Practice Essentials
Severe acute respiratory syndrome (SARS) is a serious, potentially life-threatening viral infection caused by a previously unrecognized virus from the Coronaviridae family, the SARS-associated coronavirus (SARS-CoV). Since the 2002-2003 outbreak of SARS, which initially began in the Guangdong province of southern China but eventually involved more than 8000 persons worldwide (see the image below), global efforts have virtually eradicated SARS as a threat. No further cases have been reported.
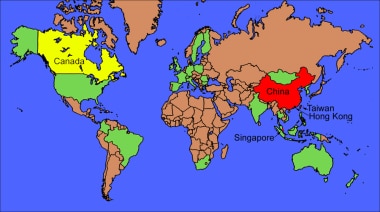 World map of severe acute respiratory syndrome (SARS) distribution from the 2002-2003 outbreak infection. The greatest number of past and new cases of SARS are in mainland China, Hong Kong, Taiwan, and Singapore (red). Canada, more specifically Toronto, Ontario (yellow), is the fifth-ranked area, although community transmission of SARS now appears to be contained, according to the US Centers for Disease Control and Prevention. Green represents the other countries reporting SARS cases.
World map of severe acute respiratory syndrome (SARS) distribution from the 2002-2003 outbreak infection. The greatest number of past and new cases of SARS are in mainland China, Hong Kong, Taiwan, and Singapore (red). Canada, more specifically Toronto, Ontario (yellow), is the fifth-ranked area, although community transmission of SARS now appears to be contained, according to the US Centers for Disease Control and Prevention. Green represents the other countries reporting SARS cases.
Signs and symptoms
The clinical course of SARS generally follows a typical pattern. Stage 1 is a flulike prodrome that begins 2-7 days after incubation, lasts 3-7 days, and is characterized by the following:
-
Fever (>100.4°F [38°C])
-
Fatigue
-
Headaches
-
Chills
-
Myalgias
-
Malaise
-
Anorexia
Less common features include the following [1, 2, 3] :
-
Sputum production
-
Sore throat
-
Coryza
-
Nausea and vomiting
-
Dizziness
-
Diarrhea
Stage 2 is the lower respiratory tract phase and is characterized by the following:
-
Dry cough
-
Dyspnea
-
Progressive hypoxemia in many cases
-
Respiratory failure that requires mechanical ventilation in some cases
See Clinical Presentation for more detail.
Diagnosis
Initial tests in patients suspected of having SARS include the following:
-
Pulse oximetry
-
Blood cultures
-
Sputum Gram stain and culture
-
Viral respiratory pathogen tests, notably influenza A and B viruses and respiratory syncytial virus
-
Legionella and pneumococcal urinary antigen testing should also be considered
Data from the 2002-2003 outbreak indicate that SARS may be associated with the following laboratory findings [1, 2, 3, 4] :
-
Modest lymphopenia, leukopenia, and thrombocytopenia: Series have shown white blood cell (WBC) counts of less than 3.5 x 109/L and lymphopenia of less than approximately 1 x 109/L
-
Mild hyponatremia and hypokalemia
-
Elevated levels of lactate dehydrogenase, alanine aminotransferase, and hepatic transaminase
-
Elevated creatine kinase level
According to guidelines from the Centers for Disease Control and Prevention (CDC), the laboratory diagnosis of SARS-CoV infection is established on the basis of detection of any of the following with a validated test, with confirmation in a reference laboratory [5, 6] :
-
Serum antibodies to SARS-CoV in a single serum specimen
-
A 4-fold or greater increase in SARS-CoV antibody titer between acute- and convalescent-phase serum specimens tested in parallel
-
Negative SARS-CoV antibody test result on acute-phase serum and positive SARS-CoV antibody test result on convalescent-phase serum tested in parallel
-
Isolation in cell culture of SARS-CoV from a clinical specimen, with confirmation using a test validated by the CDC
-
Detection of SARS-CoV RNA via reverse transcriptase polymerase chain reaction (RT-PCR) assay validated by the CDC, with confirmation in a reference laboratory, from (1) two clinical specimens from different sources or (2) two clinical specimens collected from the same source on 2 different days [7]
Chest radiography results in SARS are as follows:
-
Interstitial infiltrates can be observed early in the disease course
-
As the disease progresses, widespread opacification affects large areas, generally starting in the lower lung fields
High-resolution computed tomography (HRCT) scanning is controversial in the evaluation of SARS but may be considered when SARS is a strong clinical possibility despite normal chest radiographs. [9, 10] HRCT findings consistent with SARS include the following:
-
In early-stage SARS, an infiltrate in the retrocardiac region
-
Ground-glass opacification, with or without thickening of the intralobular or interlobular interstitium
-
Frank consolidation
See Workup for more detail.
