Overview
Switching from intravenous (IV) to oral (PO) therapy as soon as patients are clinically stable can reduce the length of hospitalization and lower associated costs. [1] While intravenous medications may be more bioavailable and have greater effects, some oral drugs produce serum levels comparable to those of the parenteral form. Medications involved in switch therapy include antibiotics, analgesics, antipsychotics, and antivirals. Pharmacist-facilitated antimicrobial stewardship initiatives can aid to IV to PO switches. [2] Studies have underscored the cost savings and effectiveness of IV to oral switch therapy. [3, 4] A study shows that for low-risk variants of Staphylococcus aureus bloodstream infection, therapy can be switched from IV to PO rapidly. [5] Even IV treatment of methicillin-resistant Staphylococcus aureus involving the skin can be shifted to PO therapy in many patients. [6]
Early switching of intravenous to oral antibiotics is possible, and positive outcomes have been reported in medical wards. [7] In addition, a meta-analysis found that early switching of intravenous to oral antibiotics is possible in moderate to severe community-acquired pneumonia (CAP). [8] In particular, quinolones can be switched effectively and rapidly from intravenous to oral formulations when patients can tolerate medications orally. [9] Many doctors continue to not be aware of guidelines for intravenous to oral switches, their thinking complicated by patients with complex problems. [10] Nevertheless guidelines can help doctors make the proper decision to do the switch in appropriate patients. [11] A useful antibiotic in IV to oral switch is linezolid. [12]
Community-acquired pneumonia
One of the most common uses of intravenous-to-oral (IV-to-PO) switch therapy is in the treatment of CAP. CAP is most commonly caused by Streptococcus pneumoniae infection. The natural history of CAP is beyond the scope of this article; see Pneumonia, Community-Acquired for more information. In terms of switch therapy, approximately 40-50% of patients admitted for intravenous antibiotics can be switched to oral antibiotics within 2-3 days.
The US Medicare Pneumonia Project database provided evidence that the routine practice of in-hospital observation after the switch from intravenous to oral antibiotics in patients with CAP can be avoided in those who are clinically stable. [13] Explicit physiological criteria must be recorded routinely to serve as a benchmark in order for the switch to be consistently successful.
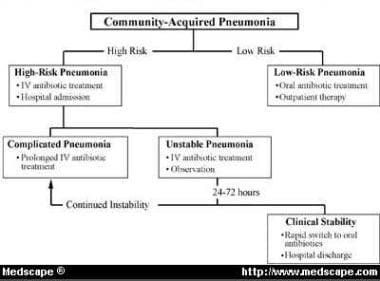
In 1999, Siegel reported that the treatment of hospitalized patients with uncomplicated CAP is changing to include a brief period of intravenous antibiotics followed by oral therapy. [14] The Classification of Community-Acquired Pneumonia (CoCAP) is a stratification tool in which patients are categorized as having low-risk pneumonia, unstable pneumonia, or complicated pneumonia (see the image below). Caregivers can achieve a structure for organizing treatment of patients with CAP by using (1) validated hospital admission criteria, (2) the CoCAP algorithm, and (3) newly evolving criteria for switching patients from intravenous to oral therapy.
A Japanese multicenter randomized study of early-switch therapy from intravenous sulbactam/ampicillin to oral garenoxacin in CAP was successful. [15]
Patients with unstable pneumonia can be discharged early if (1) their metabolic problems have reversed and comorbid conditions have stabilized and (2) they have not developed any serious pneumonia-related complications. Prolonged courses of intravenous antibiotic therapy are being replaced with 2- to 3-day courses of intravenous hydration and antibiotics; patients can be switched to oral therapy and can be discharged from the hospital after they tolerate one dose of oral therapy. The vital signs and the WBC count should be monitored, and, provided these parameters are improving (although possibly not normalized), patients can be switched to oral therapy.
