Overview
Background
The third stage of labor refers to the period following the completed delivery of the newborn until the completed delivery of the placenta. Relatively little thought or teaching seems to be devoted to the third stage of labor compared with that given to the first and second stages. A leading North American obstetrics text devotes only 4 of more than 1500 pages to the third stage of labor but significantly more to the complications that may arise immediately following delivery. [1] One respected author states: "This indeed is the unforgiving stage of labor, and in it there lurks more unheralded treachery than in both the other stages combined. The normal case can, within a minute, become abnormal and successful delivery can turn swiftly to disaster." [2]
Another interesting aspect of the third stage is the marked discrepancy in what is believed to be its appropriate and optimal conduct. A clear division exists between authorities who advocate the physiological approach and those who advocate the active approach to management. While management strategies are often controversial, for disagreement to persist when fairly compelling evidence supports one of the two approaches is somewhat unusual.
The objectives of this article are to review the issues and practices regarding the third stage of labor and to examine the evidence that supports particular management strategies.
Definition
The third stage of labor commences with the completed delivery of the fetus and ends with the completed delivery of the placenta and its attached membranes. The clinician immediately recognizes that from a practical perspective, the risk of complications continues for some period after delivery of the placenta. For this reason, many authorities have advocated a so-called fourth stage of labor, which begins with the delivery of the placenta and lasts for an arbitrary period afterward. The most commonly chosen duration is 1 hour; however, periods as long as 4 hours have been suggested. The length of the third stage itself is usually 5-15 minutes. The absolute time limit for delivery of the placenta, without evidence of significant bleeding, remains unclear. Periods ranging from 30-60 minutes have been suggested.
Significance
The third and fourth stages of labor are usually uneventful, although significant complications can occur in this period. The most common is postpartum hemorrhage (PPH). While maternal mortality rates have declined dramatically in the developed world, PPH remains a leading cause of maternal mortality.
The pregnancy-related (direct) maternal mortality rate in the United States is approximately 7-10 women per 100,000 live births. National statistics suggest that approximately 8% of these deaths are caused by PPH. [3] In the developing world, several countries have maternal mortality rates in excess of 1000 women per 100,000 live births, and World Health Organization (WHO) statistics suggest that 25% of maternal deaths are due to PPH, accounting for more than 100,000 maternal deaths per year. [4] The death of these mothers has serious implications for the newborn and any other surviving children. See the image below.
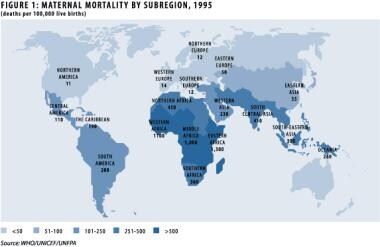 Management of the third stage of labor. Maternal morbidity by subregion, 1995. Image provided courtesy of the World Health Organization.
Management of the third stage of labor. Maternal morbidity by subregion, 1995. Image provided courtesy of the World Health Organization.
Several complications encountered in the third stage of labor may lead to maternal morbidity. PPH may cause anemia or lead to poor iron reserves, ultimately contributing to anemia. Anemia may cause weakness and fatigue. Hospitalization may be prolonged, and the establishment of breastfeeding may be affected. A blood transfusion may ameliorate the anemia and shorten the hospital stay, but it carries risks of transfusion reaction and infection. Access to safe blood is not universal, and PPH can sometimes strain the resources of the best blood bank. Severe PPH, retained placenta, and uterine inversion may require emergency anesthetic services. Any exploration or instrumentation of the uterus increases the risk of sepsis.
Physiology
Over the course of a pregnancy, maternal blood volume increases by approximately 50%, from 4 to 6 L. The plasma volume increases somewhat more than the total RBC volume, leading to a fall in the hemoglobin concentration and hematocrit values. This decrease is smaller in women who take supplemental iron, whereas the fall may be dramatic in women who do not take supplemental iron and who have limited iron stores or are anemic upon becoming pregnant. The increase in blood volume serves to fulfill the perfusion demands of the low-resistance uteroplacental unit and to provide a reserve for the blood loss that occurs at delivery. The increased blood volume also protects against hypotension caused by decreased venous return and decreased vascular tone due to high progesterone levels. [5]
Changes also occur in the coagulation system, with a marked increase in clotting factors and a decrease in fibrinolytic activity. The platelet count may fall slightly during pregnancy because of dilution related to the increased plasma volume and as a result of low-grade consumption; however, individual platelet volume is increased and activity is maintained. Although uterine contraction is initially responsible for controlling blood loss at the placental site, clot formation and fibrin deposition occur rapidly and are essential in maintaining hemostasis and promoting involution in the days following delivery. [6]
Early in pregnancy, the uterus grows dramatically, from an initial weight of roughly 70 g and a cavity volume of 10 mL to a term weight of 1.1 kg and a capacity of approximately 5 L. As with the hematological and coagulation changes, high levels of estrogen promote and allow this change in the uterus. [1] The initial growth of the uterus and the ultimate growth of the placenta and fetus require an equally impressive increase in blood flow to the uterus during pregnancy. At term, the estimated blood flow to the uterus is 500-800 mL/min, which represents 10-15% of cardiac output. Most of this flow traverses the low-resistance placental bed.
