be involved in RPF include the following:
-
Temporizing maneuvers in the form of percutaneous nephrostomy or ureteral stenting: Recommended in the presence of obstructive uropathy
-
Laparoscopic surgery: Includes complex ablative and reconstructive procedures
-
Endourologic treatment via percutaneous balloon dilatation or endoscopic incision, dilatation, and permanent wall stent [9]
-
Long-term ureteral stenting: Reasonable approach in high-risk and elderly patients to bypass ureteral obstruction
-
Short-term ureteral stenting: Adjunct to open surgical procedures
-
Relatively newer innovative surgical techniques: Ureterolysis and wrapping with Gore-Tex, excision of the ureter and reanastomosis, posterior preperitoneal flap, and kidney autotransplantation [10]
Pharmacotherapy
Medications used in the management of RPF include the following:
-
Glucocorticoids (eg, prednisolone, prednisone)
-
Immunosuppressive agents (eg, mycophenolate, azathioprine)
-
Estrogen receptor antagonists (eg, tamoxifen)
-
Rituximab
Empirical therapy includes corticosteroids, tamoxifen, and azathioprine. The possibility of autoallergic etiology has prompted the use of corticosteroids and cytotoxic drugs in managing RPF. Glucocorticoids and azathioprine are most useful in patients with signs of inflammation.
See Treatment and Medication for more detail.
Background
Retroperitoneal fibrosis (RPF) is characterized by the development of extensive fibrosis throughout the retroperitoneum, typically centered over the anterior surface of the fourth and fifth lumbar vertebrae. This fibrosis leads to entrapment and obstruction of retroperitoneal structures, notably the ureters.
The symptoms and signs associated with retroperitoneal fibrosis are nonspecific, and diagnosis requires a high degree of suspicion. Although a definitive diagnosis can only be made based on biopsy findings, intravenous urography may provide further support for the diagnosis of retroperitoneal fibrosis, particularly if the classic features are present. CT scanning or MRI is essential for evaluating the extent of the disease process.
The aims in management of retroperitoneal fibrosis include preserving kidney function, reducing the morbidity, and suppressing inflammatory processes. Although most cases are idiopathic, precipitating causes should be excluded, particularly malignancy. Surgical ureterolysis has been the preferred primary mode of treatment because it allows biopsy specimens to be obtained while ureteral obstruction is relieved. The knowledge of a possible autoallergic etiology has prompted the use of corticosteroids and cytotoxic drugs.
Pathophysiology
Anatomy of the retroperitoneum
The retroperitoneal space is bordered anteriorly by the posterior parietal peritoneum, posteriorly by the transversalis fascia, and superiorly by the diaphragm. Inferiorly, it extends to the level of pelvic brim. The anterior and posterior layers of renal fascia (Gerota fascia) subdivide the retroperitoneal space on either side of the spine into 3 compartments. The posterior space contains the pararenal fat. The intermediate space contains the kidney, the adrenal gland, and the perirenal fat. The anterior space is more extensive.
The anterior pararenal space is bordered anteriorly by the posterior parietal peritoneum, posteriorly by the anterior layer of renal fascia, and laterally by the lateral conal fascia. The anterior pararenal space contains the extraperitoneal portions of the ascending and descending colon, the duodenum, and the pancreas. The anterior pararenal space is continuous across the midline; however, collections of fluid tend to remain ipsilateral to the site of origin. Medially, the anterior layer of renal fascia blends with the connective tissue around the aorta and the inferior vena cava. The posterior layer fuses with the psoas fascia. Laterally, both layers merge to form lateral conal fascia.
Pathogenesis
Although the exact pathogenesis of retroperitoneal fibrosis has not been definitively described, good evidence supports the suggestion that it develops as an immunologic response to antigens within atherosclerotic plaques. Autopsy and CT studies have shown that fibrosis often begins around a severely atherosclerotic aorta. Thinning or breaching of the media may allow insoluble lipids, such as ceroid, an oxidized low-density lipoprotein, to leak into periaortic tissue, stimulating an immunologic reaction. This theory is supported by the presence of circulating anticeroid antibodies and the finding of ceroid-containing macrophages in nearby lymph nodes.
For this reason, Mitchinson suggested in a 1984 report that the condition should be termed chronic periaortitis. [11] The frequent association of retroperitoneal fibrosis with aortic aneurysm and the regression of fibrosis reported following aneurysm repair further supports this theory. [12] However, the occurrence of retroperitoneal fibrosis in children and those without an aneurysm suggests that other factors must be involved.
In some cases, an immune reaction to an external agent may initiate fibrosis. Drugs such as beta-blockers, methysergide, and methyldopa have been implicated, possibly by acting as haptens, leading to a hypersensitivity or autoimmune reaction. [13] The fibrous reaction associated with carcinoid tumor is believed to be the result of circulating serotonin or its metabolites. Methysergide is a strong serotonin antagonist, and the rebound release of serotonin following prolonged intake may be an alternate mechanism in this case.