Patient treatment guidelines and critical pathways are becoming widespread in disease management, and CAP is one disease in which prospective studies have demonstrated that a reduction in hospital stay is safe and agreeable with patients, caregivers, and administrators. Other treatment protocols are being explored, including a single dose of intravenous antibiotic prior to the oral switch and all-oral regimens using the newer fluoroquinolones. A study by Ramirez et al (2005) showed that the care recommended by national guidelines regarding switching from intravenous to oral therapy was not being appropriately delivered to adults with CAP in all regions of the world. [16]
Different doctors have different approaches to switch therapy. Inpatients with CAP treated by hospital clinicians had a shorter adjusted length of stay than those treated by primary care physicians, primarily because of earlier recognition of stability and more rapid conversion from intravenous to oral antibiotics. Adjusted costs were likewise reduced. However, patients treated by hospital clinicians were more often discharged with an unstable clinical variable. Other than earlier switching to oral antibiotics, less use of clindamycin and ceftazidime, and fewer consultations with infectious disease specialists, the care processes of hospital clinicians were similar to those of primary care physicians. [17]
In 2004, Wawruch et al reported on an evaluation of a group of patients selected out of 2870 patients who were hospitalized at the Clinic of Geriatric Medicine at Comenius University in Bratislava from January 1, 1999, to December 31, 2001. In their retrospective study, Wawruch et al analyzed 96 patients with CAP who were successfully treated with antibiotics. Forty-three patients received intravenous antibiotics, and 53 received IV-to-PO switch therapy (ie, intravenous administration was used at the beginning and oral administration was used when their conditions improved). [18]
According to the cost-effectiveness coefficient, the switch therapy was significantly less expensive in all evaluated antibiotics (except pefloxacin) compared with intravenous administration. For ampicillin-sulbactam, the coefficients were 93.9 versus 168.1, 90 versus 123.3 for cefuroxime, 74 versus 116.3 for amoxicillin-clavulanate, and 31.7 versus 54.1 for ciprofloxacin. Wawruch et al found that timely switching from intravenous to oral administration of antibiotics in suitable patients is an effective way to save financial resources. [18]
Oosterheert et al (2006), based on a study of 302 patients, found that early switch from intravenous to oral antibiotics in patients with severe CAP is safe and decreases the hospital stay by 2 days. [19]
Peyrani et al (2006) reported that, in a study involving 40 hospitals in 13 countries, IV-to-PO switch antibiotic therapy among hospitalized patients with CAP did not comply with evidence-based guidelines implemented by The American Thoracic Society and the Infectious Diseases Society of America. [20]
Rhew and associates investigated the effectiveness of early switch and early discharge strategies in patients with CAP by searching the MEDLINE, HealthStar, EMBASE, Cochrane Collaboration, and Best Evidence databases for the period between January 1, 1980, and March 31, 2000, for CAP studies that included specific switch criteria or recommendations to switch on a particular day. [21]
Rhew et al identified 1794 titles and reviewed 121 articles. They identified 10 prospective, interventional, CAP-specific studies that evaluated length of stay. Nine studies applied an early switch from parenteral to oral antibiotic criteria. Six different criteria for switching were applied in the 9 studies. Five of the studies that applied early-switch criteria also applied separate criteria for early discharge. Six studies applied an early-switch and early-discharge strategy to an intervention and a control group, and 5 of these provided standard deviation values for length of stay. [21]
The mean change in length of stay was not significantly (P = .05) reduced in studies of early switch and early discharge (-1.64 d; 95% CI, -3.3 to 0.02 d). However, when the 2 studies in which the recommended length of stay was longer than the control length of stay were excluded from the analysis, the mean change in length of stay was reduced by 3 days (-3.04 d; 95% CI, -4.9 to -1.19 d). Studies did not reveal significant differences in clinical outcomes between the intervention and control groups. Rhew and colleagues concluded that criteria for early switching from parenteral to oral antibiotics vary considerably for patients with CAP. Early-switch and early-discharge strategies may significantly and safely reduce the mean length of stay when the recommended length of stay is shorter than the actual length of stay. [21]
A retrospective study by Deshpande et al showed that patients with community-acquired pneumonia who switched early (by hospital day 3) from intravenous to oral antibiotics had a shorter length of stay and lower hospitalization costs than patients who remained longer on intravenous antibiotics. No increase in in-hospital mortality was associated with switching. [1]
Bacterial peritonitis, pyelonephritis, adult septic arthritis
Other uses of switch therapy can include the treatment of spontaneous bacterial peritonitis. A more cost-effective switch therapy in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis who are not receiving prophylaxis with quinolones involves the use of a cephalosporin rather than intravenous ceftazidime. [22]
In a study of 169 cases of septic arthritis in 157 adults, 12% recurred after intravenous to oral switch. Gram-negative bacteria, immunosuppression, and lack of surgical clearance of the nidus of infection complicated the switch. In most cases, 7 days of intravenous therapy was similar in outcome to 8-21 days. Fourteen days or less has the a similar outcome as 15-28 days or more than 28 days. [23]
In a study of 82 patients with putative pyelonephritis, [24] all received 2 g of intravenous ceftriaxone initially. After day 3, they were split into 2 groups of 41 one receiving 2 g of intravenous ceftriaxone and the other a single daily dose of oral cefditoren pivoxil at 400 mg. Ninety-five percent of the orally treated patients and 100% of those receiving intravenous therapy achieved a clinical cure, with 63.4% of the oral and 60% of the intravenous groups treated achieving bacteriological eradication; no difference was noted in adverse effects between the 2 groups. The authors conclude that this switch is viable, even though the pathogens of pyelonephritis are often quinolone-resistant. Perihepatitis and acute pelvic inflammatory disease treated with intravenous azithromycin switched to oral azithromycin has been found effective and practical in Japan. [25]