Following delivery of the fetus, uterine contractions continue and the placenta is sheared from the underlying endometrium. This separation primarily occurs by a reduction in the surface area of the placental site as the uterus shrinks. This decrease is caused by myometrial retraction, which is a unique characteristic of the uterine muscle to maintain its shortened length following each successive contraction. In this way, the placenta is undermined, detached, and propelled into the lower uterine segment.
The second mechanism of separation is through hematoma formation due to venous occlusion and vascular rupture in the placental bed caused by uterine contractions. As the placenta detaches, the spiral arteries are exposed in the placental bed; massive hemorrhage would occur if not for the structure of uterus. The vessels supplying the placental bed traverse a latticework of crisscrossing muscle bundles that occlude and kink-off the vessels as they contract and retract following expulsion of the placenta. This arrangement of muscle bundles has been referred to as the "living ligatures" or "physiologic sutures" of the uterus. [5]
Several agents cause uterine contraction. The sensitivity of the myometrium to oxytocin, a nonapeptide produced in the posterior pituitary, increases greatly in late pregnancy and even more so during labor. Locally produced and exogenous prostaglandins, especially those of the F series, also cause myometrial contraction. Synthetic ergot alkaloids cause strong tetanic contraction of the uterus.
Agents that cause uterine relaxation can lead to dangerous bleeding following delivery. Beta-sympathomimetics (eg, ritodrine, terbutaline, salbutamol) relax the uterus via beta-2 stimulation. Nonsteroidal anti-inflammatory agents have a dual action, with both antiprostaglandin and antiplatelet activity. The former effect makes them useful for treating dysmenorrhea and afterpains, both due to uterine cramping; however, in the postpartum period and especially following PPH, strong uterine contraction is desired. Calcium antagonists, such as nifedipine and, to a lesser extent, magnesium sulfate, may also inhibit uterine contractions. Nitroglycerin and some inhalational anesthetic agents also decrease uterine contractility.
Clinical Presentation
All women who deliver are at risk of complications in the third stage of labor. These complications include PPH, retained placenta, and uterine inversion. Others include conditions that commonly manifest for the first time during the third stage (eg, placenta accreta and its variants). The numerous risk factors for each of these conditions may be found in articles detailing these individual complications. Most complications of the third stage occur in low-risk women; therefore, caregivers and institutions must have management strategies in place to deal with these problems promptly when they arise.
Signs of placental separation
Traditionally, 4 signs of placental separation are taught. [6, 1]
The most reliable sign is the lengthening of the umbilical cord as the placenta separates and is pushed into the lower uterine segment by progressive uterine retraction. Placing a clamp on the cord near the perineum makes it easier to appreciate this lengthening. Never place traction on the cord without countertraction on the uterus above the symphysis; otherwise, one may mistake cord lengthening due to impending prolapse or inversion for that of uncomplicated placental separation.
The uterus takes on a more globular shape and becomes firmer. This occurs as the placenta descends into the lower segment and the body of the uterus continues to retract. This change may be clinically difficult to appreciate.
The uterus rises in the abdomen. The descent of the placenta into the lower segment, and finally into the vagina, displaces the uterus upward.
A gush of blood occurs. The retroplacental clot is able to escape as the placenta descends to the lower uterine segment. The retroplacental clot usually forms centrally and escapes following complete separation; however, if the blood can find a path to escape, it may do so before complete separation and thus is not a reliable indicator of complete separation. This occurrence is sometimes associated with increased bleeding and a prolonged third stage, with the delivery of the leading edge of the placenta and maternal surface first (Matthews Duncan method), rather than the cord insertion and fetal surface, which is more common (Schultze method).
Management
Preparation
Commence preparations for the third stage of labor well before delivery of the baby. In the antepartum period, discuss with the patient and her partner their preferences for the delivery process with an open dialogue regarding any risk factors present and what implications they might have for the woman. Thoroughly discuss any concerns or variations from accepted practice. It is important that the patient understand the implications and potential risks involved if management options are limited.