Management
No definitive medication protocol specific to SARS has been developed, although various treatment regimens have been tried without proven success. [11, 12] The CDC recommends that patients suspected of or confirmed as having SARS receive the same treatment that would be administered if they had any serious, community-acquired pneumonia.
The following measures may be used:
-
Isolate confirmed or suspected patients and provide aggressive treatment in a hospital setting
-
An infectious disease specialist, a pulmonary specialist, and/or a critical care specialist should direct the medical care team
-
Communication with local and state health agencies, the CDC, and World Health Organization is critical
See Treatment and Medication for more detail.
Pathophysiology
The lungs and gastrointestinal tract have been demonstrated to be the only major organ systems that support SARS-CoV replication. [14, 15]
After establishment of infection, SARS-CoV causes tissue damage by (1) direct lytic effects on host cells and (2) indirect consequences resulting from the host immune response. Autopsies demonstrated changes that were confined mostly to pulmonary tissue, where diffuse alveolar damage was the most prominent feature. (See the image below.)
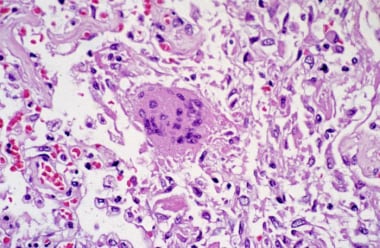 Pathologic slide of pulmonary tissue infected with severe acute respiratory syndrome–associated coronavirus. Diffuse alveolar damage is seen along with a multinucleated giant cell with no conspicuous viral inclusions. Courtesy of the US Centers for Disease Control and Prevention.
Pathologic slide of pulmonary tissue infected with severe acute respiratory syndrome–associated coronavirus. Diffuse alveolar damage is seen along with a multinucleated giant cell with no conspicuous viral inclusions. Courtesy of the US Centers for Disease Control and Prevention.
Multinucleated syncytial giant cells were thought to be characteristic of SARS but were rarely seen. Angiotensin-converting enzyme-2 (ACE-2), being a negative regulator of the local rennin-angiotensin system, was thought to be a major contributor to the development of this damage. [16]
The other mechanism was thought to be the induction of apoptosis. The SARS-CoV–3a and –7a proteins have been demonstrated to be inducers of apoptosis in various cell lines. [17]
Immunologically, SARS is characterized by a phase of cytokine storm, with various chemokines and cytokines being elevated. [15, 18]
Etiology
Sources
Coronaviruses (CoVs) are found in a wide range of animal species, including in cats, dogs, pigs, rabbits, cattle, mice, rats, chickens, pheasants, turkeys, and whales, as well as in humans. [19] They cause numerous veterinary diseases (eg, feline infectious peritonitis, avian infectious bronchitis); they can also cause upper and, more commonly, lower respiratory tract illness in humans (group 1 [human CoV 229E] and group 2 [human CoV OC43]).
The near absence of SARS-CoV antibodies in persons who did not have SARS demonstrated that SARS-CoV had not circulated to any significant extent in humans before 2003 and was introduced into humans from animals. [20] Preliminary data after the outbreak started suggested that animals in the markets of Guangdong province in China may have been the source of human infection. However SARS-CoV ̶ like viruses were not found in animals prior to arrival in the markets.