Antibiotics
Switch therapy options
Switch therapy is possible with various oral antibiotics. Antibiotics ideal for intravenous-to-oral (IV-to-PO) switch programs include chloramphenicol, clindamycin, metronidazole, trimethoprim-sulfamethoxazole, fluconazole, itraconazole, voriconazole, doxycycline, minocycline, levofloxacin, moxifloxacin, and linezolid. [26]
Sequential antibiotic therapy ensures an early switch to the oral route when a patient is clinically stable. This increasingly used strategy is safe and improves the quality and cost-effectiveness of health care. Timely and appropriate switch therapy must be underpinned by clear guidelines and supported by a multidisciplinary team. According to some authorities, approximately 40% of patients starting on intravenous antibiotics are candidates for a switch to oral antibiotics after 2-3 days of therapy. Harvey et al found that the most common timing for a review of a possible switch to oral administration was 48-72 hours from the initiation of intravenous antibiotic therapy. [27]
In 2004, Vogtlander et al, at the Department of General Internal Medicine, University Medical Center Nijmegen, in the Netherlands, involved the departments of internal medicine, surgery, and neurology and the emergency department at a tertiary referral university medical center in a study of all consecutive patients receiving therapeutic antibiotics. Dosages, timing of first doses, dosing intervals, administration routes, and adjustment of the chosen drug to clinical data were investigated. After the preintervention period, barriers to change were identified, followed by specific interventions and a postintervention measurement. In the preintervention and postintervention periods, 247 and 250 patients were enrolled, receiving 563 and 598 antibiotic prescriptions, respectively. [28]
The mean time from the order to first dose at the wards improved from 2.7 to 1.7 hours in potentially severe cases (P = .003). Dosage adjustment per renal function remained unchanged at 45% versus 52% (P = .09) of cases when necessary. Switching of therapy from an intravenous route to an oral route improved from 46% to 62% (P = .03) and was performed a mean of 1.6 days earlier (P = .002). Streamlining was performed correctly in most cases; thus, no interventions were necessary. Timing of antibiotic therapy and switch therapy may be improved with a combination of interventions. Other strategies are needed to improve the poor adjustment of dosing per renal function. In this study, streamlining was already correct in most cases. [28]
A retrospective study by Gasparetto et al demonstrated that intravenous-to-oral antibiotic switch therapy was associated with a shorter duration of stay in the intensive care unit for patients with sepsis. No difference in mortality was found between patients who received switch therapy and those who did not. [29]
A randomized controlled trial by Keij et al showed that among neonates with probable bacterial infection, reinfection rates were similar between patients who were switched from intravenous to oral antibiotics (amoxicillin-clavulanic acid) and those who received a full course of intravenous therapy. No increase in adverse events was observed in the neonates who were switched to oral antibiotics. [30]
Fluoroquinolones
Fluoroquinolones are suitable for switch therapy.
Levofloxacin and ofloxacin
The intravenous and oral formulations of levofloxacin have same-dose bioequivalence, allowing for switch or step-down therapy from parenteral to oral formulations of the same agent at the same dose. In the late 1990s, ofloxacin was also used for switch therapy, but its role is unclear in switch therapy because it is a twice-a-day medication, whereas levofloxacin is a once-a-day medication. Fluoroquinolones should not be used in children because of a possible adverse effect on cartilage.
Levofloxacin provides almost complete (≥99%) oral bioavailability, suggesting that oral administration may provide exposure that is comparable to that of the intravenous regimen. The overall clinical success rate in such a switch is 94.1%. In several randomized controlled trials, 5-14 days of treatment with intravenous and/or oral levofloxacin proved to be an effective therapy for patients with upper and lower respiratory tract infections. In patients with mild-to-severe community-acquired pneumonia (CAP), intravenous and/or oral levofloxacin at a dose of 500 mg once or twice daily was as effective as clarithromycin, azithromycin, and amoxicillin/clavulanic acid. Overall, clinical response rates with levofloxacin were 86-95% versus 88-96% with comparator agents; bacteriological response rates were 88-95% and 86-98%, respectively.
In 2005, Pablos et al reported on a study of the consumption of quinolones (eg, ofloxacin, levofloxacin, ciprofloxacin) 6 months before and after the implementation of a sequential therapy program in hospitalized patients. A program was calculated for each antibiotic, in its oral and intravenous forms, in "defined daily dose/100 stays per day" and in economic terms (drug acquisition cost). At the beginning of the program, ofloxacin was replaced by levofloxacin and, because their clinical uses are similar, the consumption of both drugs was compared during the period. [31]
In economic terms, the consumption of intravenous quinolones decreased 60%, whereas the consumption of oral quinolones increased 66%. In "defined daily dose/100 stays per day," consumption of intravenous forms decreased 53% and consumption of oral forms increased 36%. Pablos et al focused on quinolones and their use in implementing a sequential therapy program based on promoting an early switch from an intravenous regimen to an oral regimen. They proved the program's capacity to alter the use profile of these antibiotics. During the period under consideration, the program achieved a global drug savings of $41,420 for the hospital. [31]
Levofloxacin (Levaquin) has activity against pneumococci, including penicillin-resistant isolates, and activity against aerobic gram-negative rods but not Pseudomonas. It can be used to treat CAP. The adult dose is 500 mg PO qd for 7-14 d. It is not recommended for children. Levofloxacin is a pregnancy category C medication.
Ofloxacin (Floxin) penetrates the prostate well and is effective against Chlamydia trachomatis. It is derived from pyridine carboxylic acid and has broad-spectrum bactericidal effect. It is specifically used to treat prostatitis and UTI. In adults, the dosing for prostatitis is 400 mg PO once, while dosing for chronic prostatitis is 200-400 mg PO q12h. It is not recommended for children. Ofloxacin is also a pregnancy category C medication.