Physiological versus active management
The controversy surrounding third-stage management exists between authorities who advocate the physiological, or expectant, approach and those who advocate the active approach. The basic components of the two management strategies are outlined in Table 1.
Table 1. Physiological Versus Active Management (Open Table in a new window)
. . . |
Physiological Management |
Active Management |
Uterotonic |
None or after placenta delivered |
With delivery of anterior shoulder or baby |
Uterus |
Assessment of size and tone |
Assessment of size and tone |
Cord traction |
None |
Application of controlled cord traction* when uterus contracted |
Cord clamping |
Variable |
Early |
*Gentle downward cord traction with countertraction on the uterine body |
||
Proponents of physiological management argue that the natural processes outlined above promote normal separation and delivery of the placenta and lead to fewer complications. If PPH develops, it may be effectively managed with available techniques and drugs. Proponents express concern that active management increases PPH and uterine inversion rates due to cord traction and increases the risk of retained placenta due to entrapment caused by uterotonic agents. Delivery of the placenta occurs by uterine contractions and maternal expulsive efforts, and cord traction is prohibited. Concern also exists regarding the case of an undiagnosed second twin if uterotonics are routinely used at the time of delivery.
Advocates of active management argue that administering prophylactic uterotonic agents promotes strong uterine contractions and leads to faster retraction and placental delivery. This decreases the amount of maternal blood loss and the rate of PPH. [7] They also argue that the more effective uterine activity leads to a reduction in the incidence of retained placenta.
Gentle cord traction is only applied when the uterus is well contracted, and the uterus is manually controlled above the level of the symphysis with countertraction (Brandt-Andrews maneuver). This maneuver is referred to as controlled cord traction (CCT). [7] Cord traction must never be applied in the absence of countertraction and is applied in the axis of the birth canal. Advocates point out that an undiagnosed second twin is an increasingly rare problem and that clinical assessment in labor and following delivery of the first baby can establish the diagnosis before uterotonic administration. [8]
Several large, randomized, controlled trials have addressed the question of whether physiological management or active management is preferable. These trials have consistently shown that active management leads to several benefits compared to physiological management. These trials use 1 of 3 uterotonic agents: ergonovine, oxytocin, or Syntometrine, (a combination of ergometrine and oxytocin).
Seven trials have been the subject of a meta-analysis in the Cochrane Library, [9] in which active management showed a reduction in the average risk of maternal primary hemorrhage (more than 1000 mL) at birth and of maternal hemoglobin less than 9 g/dL following birth. No difference was found in the incidence of admission to neonatal units or of infant jaundice requiring treatment.
The authors also reported a significant decrease in primary blood loss greater than 500 mL with active management, as well as a decrease in mean maternal blood loss at birth, maternal blood transfusion, and therapeutic uterotonics. Significant increases in maternal diastolic blood pressure, vomiting after birth, after pains, and analgesia use from birth to discharge were reported. Decrease in the infant's birthweight was also found with active management. [9]
Choice of uterotonic agent
Randomized trials have examined the use of oxytocin alone, ergot alone, misoprostol alone, and Syntometrine in active management protocols compared with physiological management. Trials have also compared the various uterotonics to each other in active management protocols and have been the subject of meta-analyses in the Cochrane Library.
Trial findings suggest that while Syntometrine may have a slight advantage in reducing PPH by 500 mL or more (RR, 0.74; 95% CI, 0.65-0.85) and possibly by 1000 mL or more (RR, 0.79; 95% CI, 0.59-1.06), oxytocin alone is very effective and does not have the adverse effect profile associated with preparations containing ergot. [10] Trial results suggest that increasing the intramuscular dose of oxytocin from 5 IU to 10 IU increases the effectiveness of oxytocin. An additional randomized controlled trial found that infusing 80 U or 40 U, as opposed to the usual 10 U, did not decrease postpartum hemorrhage overall; however, it did decrease the need for additional oxytocin infusion and of the risk of a decline in hematocrit of 6% or more. [11] It has also been suggested that initial or continued intravenous administration of oxytocin increases effectiveness. Do not administer more than 5 IU of oxytocin as a bolus intravenous injection.
Trials using oxytocin alone showed reduced rates of manual removal of the placenta, whereas those using ergot preparations demonstrated increased rates. The slight trend of increased manual removal mentioned in the Cochrane meta-analysis above was entirely due to the results of the single trial that used intravenous ergot. [12] The increases in nausea, vomiting, and blood pressure are all exclusively observed in the trials using ergot preparations. [13]
The likely explanation for these differences is that ergot preparations act systemically on smooth muscle, whereas oxytocin is specific for uterine smooth muscle. Oxytocin causes increased contraction strength and frequency, but the uterus does not undergo tetanic contraction, as is the case with an ergot. Studies undertaken by the WHO also favor oxytocin because it is more stable when exposed to heat and light compared to ergot preparations. This makes oxytocin more useful in settings where storage capabilities, especially refrigeration, may be an issue. A potential benefit of ergot preparations is a longer duration of action. Trials have been performed using a synthetic oxytocin analogue, carbetocin, which has a prolonged action. [14, 15] A Cochrane review concluded that carbetocin should not be used as a first-line agent in place of other proven uterotonic agents. [16] Carbetocin is not available in the United States.