A wide range of other coronaviruses in bats has been found, [21, 22] suggesting that bats are the most likely animal reservoir for the SARS outbreak. SARS infection in animals before arrival in the markets was uncommon, and these animals were probably not the original reservoir of the outbreak, although they may have acted as amplifying hosts. The proximity in which humans and livestock live in rural southern China may have led to the transmission of the virus to humans. [21] In 2004, the CDC banned the importation of civets when a SARS-like virus was isolated in animals captured in China. [3]
Cellular binding
Single-stranded ribonucleic acid (RNA) viruses such as the SARS-CoV have no inherent proofreading mechanism during replication. Accordingly, mutations in the RNA sequence replication of coronaviruses are relatively common. Such mutations can cause the resulting new virus to be either less or more virulent. [23]
The surface envelop S protein of SARS-CoV is thought to be a major determinant in establishing infection and cell and tissue tropism. [24] This protein, after binding to its receptor—which is thought to be angiotensin-converting enzyme 2 (ACE-2) and is expressed in a variety of tissues, including pulmonary, intestinal, and renal—undergoes conformational change and cathepsin L–mediated proteolysis within the endosome. [25, 26]
The binding of SARS-CoV to DC-SIGN (dendritic cell–specific intercellular adhesion molecule–grabbing nonintegrin), which recognizes a variety of microorganisms, does not lead to entry of the virus into dendritic cells. It instead facilitates the transfer and dissemination within the infected host. [27]
Immune response
The type I interferon (IFN-alfa/beta) system represents a powerful part of the innate immune system and has potent antiviral activity. However, SARS-CoV discourages attack by the IFN system. Replication of the virus occurs in cytoplasmic compartments surrounded by a double membrane layer. Such concealment within cells probably causes a spatial separation of the viral pathogen-associated molecular patterns (PAMPs) and the cellular cytoplasmic pattern recognition receptors (PRRs). [28, 29, 30, 31]
In addition, the activation of IFN regulatory factor–3 (IRF-3) is actively inhibited by SARS-CoV, with IRF-3 being targeted by 5 known SARS-CoV proteins in order to prevent IFN-system activation. IFN induction can also be affected by unspecific degradation of host messenger RNA (mRNA). [31]
These defensive measures prevent tissue cells from mounting an antiviral IFN attack following SARS-CoV infection. Ultimately, however, an IFN immune response can occur. Plasmacytoid dendritic cells (pDCs) use Toll-like receptors (TLRs) to recognize pathogen structures and use IRF-7 to induce IFN transcription. Large amounts of IFN are thus produced by the pDCs following infection with SARS-CoV. [32, 31]
In a study that examined 40 clinically well-defined human SARS cases, high levels of IFN were found in the infection’s early stages, except in more severe cases, and early production of IFN correlated with a beneficial outcome for the infected individuals. [31, 33]
Nuclear factor
SARS-CoV membrane protein, most likely by interacting directly with IkappaB kinase (IKK), also suppresses nuclear factor-kappaB (NF-kappaB) activity and reduces cyclooxygenase-2 (COX-2) expression. These disturbances may aid SARS pathogenesis. [23, 34]
Middle East respiratory syndrome coronavirus (MERS-CoV)
Middle East respiratory syndrome coronavirus (MERS-CoV; formerly referred to as novel coronavirus [NCoV]), a new virus from the same family as the common cold virus and SARS-CoV, emerged in the Middle East in 2012, with some recent recorded cases in Britain and France among travelers to the Middle East. [35, 36] Although only distantly related to SARS-CoV, MERS-CoV is also apparently of zoonotic origin and causes severe respiratory illness, fever, coughing, and breathing difficulties. Interferons have been shown to efficiently reduce MERS-CoV replication in human airway epithelial cell cultures, suggesting a possible mode of treatment in the event of a large-scale outbreak.
According to the WHO, it is possible for MERS-CoV to be passed between humans, but only after prolonged contact. [37] So far, however, there is no evidence that the virus is able to sustain generalized transmission in communities, a scenario that would raise the specter of a pandemic. Although no specific vaccine or medication is currently available for MERS-CoV, patients have been responding to treatment.
Background
Severe acute respiratory syndrome (SARS) is a serious, potentially life-threatening viral infection caused by a previously unrecognized virus from the Coronaviridae family. [38] This virus has been named the SARS-associated coronavirus (SARS-CoV). Previously, Coronaviridae was best known as the second-most-frequent cause of the common cold. (See the images below.)
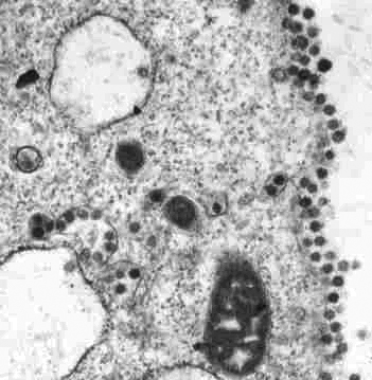 Thin-section electron micrograph of the severe acute respiratory syndrome–associated coronavirus isolated in FRhK-4 cells. Courtesy of the Government Virus Unit, Department of Health, Hong Kong SAR, China.