Ciprofloxacin
Ciprofloxacin also has a role in IV-to-PO switch therapy. Giamarellou and colleagues demonstrated that high-dose ciprofloxacin administered intravenously for at least 3 days and then orally is therapeutically equivalent to the routine regimen of intravenous ceftazidime plus amikacin, even in febrile patients with severe neutropenia (ie, polymorphonuclear leukocyte count, < 100/µL). [32]
Solomkin and colleagues studied patients with complicated intra-abdominal infections, who were randomized to receive either (1) intravenous ciprofloxacin plus metronidazole or intravenous imipenem throughout their treatment course or (2) intravenous ciprofloxacin plus metronidazole and treatment with oral ciprofloxacin plus metronidazole when oral feeding was resumed. The study demonstrated statistical equivalence between intravenous ciprofloxacin plus metronidazole and intravenous imipenem in both the intent-to-treat and valid populations. Conversion to oral therapy with intravenous ciprofloxacin plus metronidazole appeared as effective as continued intravenous therapy in patients able to tolerate oral feedings. [33]
Ciprofloxacin (Cipro) is a fluoroquinolone with activity against pseudomonads and most gram-negative organisms but no activity against anaerobes. It inhibits bacterial DNA synthesis and, consequently, growth. Continue treatment for at least 2 d (7-14 d typical) after signs and symptoms have disappeared. The adult dose is 250-500 mg PO bid for 7-14 d. It is not indicated in children. Ciprofloxacin is a pregnancy category C medication.
Moxifloxacin
In 2003, a trial reported by Drummond et al compared sequential intravenous/oral monotherapy with moxifloxacin (400 mg/d) to intravenous/oral co-amoxiclav (1.2 g IV/625 mg PO tid) with or without clarithromycin (500 mg bid) for 7-14 days in hospitalized patients with CAP and found that intravenous/oral monotherapy with moxifloxacin shows clinical benefits, including increased speed of response, and is cost-effective compared with intravenous/oral co-amoxiclav with or without clarithromycin. [34]
Similarly, in 2002, Finch et al noted that monotherapy with moxifloxacin is superior to a standard combination regimen of a beta-lactam and a beta-lactamase inhibitor (co-amoxiclav) with or without a macrolide (clarithromycin) in the treatment of patients with CAP admitted to a hospital. [35]
Specifically, Finch et al noted the superiority of moxifloxacin irrespective of the pneumonia severity and regardless of whether the combination therapy included a macrolide. The time to resolution of fever was also statistically significantly faster in patients who received moxifloxacin (median time, 2 vs 3 d), and the duration of hospital admission was approximately 1 day less among patients who received moxifloxacin. The treatment was converted to oral therapy immediately after the initial mandatory 3-day period of intravenous administration for a larger proportion of patients in the moxifloxacin group than patients in the comparator group (151 [50.2%] vs 57 [17.8%] patients). Fewer deaths (9 [3%] vs 17 [5.3%]) and fewer serious adverse events (38 [12.6%] vs 53 [16.5%]) were reported in the moxifloxacin group than in the comparator group. [35]
Delafloxacin
In June 2017, FDA approved Baxdela (delafloxacin) for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Approvals for delafloxacin were supported by two phase III studies in patients with ABSSSI demonstrating that IV and oral delafloxacin monotherapy was statistically non-inferior to the combination of vancomycin plus aztreonam at the FDA primary endpoint of early clinical response at 48–72 hours [36] . [37]
Baxdela exhibits activity against both gram-positive and gram-negative pathogens, including MRSA (methicillin-resistant Staphylococcus aureus), and is available in both intravenous (IV) and oral formulations. The oral bioavailability is 58.8%, with 450 mg taken orally being equivalent to IV infusion of 300 mg for 1 h, permitting oral sequencing. Delafloxacin is excreted renally and unaffected by cytochrome P450. The recommended dosage for IV use is 300 mg every 12 h, and orally, 450 mg every 12 h. [38]
Cephalosporins
Similar switches can be effective with cephalosporins. Validated treatment algorithms, such as the Classification of Community-Acquired Pneumonia (CoCAP), now enable decisions concerning which patients with CAP require hospitalization and which patients will benefit from early switch therapy. Generally, unstable patients with CAP are suitable candidates for early switch therapy, which consists of rapid initiation of 1-2 days of intravenous therapy followed by 5 days of oral therapy, with early hospital discharge after the administration of 1-2 doses of oral antibiotic.
Cefuroxime, cefuroxime axetil, and cefetamet pivoxil
Studies of intravenous cefuroxime followed by oral cefuroxime axetil suggest this regimen is both effective and well-tolerated as rapid switch therapy and has the potential to reduce overall health care costs and improve patient satisfaction. Specifically, Van den Brande and colleagues noted that intravenous cefuroxime twice daily followed by oral cefuroxime axetil is a simple and effective sequential therapy regimen for the treatment of CAP. [39]
Hamilton-Miller found that switch therapy to cefixime after 2-3 days used to treat serious infections resulted in excellent clinical outcomes. [40] Similarly, Dagan and colleagues found that 1 or 2 days' treatment with parenteral ceftriaxone before switching to oral cefetamet pivoxil was safe and effective in the treatment of childhood pneumonia. [41] This suggests that parenteral-to-oral switch therapy is a feasible treatment option in the treatment of serious pediatric CAP.