Misoprostol has shown early promise in the treatment of PPH. Additionally, its low cost, pill form, and heat stability make it a potentially excellent agent for prophylaxis in the third stage of labor. Unfortunately, randomized trials have shown it to be inferior to injectable uterotonics and to not be significantly more effective than placebo. [17] Adverse effects, such as shivering and fever, are common; in regimens using higher doses, nausea, vomiting, and diarrhea occur more frequently. [18] Clearly, the presence of prostaglandin-induced pyrexia and shivering in the postpartum period may lead to confusion in the diagnosis of sepsis.
Misoprosotol may still be a useful uterotonic in some settings. A recent trial in a low resource setting showed it to be as effective as intramuscular oxytocin following vaginal delivery. [19] Many have also pointed out that in low resource settings, even if misoprostol is somewhat less effective than injectable uterotonics, that it’s low cost and ease of storage and use should mandate it’s widespread availability. Another trial has shown that buccal misoprostol given at the time of cesarean section reduces the need for the use of additional uterotonic agents. [20] Other prostaglandins have not been sufficiently investigated to warrant recommending them over oxytocin, and they all elicit more adverse effects.
Early suckling or nipple stimulation is thought to increase uterine contractility; however, a trial involving 4227 women did not suggest that early suckling reduced the rate of PPH of 500 mL or more (odds ratio [OR], 0.93; 95% CI, 0.75-1.17) or other adverse outcomes. [21] Early suckling should still be encouraged because it promotes bonding and breastfeeding and may help maintain uterine tone.
Mode of uterotonic administration
Active management protocols involve administering a uterotonic agent with the delivery of the anterior shoulder or with the completed delivery of the newborn. In practical terms, these 2 events are separated by only seconds, and the distinction is unlikely to be important. Importantly, the anterior shoulder must be delivered prior to uterotonic administration. In the event of shoulder dystocia, strong uterine contractions serve to further impact the anterior shoulder, make curative maneuvers more difficult, and decrease already compromised fetal oxygenation. Immediately following delivery, the fundal position and size of the uterus is determined. This serves to exclude the presence of a second baby and to establish the baseline size of the uterus.
Avoid uterine massage, or "fundus fiddling," as it has been called, before placental delivery. Draw up the uterotonic before the delivery in order to facilitate rapid administration. Typically, at vaginal delivery, a dose of 10 IU of oxytocin is administered intramuscularly. In patients with intravenous access in place, 10-20 IU is placed in 500-1000 mL of crystalloid and run quickly. With cesarean deliveries, 5 IU is often administered as an intravenous bolus, followed by a similar infusion. Some authorities advocate the same practice for vaginal deliveries.
Higher-dose infusions may somewhat decrease the risk of subsequent atony, but they result in more fluid retention. [22] Keep in mind the adverse effects of oxytocin, although they are rarely problematic in this setting. Administer ergot-containing preparations intramuscularly. The usual dose of ergonovine is 0.2-0.25 mg, although some trials have used 0.5 mg. (Syntometrine contains 0.5 mg of ergonovine with 5 IU of oxytocin.) Avoid ergot preparations in patients with hypertension, history of migraine, and, possibly, Raynaud phenomenon.
Routine administration of a uterotonic following delivery of the placenta using physiological management has not been shown to provide the same benefits observed in active management. [8] A trend in the reduction of PPH is present, but the effect size is less than that observed with active management. The need for therapeutic uterotonics may be reduced compared with no uterotonic following placental delivery. Findings of a much more recent trial suggest that if CCT is used, the timing of oxytocin administration (with presentation of the anterior shoulder or immediately following placental delivery) does not alter the rate of PPH, manual removal, or other outcomes. [23] There was a trend to less PPH and the need for manual removal was not increased in the early oxytocin administration group. Both of these findings support the practice of administering oxytocin with delivery of the anterior shoulder as in true active management.
Cord management
Cord traction is applied during active management only when countertraction is applied. Countertraction is performed by trapping the body of the uterus above the symphysis pubis and directing it cephalad and back. Traction is applied in a continuous, downward manner only when the uterus is well contracted. A delay occurs between the administration of the uterotonic and good contraction of the uterus. Several issues must be considered during this interval.