Thin-section electron micrograph of the severe acute respiratory syndrome–associated coronavirus isolated in FRhK-4 cells. Courtesy of the Government Virus Unit, Department of Health, Hong Kong SAR, China.
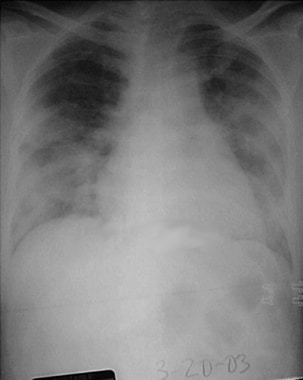 Chest radiograph of a 52-year-old symptomatic woman with severe acute respiratory syndrome (March 20, 2003) taken 5 days after presentation. Moderately severe-to-severe ground-glass and consolidative bilateral changes are noted in the lung fields and are somewhat worse on the left side. Courtesy of Michael E. Katz, MD.
Chest radiograph of a 52-year-old symptomatic woman with severe acute respiratory syndrome (March 20, 2003) taken 5 days after presentation. Moderately severe-to-severe ground-glass and consolidative bilateral changes are noted in the lung fields and are somewhat worse on the left side. Courtesy of Michael E. Katz, MD.
The SARS-CoV strain is believed to have originated in Guangdong province in southern China prior to its spread to Hong Kong, neighboring countries in Asia, and Canada and the United States during the 2002-2003 outbreak. [1, 2, 3, 39, 40] In early 2004, several new cases of SARS were investigated in Beijing and in the Anhui province of China. The most recent outbreak was believed to have been successfully contained without spread into the general population. There have subsequently been three instances of laboratory-acquired infection, and one reintroduction from animals in Guangdong Province, China. (See Epidemiology.) [41, 42]
Despite concerns that new cases of SARS would emerge in the region, no new human-to-human transmission has been reported. The reasons for this maybe (1) a very high prevalence of serious illness, making identification of cases and transmission easier and (2) a low risk of transmission before the development of severe illness.
The World Health Organization’s (WHO’s) timely updates on where SARS cases were occurring, the clinical and epidemiologic features of infection, laboratory methods, strategies to control the disease’s spread, and the intensive collaborative global response to SARS were also responsible for the effective prevention of a global pandemic. (See Epidemiology, Workup, and Treatment.) [43, 44]
Global efforts to acknowledge and research the CoV have virtually eradicated SARS as a threat. Although much has already been learned about the virus, ongoing efforts are being made to better understand it in hopes of developing medications and vaccinations to maintain its suppression. Global organizations, including WHO, the Centers for Disease Control and Prevention (CDC), and the National Institutes of Health (NIH) are still facilitating research on the virus and its family. (See Etiology, Workup and Treatment.)
Epidemiology
In November 2002, an unusual epidemic of severe pneumonia of unknown origin in Guangdong Province in southern China was noted. There was a high rate of transmission to health care workers (HCWs). [1, 2] Some of these patients were positive for SARS-CoV in the nasopharyngeal aspirates(NPA), whereas 87% patients had positive antibodies to SARS-CoV in their convalescent sera. Genetic analysis showed that the SARS-CoV isolates from Guangzhou had the same origin as those in other countries, with a phylogenetic pathway that matched the spread of SARS to other parts of the world.
The 2002-2003 SARS outbreak predominantly affected mainland China, Hong Kong, Singapore, and Taiwan. In Canada, a significant outbreak occurred in the area around Toronto, Ontario. In the United States, 8 individuals contracted laboratory-confirmed SARS. All patients had traveled to areas where active SARS-CoV transmission had been documented. [1, 2, 3, 44]
SARS is thought to be transmitted primarily via close person-to-person contact, through droplet transmission. [45] Most cases have involved persons who lived with or cared for a person with SARS or who had exposure to contaminated secretions from a patient with SARS. Some affected patients may have acquired SARS-CoV infection after their skin, respiratory system, or mucous membranes came into contact with infectious droplets propelled into the air by a coughing or sneezing patient with SARS.
Leaky, backed-up sewage pipes; fans; and a faulty ventilation system were likely responsible for a severe outbreak of SARS in the Amoy Gardens residential complex in Hong Kong. Transmission may have occurred within the complex via airborne, virus-laden aerosols. [46]
The worldwide number of SARS cases from the original outbreak (November 2002 through July 31, 2003) reached more than 8000 persons, including 1706 healthcare workers. Of those cases, 774 resulted in death, with a case fatality ratio of 9.6% deaths, and 7295 recoveries. The majority of these cases occurred in mainland China (5327 cases, 349 deaths), Hong Kong (1755 cases, 299 deaths), with Taiwan (346 cases, 37 deaths), and Singapore (238 cases, 33 deaths).