Cefuroxime (Ceftin, Kefurox, Zinacef) is a second-generation cephalosporin that maintains gram-positive activity of first-generation cephalosporins and adds activity against Proteus mirabilis, Haemophilus influenzae, Escherichia coli, Klebsiella pneumoniae, and Moraxella catarrhalis. The condition of the patient, severity of the infection, and susceptibility of the microorganism determine proper dose and route of administration. The adult dose is 500 mg PO bid for 20 d or, alternatively, 750-1500 mg IV/IM q8h, not to exceed 6 g/d. In children, dosing is 250 mg PO bid for 20 d. Use the adult dose for adolescents. Cefuroxime is a pregnancy category C medication.
Cefixime (Suprax) has activity against aerobic gram-negative rods. It arrests bacterial cell-wall synthesis and inhibits bacterial growth by binding to one or more of the penicillin-binding proteins. The adult dose is 400 mg PO qd (recommended for gonococcal infections); alternatively, 200 mg PO q12h or 400 mg PO qd or divided q12h can be used. In children < 12 years, dosing is 8 mg/kg PO qd or 4 mg/kg bid. In children >50 kg or >12 years, administer cefixime as in adults. Cefixime is a pregnancy category B medication.
Ceftriaxone and ceftibuten
Fernandez and San Martin studied 40 patients admitted to the hospital because of CAP. Initially, these patients were treated with intravenous ceftriaxone (1 g/d) and showed clinical improvement after 3 days of therapy. They were randomly assigned to continue intravenous ceftriaxone therapy for a total of 10 days or to switch to ceftibuten (400 mg/d) for 7 days. Twenty-one of the patients continued intravenous treatment, and 19 were switched to ceftibuten. In terms of clinical cure, radiological improvement, and normalization of WBC count, no differences were noted between the 2 groups. [42] These findings support the viability of switch therapy in this context.
Ceftriaxone (Rocephin) is a third-generation cephalosporin with broad-spectrum, gram-negative activity. It has lower efficacy against gram-positive organisms. Ceftriaxone arrests bacterial growth by binding to one or more penicillin-binding proteins. In adults with uncomplicated infections, dosing is 250 mg IM once, not to exceed 4 g. In adults with severe infections, dosing is 1-2 g IV qd or divided bid, not to exceed 4 g/d. In neonates >7 days, dosing is 25-50 mg/kg IV/IM qd, not to exceed 125 mg/d. Dosing in infants and children is 50-75 mg/kg IV/IM qd divided q12h, not to exceed 2 g/d. Ceftriaxone is a pregnancy category B medication.
Ceftibuten (Cedax) is a third-generation bactericidal cephalosporin that inhibits cell-wall mucopeptide synthesis. In adults, dosing is 400 mg PO qd for 10 d, not to exceed 400 mg/d. If the CrCl is 30-49 mL/min, administer 4.5 mg/kg or 200 mg PO qd. If the CrCl is < 30 mL/min, administer 2.25 mg/kg or 100 mg PO qd. In children < 12 years, dosing is 9 mg/kg PO qd for 10 d, not to exceed 400 mg/d. Administer as in adults in children >12 years. Ceftibuten is a pregnancy category B drug.
Macrolides
Macrolides that can be used for switch therapy include azithromycin and clarithromycin.
Azithromycin
The macrolide azithromycin appears to be superior to the cephalosporin cefuroxime in intravenous therapy and a subsequent switch to oral therapy. This was shown in a cost-effectiveness analysis of IV-to-PO switch regimens of azithromycin versus cefuroxime with or without erythromycin in the treatment of patients hospitalized with CAP.
Azithromycin (Zithromax) is active against gram-positive bacteria and organisms responsible for atypical pneumonia but resistant to erythromycin-resistant pneumococci. It inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNA-dependent protein synthesis to arrest. It is used to treat mild-to-moderate microbial infections. In adults, dosing is 500 mg PO on day 1 and 250 mg PO qd on days 2-5. Alternatively, 1 g PO once can be used. Dosing in children < 6 months has not been established. In children >6 months, dosing is 10 mg/kg PO once (not to exceed 500 mg/d) on day 1 and 5 mg/kg PO qd (not to exceed 250 mg/d) on days 2-5. Azithromycin is a pregnancy category B medication.
Clarithromycin
Clarithromycin can also be used to in an IV-to-PO switch regimen. In 2000, Parola et al reported on 290 patients with CAP who were given clarithromycin at 500 mg twice daily, first given intravenously in 250 or 500 mL of saline solution and then switched after 4-5 days to the same dose given orally. Within 10-15 days, 261 (90%) of 290 patients improved clinically and radiologically. [43]
Other antibiotics
Other antibiotics that can be used for switch therapy include clindamycin, ertapenem, linezolid, metronidazole, and trimethoprim-sulfamethoxazole.