Most active management protocols include early clamping of the cord. However, of the 3 active management components, this practice seems the least important in conferring the observed benefits. Early cord clamping may be indicated in order to facilitate newborn assessment or resuscitation. Barring these indications, rushing to clamp the cord is unnecessary because traction cannot be applied until the uterus is well contracted. Delaying cord clamping until the cord is pulseless, usually 2-4 minutes, results in higher hemoglobin and hematocrit values in the newborn and, possibly, lower levels of early childhood anemia and greater iron stores. [24, 25] These effects are probably more profound in preterm infants and may result in fewer transfusions in the neonatal period [26] and lower rates of neonatal intraventricular hemorrhage and sepsis. [27]
Balance these potential benefits against the potential for an increase in newborn polycythemia and jaundice; however, these risks may be overstated. Recent reviews have yielded conflicting results with regard to adverse effects but not potential benefits. One meta-analysis suggested that delayed cord clamping did not result in any increase in respiratory distress or statistically significant increases in bilirubin levels or use of phototherapy in newborns. [28] However, a second meta-analysis did show an increased risk of jaundice requiring phototherapy (RR 0.59, 95% CI, 0.38-0.92; 5 trials of 1,762 infants). [29]
Regardless, holding the newborn below the level of the placenta or "milking" the cord toward the baby to exaggerate this transfer is discouraged. [30] Parents may have a preference regarding the timing of cord clamping and the position of the baby immediately following delivery. Barring any contraindication, follow such preferences. The issues around the timing of cord clamping have not been extensively studied, and the practice of early cord clamping is not based in strong evidence. This observation is especially true with respect to newborn implications.
Given the current evidence, the recommendation to give oxytocin with delivery of the anterior shoulder and then to wait 1-2 minutes before clamping and dividing the cord if the baby appears well, does not seem unreasonable. [31]
In the instance of a nuchal cord, attempt to avoid clamping and cutting the cord before delivering the baby. This may be accomplished by passing the loop(s) of cord from back to front over the baby's head or by delivering the baby through the loop of cord. While these maneuvers are preferable and usually successful, clamps must be ready in case the maneuver fails or the cord is inadvertently torn. Clamping and dividing a nuchal cord is most problematic when it is followed by a shoulder dystocia. The divided cord prevents what little placental support that would have been present from reaching the baby. Additionally, no intrauterine resuscitation can occur if the clinician resorts to a Zavanelli (cephalic replacement) maneuver.
At the time of cord clamping, the cord should be singley clamped; a second clamp should then be placed after the blood has been milked from the segment of cord between the 2 clamps. The cord is then divided between the clamps in a relatively bloodless manner. Place the clamps a reasonable distance from the newborn so that the newborn caregivers can place the cord tie or disposable cord clamp at the appropriate place. Attempting to immediately place the cord clamp or tie on the newborn is generally not time well spent. A hastily placed clamp may need to be replaced if the cord stump is too long or, worse, may interfere with access to the cord vessels or lead to later problems with the site if placed too close to the abdominal wall. This practice also minimizes the risk of damaging vital structures in the rare case of abnormalities at the cord insertion site.
Cord blood may be taken following cord clamping and division if no signs of placental separation are observed. Practices vary, but commonly taken specimens include those for a CBC count, group and screen, and, possibly, blood gas analysis. Some physicians choose to draw the samples from an isolated piece of cord or from the delivered placenta and cord following the third stage. The emergence of fetal stem cell harvesting has created new issues in this area. This practice should not compromise the care of the mother or the newborn, and the caregiver must not become distracted by the procedure at this critical time. Ideally, an additional designated member of the team can perform this procedure. Cord blood can be efficiently collected following delivery of the placenta by having an assistant hold the placenta above the level of the cord.
Cord blood harvesting (CBH) should not delay uterotonic administration. In fact, uterotonics may increase the amount of blood harvested due to placental compression. CBH has not been shown to increase the risk of PPH; however, there was a trend to increased PPH in a recent meta-analysis. (RR 1.22, 95% CI, 0.96-1.55, 5 trials including 2,236 women). [29] Further research on the optimal and safest techniques for CBH at both vaginal and cesarean deliveries should be undertaken.
The potential benefit of completely draining the cord of blood is unclear. This measure would be performed after taking cord blood samples or setting aside a clamped cord segment for sampling. Some investigators believe that this practice promotes placental separation. Limited evidence supports this belief. [32, 33, 34, 35] Before the advent of Rh D immune globulin prophylaxis, interest existed regarding whether fetomaternal bleeding was reduced by the maneuver, which might therefore reduce the risk of maternal sensitization. Findings from small, nonrandomized studies from the early 1970s suggested a reduction, but further work has not been performed. [36] Draining the cord reduces the potential for caregivers being splashed with blood in the rare case of cord avulsion; however, the routine exposure to this blood during and after drainage may also carry a slight risk.