In North America, there were 251 cases, with 43 resulting in death (all in Canada). [43] The map below shows the worldwide distribution of SARS cases during the 2002-03 outbreak.
 World map of severe acute respiratory syndrome (SARS) distribution from the 2002-2003 outbreak infection. The greatest number of past and new cases of SARS are in mainland China, Hong Kong, Taiwan, and Singapore (red). Canada, more specifically Toronto, Ontario (yellow), is the fifth-ranked area, although community transmission of SARS now appears to be contained, according to the US Centers for Disease Control and Prevention. Green represents the other countries reporting SARS cases.
World map of severe acute respiratory syndrome (SARS) distribution from the 2002-2003 outbreak infection. The greatest number of past and new cases of SARS are in mainland China, Hong Kong, Taiwan, and Singapore (red). Canada, more specifically Toronto, Ontario (yellow), is the fifth-ranked area, although community transmission of SARS now appears to be contained, according to the US Centers for Disease Control and Prevention. Green represents the other countries reporting SARS cases.
Prognosis
WHO data indicate that mortality from SARS is highly variable. The mortality rate has been found to range from less than 1% in patients below age 24 years to more than 50% in patients aged 65 and older. Certain risk factors, including the following, have been associated with a poorer prognosis [47, 48] :
-
Older age
-
Chronic hepatitis B infection
-
Laboratory features - Including marked lymphopenia and leukocytosis, elevated lactate dehydrogenase level, hepatitis, high SARS-CoV viral load, and comorbidities such as diabetes mellitus
Elevated levels of interferon-inducible protein 10 (IP-10), monokine induced by IFN-gamma (MIG), and interleukin 8 (IL-8) during the first week, as well as an increase of MIG during the second week, have also been associated with a poor prognosis. [49]
A study of SARS survivors found that most of these had significant improvement clinically, radiographically, and in their pulmonary function studies. However, 27.8% of patients still exhibited abnormal radiographs at 12 months. Significant reductions in the diffusing capacity of carbon monoxide and in exercise ability (6-min walking distance) were also documented at 12 months. [50] Polyneuropathy and myopathy associated with critical illness, avascular necrosis (possibly steroid induced), steroid toxicity, and psychosis were some of the other long-term sequel observed in the SARS survivors. [13]
Morbidity and mortality
SARS can result in significant illness and medical complications that require hospitalization, intensive care treatment, and mechanical ventilation. [51]
Morbidity and mortality rates were observed to be greater in elderly patients. The overall mortality rate of SARS has been approximately 10%. According to the CDC and WHO, the death rate among individuals older than age 65 years exceeds 50%.
-
Thin-section electron micrograph of the severe acute respiratory syndrome–associated coronavirus isolated in FRhK-4 cells. Courtesy of the Government Virus Unit, Department of Health, Hong Kong SAR, China.
-
World map of severe acute respiratory syndrome (SARS) distribution from the 2002-2003 outbreak infection. The greatest number of past and new cases of SARS are in mainland China, Hong Kong, Taiwan, and Singapore (red). Canada, more specifically Toronto, Ontario (yellow), is the fifth-ranked area, although community transmission of SARS now appears to be contained, according to the US Centers for Disease Control and Prevention. Green represents the other countries reporting SARS cases.
-
Pathologic slide of pulmonary tissue infected with severe acute respiratory syndrome–associated coronavirus. Diffuse alveolar damage is seen along with a multinucleated giant cell with no conspicuous viral inclusions. Courtesy of the US Centers for Disease Control and Prevention.
-
Severe acute respiratory syndrome case definition put forth by the US Centers for Disease Control and Prevention (CDC) on April 29, 2003. Courtesy of the CDC.
-
Clinical and laboratory criteria for severe acute respiratory syndrome cases and infection per the US Centers for Disease Control and Prevention (CDC) on April 29, 2003. Courtesy of the CDC.
-
Chest radiograph of a 52-year-old symptomatic woman with severe acute respiratory syndrome (March 20, 2003) taken 5 days after presentation. Moderately severe-to-severe ground-glass and consolidative bilateral changes are noted in the lung fields and are somewhat worse on the left side. Courtesy of Michael E. Katz, MD.