Clindamycin
Martinez and associates found that switch therapy can be used when administering clindamycin. [44] Specifically, a multicenter, prospective, controlled study compared the clinical efficacy, safety, and economic impact of pharmacist intervention to promote sequential IV-to-PO clindamycin conversion. Clindamycin was prescribed for respiratory tract infections in 38.9% of patients and for prophylaxis in surgery in 25.4% (71% were contaminated during surgery). A total of 473 patients receiving intravenous clindamycin for at least 72 hours were included in the study. Two groups were established. Those in the intervention group (204 patients) were given an informative sheet recommending the sequential treatment, and the other group consisted of 269 control patients. Outcomes appeared similar.
Clindamycin (Cleocin) is a lincosamide used to treat serious skin and soft tissue staphylococcal infections. It is also effective against aerobic and anaerobic streptococci (except enterococci). It inhibits bacterial growth, possibly by blocking dissociation of peptidyl t-RNA from ribosomes, causing RNA-dependent protein synthesis to arrest. In adults, dosing is (1) 150-450 mg/dose PO q6-8h, not to exceed 1.8 g/d, or (2) 600-1200 mg/d IV/IM divided q6-8h, depending on the severity of infection. In children, dosing is 8-20 mg/kg/d PO as hydrochloride or 8-25 mg/kg/d as palmitate divided tid/qid. Alternatively, use 20-40 mg/kg/d IV/IM divided tid/qid. Clindamycin is a pregnancy category C medication.
Ertapenem
The efficacy and safety of intravenous ertapenem (1 g/d) with the option to switch to an oral agent for treatment of adults with complicated urinary tract infections were compared with those of intravenous ceftriaxone (1 g/d) with the same oral switch option in a multicenter, double-blinded, prospective randomized study. The frequency and severity of drug-related adverse events were generally similar in both treatment groups. In this study, ertapenem was as effective as ceftriaxone for the initial treatment of complicated urinary tract infections in adults, was generally well tolerated, and had a similar overall safety profile. [45]
Linezolid
In 2003, Li et al noted that intravenous linezolid can be followed by oral linezolid; these regimens were found to shorten hospital stays. [46] The exact timing of the switch depends on the clinical condition of the patient; oral and intravenous linezolid are relatively similar in effect. Linezolid is available in intravenous, film-coated tablet, and oral suspension forms. Linezolid can be assayed in serum and body fluids and has good bioavailability, with a maximum blood concentration at 0.5-2 hours.
Metronidazole
Metronidazole can be part of regimens for switching patients from intravenous to oral therapy. Treatment between prolonged intravenous therapy and intravenous therapy followed by conversion to oral antibiotic therapy is equivalent in children with perforated appendicitis. Similarly, a study noted 8 patients with brain abscesses who refused prolonged hospitalization and were treated with a short course (6-12 d) of intravenous antibiotics followed by prolonged treatment (15-19 wk) with an oral antibiotic regimen consisting of metronidazole, ciprofloxacin, and amoxicillin. All patients responded favorably based on clinical findings and imaging studies.
In 2003, Starakis et al compared the efficacy and safety of sequential intravenous/oral ciprofloxacin plus intravenous/oral metronidazole with that of intravenous ceftriaxone plus intravenous/oral metronidazole in the treatment of complicated intra-abdominal infections in 135 patients. Conversion to oral therapy with ciprofloxacin/metronidazole was as effective as continued intravenous therapy with ceftriaxone and oral metronidazole in patients who were able to tolerate oral feeding. [47]
Similarly, in 1996, Solomkin et al reported a study in which patients were randomized to either (1) ciprofloxacin plus metronidazole intravenously or imipenem intravenously throughout their treatment course or (2) ciprofloxacin plus metronidazole intravenously and treatment with oral ciprofloxacin plus metronidazole when oral feeding was resumed, with equal outcomes. [33]
Trimethoprim-sulfamethoxazole
Trimethoprim-sulfamethoxazole (Bactrim) can also be used as part of IV-to-PO switch regimens. In 2002, Gollin et al reported on a study of 80 children who underwent appendectomy for perforated appendicitis. The children were safely discharged home on a 7-day course of oral trimethoprim-sulfamethoxazole and metronidazole when enteral intake was tolerated, regardless of fever or leukocytosis. [48]
Antifungals
Antifungals that can be used for switch therapy include itraconazole and fluconazole.