Trials that have examined administering uterotonics at the time of delivery but then taking an expectant approach to placental delivery suggest that some reduction in PPH rates may occur, but that the effect is less than with an active management protocol. Additionally, there is a trend toward an increase in retained placenta and no reduction in the number of patients receiving blood transfusions.
A single trial examined the effect of CCT with and without the administration of oxytocin upon delivery of the baby. The results suggest that CCT alone does not reduce the incidence of PPH or severe PPH. Another trial, discussed earlier, found that CCT used in conjunction with oxytocin immediately following placental delivery resulted in outcomes similar to those with true active management. [23] A third trial showed that true active management resulted in lower PPH rates when compared with CCT followed by oxytocin at the time of placental delivery. [37]
Some authors advocate the use of uterine massage and CCT if a uterotonic agent is not available for prophylactic use. No good evidence supports this recommendation and many are strongly against any form of fundus fiddling. The risks of cord traction when the uterus is not well contracted are substantial.
Uterine assessment
The fundus is assessed immediately following delivery of the baby, thus excluding an undiagnosed twin and giving a baseline fundal height. A uterotonic, preferably oxytocin, is then administered. If twin pregnancy has been previously excluded, as is usually the case in the developed world, a uterotonic may be administered prior to fundal assessment. The fundus is periodically assessed to determine when uterine contraction occurs, at which time CCT is applied.
Oxytocin-induced contractions are periodic; when the uterus relaxes, stop CCT until the next contraction. The direction of cord traction mirrors that for an instrumental delivery from the mid cavity because the placenta must follow the same path through the birth canal. Traction should initially be downward, then parallel to the floor, and finally upward as the placenta delivers. Do not perform uterine massage before delivery of the placenta, and never apply downward fundal pressure.
Periodic assessment of the uterus also serves to detect the signs of placenta separation and to assess whether an atonic uterus is becoming distended with blood. If uterine signs of placental separation are present, the cord has lengthened, but no gush of blood has occurred and the placenta remains undelivered, the placenta may be detached but remain at the level of the internal os. Blood trapped behind the placenta in this position can distend the uterus, preventing further retraction and increasing the likelihood of PPH. Gently running a finger up the cord to feel if the insertion site and the placenta are at the cervix may be helpful. If the placenta is at this level, it may be delivered with the aid of maternal expulsive effort or slightly more aggressive CCT.
Placental delivery
The placenta usually presents with the cord insertion and the fetal side of the placenta. Ensure that only the placenta is delivering because a uterine inversion has a similar (although more massive) appearance. Fortunately, most clinicians never experience this rare complication. If inversion is encountered, leave the placenta attached and promptly replace the uterus using the "last out, first in" principle as discussed in Uterine inversion.
The membranes trail the placenta, and measures to prevent them from tearing include slowly rotating the placenta about the insertion site as it descends or grasping the membranes with a clamp. Assessment of the placenta and membranes as they are being delivered provides a good idea of whether they are intact, but delay detailed examination until it is clear that the uterus is well contracted and bleeding is minimal.
Uterine exploration
Routine exploration of the uterus is no longer recommended for normal deliveries or those following previous cesarean delivery. The procedure is uncomfortable and probably increases the risk of complications, especially infectious morbidity. Exploration is justified in patients with bleeding originating high in the genital tract despite the uterus being well contracted. The cervix should be visualized after all forceps deliveries.
Fourth stage
The delivery of the placenta does not mark the end of risk for bleeding; on the contrary, the uterus may have a tendency to relax slightly following placental delivery, and this is the point at which problems most commonly begin. The prophylactic use of a uterotonic helps ensure that the uterus continues to contract and retract, but the caregiver must remain vigilant. Nearly every clinician can recount an episode of being briefly distracted at this point only to have his or her attention abruptly reclaimed by a cascade of blood.
Following delivery of the placenta, palpate the abdomen to assess and monitor uterine tone and size. At this point, uterine massage is reasonable, especially if concern exists regarding uterine tone. Uterine massage can be uncomfortable; therefore, explain the rationale to the patient. If intravenous access is in place, a continuous infusion of oxytocin for a period following delivery is reasonable. If ongoing concerns exist regarding uterine tone, then start an oxytocin infusion or administer a longer-acting agent. Encourage early breastfeeding to promote endogenous oxytocin release.