The association of retroperitoneal fibrosis with other connective-tissue diseases and reported familial occurrences suggest that genetic factors may also play a role. [14] The human leukocyte antigen (HLA)–B27 cell marker has been demonstrated in several patients with retroperitoneal fibrosis. [15]
Retroperitoneal fibrosis can be a presentation of immunoglobulin G4 (IgG4)–related disease, an insidiously progressive immune-mediated fibro-inflammatory condition typified by formation of tumor-like masses in any of several organs, including the salivary glands, lacrimal glands/orbit, lymph nodes, and pancreas. [16, 17] The etiology is unknown but increasing evidence supports an autoimmune basis, with IgG4 generated in response to autoantigens; the IgG4 antibody itself is not thought to be pathogenic. IgG4-related disease has a 2:1 male preponderance, and the median age of affected patients at diagnosis is in the sixth to seventh decade of life. [17]
A study in a large Chinese cohort found that, compared with idiopathic retroperitoneal fibrosis, the features significantly more likeliy to be found in IgG4-related retroperitoneal fibrosis include the following [18] :
-
Involvement of other organs (eg, lymph nodes, salivary glands) in most patients
-
Elevated C-reactive protein (CRP) level
-
High serum IgG4 concentration
-
High serum IgE concentration
-
Eosinophilia
-
Reduced serum C4 concentration
A 2004 case-control study concluded that occupational exposure to asbestos increases the risk of retroperitoneal fibrosis. Other environmental risk factors identified were use of ergot derivates and tobacco smoking for more than 20 pack-years. [19]
Ureteral obstruction in retroperitoneal fibrosis often appears minimal despite severe kidney failure. This suggests that the obstruction relates to impairment of normal ureteric peristalsis by fibrotic tissue rather than to mechanical obstruction.
Etiology
In most cases, the etiology is unknown. However, the occasional association of RPF with autoimmune diseases and its response to corticosteroids and immunosuppressive therapy suggest it is probably immunologically mediated. [20, 21] Approximately 8% of cases are associated with metastatic malignancy. [22]
Studies have linked smoking tobacco and asbestos exposure to increased risk of RPF. [2]
Epidemiology
Retroperitoneal fibrosis is a relatively uncommon disease. The estimated incidence is 0.1–1.3 cases per 100,000 persons per year. [1, 2]
Retroperitoneal fibrosis has no reported racial predilection. The condition occurs twice as commonly in males as in females. The peak incidence of retroperitoneal fibrosis is in adults aged 40-60 years. [22, 23] Childhood presentation is extremely rare. To date, approximately 33 cases in children younger than 18 years have been reported. [24]
Prognosis
The natural history of retroperitoneal fibrosis (RPF) has not been clearly established. However, the outcome of nonmalignant RPF is generally good. [25, 26, 27, 28, 29, 30, 31, 32] Recovery of kidney function is usually observed within the first 2 weeks. Checking these patients periodically is always better because some patients may regain kidney function much later. Idiopathic RPF carries a good prognosis, with little effect on long-term morbidity or mortality.
Malignant RPF is associated with a poor prognosis. Average survival is only approximately 3-6 months. [33]
Because of the nonspecific nature of the symptoms, the diagnosis of retroperitoneal fibrosis is often delayed. This may lead to progressive loss of kidney function.
Envelopment of the inferior vena cava and lymphatics may result in compression or thrombosis and lead to lower-limb edema. In addition, involvement of gonadal vessels may cause scrotal edema. Occasionally, the duodenum, biliary tract, pancreas, large bowel, and mesentery are involved. [34, 35]
Patient Education
Patients with kidney failure should be educated about the following:
-
The importance of compliance with secondary preventive measures
-
Natural disease progression
-
Prescribed medications (highlighting their potential benefits and adverse effects)
-
Diet
-
Intravenous urogram in a patient with retroperitoneal fibrosis shows medial deviation of the middle part of both ureters.
-
Retrograde ureterogram in a patient with retroperitoneal fibrosis reveals smooth narrowing and medial shift of the ureter.
-
Retroperitoneal fibrosis. Retrograde pyelogram demonstrates hydronephrosis.
-
Retroperitoneal fibrosis. Contrast-enhanced CT scan demonstrates a periaortic soft tissue attenuating mass.
-
Retroperitoneal fibrosis. Noncontrast CT scan shows periaortic fibrotic reaction associated with an inflammatory aortic aneurysm. Note bilateral ureteric stents.
-
Management algorithm of retroperitoneal fibrosis.
-
Postureterolysis intravenous urogram in a patient with retroperitoneal fibrosis demonstrates lateral displacement of both ureters and a double J stent on the right side.
-
Retroperitoneal fibrosis. Retrograde pyelogram shows satisfactory positioning of a wall stent in a patient with postureterolysis obstruction.
-
Retroperitoneal fibrosis. Abdominal radiograph demonstrates a wall stent on the right side.