Itraconazole and fluconazole
The efficacy and safety of intravenous and oral itraconazole and intravenous and oral fluconazole for long-term prophylaxis of fungal infections in transplantation patients have been established; itraconazole is better tolerated. Generally, in patients who can take oral medications, itraconazole and fluconazole can be given orally with no adverse effects or effect on outcomes. Similarly, in 2002, Purkins et al noted that switching from intravenous to oral voriconazole can be effectively achieved. [49]
Specifically, in 2002, Winston and Busuttil reported a study in which adult liver transplant recipients were randomized to receive either an oral itraconazole solution (200 mg q12h) or intravenous/oral fluconazole (400 mg/d). Each study drug was started immediately before the transplantation surgery and continued for 10 weeks after transplantation. Patients were evaluated for fungal colonization, proven invasive or superficial fungal infection, drug-related adverse effects, and death. Results were similar. [50]
In patients with candidemia, successful protocols in a study of 37 patients have been developed to step down intravenous echinocandin or voriconazole to oral fluconazole, with only one failure. [51]
Antidepressants
Citalopram
Recently, the selective serotonin reuptake inhibitor citalopram has been administered as an intravenous infusion to patients with severe depression. The results from both open and double-blinded clinical studies with intravenous citalopram suggest that it is an effective and well-tolerated treatment for depression. Moreover, when infusion treatment is initiated and continued orally, citalopram is at least as effective as clomipramine, doxepin, and viloxazine. As with oral treatment, adverse events are mild to moderate in severity, and 50% of patients report no adverse events.
The high bioavailability of citalopram indicates that the switch from intravenous to oral citalopram prevents a deterioration of symptoms because plasma drug concentrations are maintained. Thus, citalopram, the only selective serotonin reuptake inhibitor available as an intravenous formulation, may be a useful addition in the treatment of patients with severe depression who may benefit from more intensive therapy. [52]
Citalopram (Celexa) enhances serotonin activity because of selective reuptake inhibition at neuronal membrane. The adult dose is 20-40 mg PO qd. It is not indicated in children. Citalopram is a pregnancy category C drug, but use late in the third trimester is associated with complications in newborns and may result in prolonged hospitalization, respiratory support, and tube feeding.
Dose-dependent QT prolongation has been reported with citalopram. Because of the risk for QT prolongation, citalopram is contraindicated in individuals with congenital long QT syndrome and the dose should not exceed 40 mg/d. [53, 54]
Doxepin
In 1997, Adler et al conducted a randomized, double-blinded, placebo-controlled study on doxepin to evaluate the effect of a switch from parenteral to oral administration upon symptoms of endogenous depression. They tested the hypothesis that the treatment response significantly worsens during the switch and concluded that this hypothesis must be rejected based on objective and subjective psychometric test findings. In fact, they noted continuous improvement. Preconditions included selection of patients with typical endogenous depression and maintenance of at least constant plasma levels of the active antidepressants. [55]
In patients younger than 65 years, doxepin plasma levels can be kept constant by switching in a ratio of 125 mg intravenous to 250 mg oral. Individual case studies indicated that declining progress after switching was correlated with a decreasing plasma level of the active drug. An already-low plasma level during the infusion period, insufficient response, and questionable compliance with the oral medication were associated factors. Owing to large (by a factor of 10) interindividual differences of plasma levels, measurements before and after switching were required.
Doxepin (Adapin, Sinequan) increases the concentration of serotonin and norepinephrine in the CNS by inhibiting their reuptake by presynaptic neuronal membrane. Effects are associated with a decrease in symptoms of depression. Adult dosing is 30-150 mg/d PO hs or 2-3 divided doses, gradually increased to 300 mg/d prn. In children < 12 years, doxepin is not recommended. In those >12 years, dosing is 25-50 mg/d PO hs or bid/tid, gradually increased to 100 mg/d. Doxepin is a pregnancy category C drug.
Analgesics
Acetaminophen
Promptly switching intravenous acetaminophen to oral acetaminophen is possible in patients with severe pain outbreaks if certain steps have been taken, including the establishment of a local consensus process, presentation of a short educational program, display of posters in all nurses' offices, and feedback regarding the practice 6 months after implementation of guidelines.
Antivirals
Acyclovir
Carcao and colleagues noted that immunocompromised children are at risk for disseminated varicella infections and that standard treatment involves hospitalization and intravenous acyclovir for 7-10 days. Carcao et al undertook a pilot study to assess the safety and efficacy of an alternative approach that involved a combination of intravenous followed by oral acyclovir in a cohort of immunocompromised children. Specifically, the cohort consisted of 26 immunocompromised children aged 1.5-12.7 years (mean age, 6.3 y). [56]
Therapy was commenced with intravenous acyclovir (1500 mg/m2/d in 3 divided doses). Concurrent treatment included holding or reducing immunosuppressive therapy (by 50%) and administering varicella-zoster immunoglobulin in 11 (69%) of 16 patients in whom exposure to chickenpox was recognized. Patients were eligible to switch to oral therapy after receiving a minimum of 48 hours of intravenous acyclovir therapy, provided they were afebrile, had no new lesions for 24 hours, had no internal organ involvement, and were able to tolerate oral medications. [56]
Patients were observed in the hospital for another 24 hours and were then discharged provided they remained well. Oral acyclovir was continued for a total of 7-10 days (intravenous plus oral). Carcao et al found that 25 of the 26 patients were successfully switched from intravenous to oral administration after 4.1 (mean) ± 1.2 days (standard deviation) (range, 2.3-6 d). Children had fever for a mean of 2 ± 1.6 days (range, 0-5 d) and developed new lesions for 2.9 ± 0.7 days (range, 2-4 d). [56]
Disease resolved in all 25 patients who switched to oral therapy, and no patient required resumption of intravenous therapy. Carcao and associates concluded that the sequential use of intravenous acyclovir followed by oral acyclovir is feasible in the treatment of varicella infection in immunocompromised children and results in a reduced duration of intravenous therapy and hospitalization. [56]
Acyclovir (Zovirax) inhibits activity of both HSV-1 and HSV-2. It has affinity for viral thymidine kinase and, once phosphorylated, causes DNA chain termination when acted on by DNA polymerase. Patients experience less pain and faster resolution of cutaneous lesions when used within 48 h of rash onset. It may also prevent recurrent outbreaks. Early initiation of therapy is imperative. Adult dosing is 600-800 mg PO 5 times/d for 7 d or 10 mg/kg/dose IV q8h; initiate treatment immediately upon onset of symptoms of recurrent episodes. In immunocompromised adults, dosing is 800 mg PO q4h (5 times/d) for 7-10 d. In children, dosing is (1) 250-600 mg/m2/dose PO 4-5 times/d for 7-10 d or (2) 1500 mg/m2/d IV divided q8h or 10 mg/kg/dose IV q8h for 7 d.