Once good, sustained uterine tone has been established, the presence of any bleeding from the lower genital tract can be assessed. If bleeding is minimal, assess the placenta for completeness. (First, manage any significant lower genital tract bleeding.) Assessment of the placenta before repair of an episiotomy or any lacerations is advised in order to avoid disrupting these repairs if uterine exploration or instrumentation is necessary.
Examine the fetal side for any evidence of vessels coursing to the edge of the placenta and into the membranes. Such vessels suggest the presence of a succenturiate placental lobe. If the vessels are torn and the lobe is not present, it is quite likely retained and may subsequently lead to bleeding or infection. Turn the placenta over and lay it on a flat surface to examine the maternal side, with special attention to any defect suggestive of a missing, retained cotyledon. Note other abnormalities of the placenta, and consider whether pathological examination is warranted. Cultures of the placenta seem to be of little value in the diagnosis or management of fetal or uterine infection.
The lower genital tract is examined using adequate lighting and appropriate positioning and analgesia. Any episiotomy or lacerations are repaired. During this time, note any ongoing blood loss from the upper vagina, and, if present, reassess uterine tone and size. Closely observe the patient for blood loss over the next hour, with skilled assessment of uterine tone and size at least every 15 minutes. The duration of close observation and the presence and/or length of any uterotonic administration depends on the risk factors present and the clinical course.
Complications
Postpartum hemorrhage
The most common complication of the third stage of labor is PPH. Active management of the third stage has clearly been shown to reduce the frequency of this complication and therefore most likely has a positive impact on maternal mortality and longer-term morbidities such as anemia.
Retained placenta
Retained placenta is defined in various ways. The most common definition is retention of the placenta in utero for more than 30 minutes. This is an arbitrary definition, and management is greatly influenced by the clinical assessment of whether significant bleeding is occurring. This bleeding may be visible or may manifest only by the increasing size of the uterus. In the absence of any evidence of placental detachment, consider the diagnosis of complete placenta accreta or a variant. This condition may be present with bleeding if only a portion of the placenta is abnormally implanted.
Ensuring that the bladder is empty may speed the delivery of the placenta and at least aid in the assessment and control of the uterus. Ideally, women should have an empty bladder at the time of delivery. This usually occurs naturally because of pressure from the presenting part and maternal expulsive effort. Encouraging the woman to attempt to void late in the second stage or following delivery is not unreasonable, although this may be difficult. Emptying the bladder is mandatory before any attempt at assisted vaginal delivery.
A number of trials have evaluated the role of injection into the umbilical cord in the management of retained placenta in women not experiencing significant bleeding. [38] The definitions of retained placenta range from 15-60 minutes without placental delivery but are most commonly 20-30 minutes. Injections into the cord vein have used isotonic sodium chloride solution (normal saline), oxytocin and saline, prostaglandin and saline, misoprostol and saline, and dextran 70.
The studies comparing injection of oxytocin (commonly, 10 IU) and saline (commonly, 20 mL) with expectant management (OR, 0.7; 95% CI, 0.48-1.02) or saline injection alone (OR, 0.59; 95% CI, 0.43-0.82 and NNT, 8; 95% CI, 5-20) suggest that this practice indeed reduces the need for manual removal of the placenta. A recent study comparing misoprostol 800 mcg to oxytocin 50 IU, each in 30 mL of normal saline, found that misoprostol was significantly more effective in this setting. [39]
The additional use of intraumbilical injection of oxytocin may also significantly shorten the third stage of labor and reduce postpartum blood loss. A recent study noted lower blood loss, higher rate of placental delivery by 15 minutes, and shorter time to placental delivery in subjects administered intraumbilical oxytocin compared with subjects getting standard active management alone. [40]
The technique is carried out by inserting a size 10 nasogastric tube into the umbilical vein and advancing it to the placental insertion site of the cord. Resistance is met at this point. The nasogastric tube is then retracted by 3-4 cm to ensure the tip is not in a placental branch. The uterotonic solution is then injected and the cord is clamped with the tube in place. This intervention seems reasonable in stable women with minimal bleeding while preparations for a manual removal are being made.
Manual removal of the placenta is warranted if the above maneuvers have failed to deliver the placenta or if significant bleeding occurs. The retained or partially detached placenta interferes with uterine contraction and retraction and leads to bleeding. The risk of significant PPH increases the longer the placenta remains in situ and is increased 6-fold if the placenta remains undelivered after 30 minutes. [41]
Perform manual removal with a level of analgesia that matches the clinical urgency of the situation. The cessation of an oxytocin infusion or the administration of uterine relaxants to promote uterine exploration and manual removal is of questionable value and may lead to increased bleeding. Ultrasonography may be useful in select cases.
When possible, an elbow-length glove is worn and attention is paid to asepsis. The perineum and vagina must be prepared. The vaginal hand may be immersed in povidone-iodine solution (Proviodine) to facilitate easier entry. The hand is passed into the vagina through the cervix and into the lower segment following the umbilical cord. Care is taken to minimize the profile of the hand as it enters, keeping the thumb and fingers together in the shape of a cone to avoid damage.
Control of the uterine fundus with the nonvaginal hand is essential. If the placenta is encountered in the lower segment, it is removed. If the placenta is not encountered, the placental edge is sought. Once found, the fingers gently develop the space between the placenta and uterus and shear off the placenta. The placenta is pushed to the palmar aspect of the hand and wrist; when it is entirely separated, the hand is withdrawn. Ensure that an oxytocin infusion is running rapidly as the hand is withdrawn in order to encourage strong uterine contraction, and then perform uterine massage. Care must be taken to tease out the membranes. Once uterine contraction is established, examine the placenta and membranes to determine whether further exploration or curettage is necessary. The administration of antibiotics following manual removal is sometimes advocated. Evidence is very limited, but a single, small, randomized trial supports the practice. [42]
How to gain experience with potentially lifesaving procedures such as manual removal poses a dilemma. The days of regional anesthesia being an indication for manual removal are hopefully past, and this opportunity no longer exists. Manual removal at cesarean delivery allows the clinician to gain the most critical skills needed for this procedure. However, recent trials show that manual removal in this setting is associated with increased blood loss and infectious morbidity and therefore should not be routinely practiced. [43]
Uterine inversion
This condition is very rare. The risk of uterine inversion is increased in abnormalities of placentation, such as accreta, and is more likely with fundal cord insertions and any condition that predisposes patients to uterine atony and prolapse. Cord traction should never occur without countertraction or in the absence of uterine contraction. Leave the placenta attached, and focus management on maternal resuscitation and rapid return of the uterus to the abdominal cavity.
The fingers are formed into a single cone-shaped unit and placed at the most dependent portion of the protruding mass, which represents the inverted uterine fundus. Gentle upward pressure is exerted in the axis of the birth canal with the fingers and thumb together to minimize the risk of uterine perforation. The action has been likened to that of placing the fingers at the toe of an inside-out sock and pushing to make the sock right-side out. Following uterine replacement vigorous massage and uterotonic administration should undertaken.
Manual removal of the placenta may be performed when the mother's vital signs are stable unless concern exists regarding abnormal placentation. Uterine relaxants, such as nitroglycerin, may be helpful.
Placenta accreta
Placenta accreta and its variants are not complications of third-stage management but are most commonly recognized during the third stage. These life-threatening abnormalities of placentation may occur spontaneously; however, they are much more common in situations in which the placenta has implanted over a previously scarred uterus. The routine use and improving capabilities of ultrasound may suggest this diagnosis in the antepartum period, and the diagnosis should be considered in high-risk situations. The possibility of placenta accreta mandates that preparations for the management of severe PPH are in place and, if suggested based on ultrasound findings, that expertise is available to deal with the complications of placenta percreta.
Patient Education
As a component of antepartum care, discuss the issues surrounding the third stage of labor with the patient. This is true of all aspects of care as they pertain to the antepartum, intrapartum, and postpartum periods. The preferences of the patient and the practices of the caregiver should not come as a surprise or become the source of misunderstandings between the health care team and the parents. Identify patients at high risk for complications in the third stage, and discuss specific management issues with them. Whether emerging technologies, such as cord blood harvesting, should be routinely discussed is debatable but worth consideration.
For excellent patient education resources, visit WebMD's Pregnancy Center. Also, see WebMD's patient education article Labor Signs.
Summary
The period following delivery of the baby is a time of relief and joy for all involved; however, this period holds great potential danger for the mother. Complications of the third stage, especially PPH, account for much maternal mortality and morbidity. Compelling evidence suggests that active management of the third stage results in a decrease in complications and morbidity. The practice of prophylactic oxytocin administration with delivery of the baby and CCT with countertraction when the uterus is well contracted is strongly advocated. Caregivers must be prepared to diagnose and manage the complications that arise in the third stage in a timely and systematic manner.
Medications
Medications commonly used in the management of the third stage of labor include oxytocin, ergometrine/ergonovine, Syntometrine, misoprostol, carboprost tromethamine (Hemabate), and carbetocin.
-
Management of the third stage of labor. Maternal morbidity by subregion, 1995. Image provided courtesy of the World Health Organization.
-
Management of the third stage of labor. Active versus expectant management of the third stage of labor.