Famciclovir and valacyclovir
The bioavailability of acyclovir is approximately 8%. The bioavailability of famciclovir and valacyclovir is approximately 50%. Now that famciclovir and valacyclovir have been approved, choosing either agent seems advisable when switching from intravenous acyclovir to an oral agent.
Inpatient Care
Inpatients with nonsevere community-acquired pneumonia (CAP) can be effectively and safely treated with oral antimicrobials from the time of admission, whereas those with severe pneumonia can be treated with early switch therapy. Once a hospitalized patient with CAP is clinically stable, switching from intravenous to oral antibiotics, even if the bacteremia was initially documented to be caused by S pneumoniae, is safe.
Numerous factors must be weighed before switching hospitalized patients from intravenous to oral antibiotics. In a study by Halm et al, the following factors were rated as very important to the antibiotic conversion decision [57] :
-
Absence of suppurative infection (93%)
-
Ability to maintain oral intake (79%)
-
Respiratory rate at baseline (64%)
-
No positive blood culture findings (63%)
-
Normal temperature (62%)
-
Oxygenation at baseline (55%)
-
Mental status at baseline (50%)
Fifty-eight percent of physicians believed that "patients should be afebrile for 24 hours before conversion to oral antibiotics," and 19% said "patients should receive a standard duration of intravenous antibiotics." The median thresholds at which physicians believed a typical patient could be converted to oral therapy were as follows [57] :
-
Temperature of less than or equal to 100°F (37.8°C)
-
Respiratory rate of less than or equal to 20 breaths per minute
-
Heart rate of less than or equal to 100 beats per minute
-
Systolic blood pressure of 100 mm Hg or higher
-
Room air oxygen saturation of 90% or higher
In univariate analyses, pulmonary and infectious disease physicians were the most predisposed toward early conversion to oral antibiotics, and other medical specialists were the least predisposed, with generalists being intermediate (P< .019). In multivariate analyses, practice beliefs were associated with age, inpatient care activities, attitudes about guidelines, and agreeableness on a personality inventory scale. In summary, physicians believed that patients could be switched to oral antibiotics once vital signs and mental status had stabilized and oral intake was possible. However, antibiotic practice beliefs varied considerably. [57]
Regarding the management of CAP, Ramirez reported in 2001 that switch therapy can reduce costs associated with drug administration and length of hospital stay. He stated that switch therapy can be safely implemented when the following 4 criteria are met: (1) cough and respiratory distress improve, (2) fever abates for at least 8 hours, (3) the WBC count is returning to within the reference range, and (4) the patient can take drugs orally. In prospective clinical studies conducted at his institution, the clinical cure rate with switch therapy was 99% and the mean length of hospital stay was reduced by more than 2 days. Early switch, coupled with hospital discharge, may be possible in nearly half of all patients with CAP. Ramirez concluded that universal use of switch therapy in the United States could result in a total reduction of approximately 440,000 hospital days annually and an overall savings of $400 million. [58]
In 1999, Ramirez and colleagues studied early switching to oral antibiotics (within the first 3 d of hospitalization) in 133 patients (67%). Clinical failure was documented in 1 patient. Early switch and early discharge was achieved in 88 patients (44%). The mean length of hospital stay for this group was 3.4 days. The most common reason for prolonged hospitalization after the switch to oral antibiotics was the need for a diagnostic workup. More than 95% of patients were satisfied with the care they had received. Ramirez and colleagues concluded that, based on simple clinical and laboratory criteria, a significant proportion of hospitalized patients with CAP (44%) can be treated with early switch and early discharge. This model did not affect patient outcome, but it did decrease the length of hospitalization and was associated with a high level of patient satisfaction. [59]
Early IV to oral switch protocols can aid in the care and discharge planning in patients with prosthetic hip infection that was treated with 1- or 2-stage replacement. [60]
Implementation of Switch Therapy Protocol
Important questions include how to identify candidates for an early switch and how to effect the IV-to-PO switch. Releasing IV-to-PO switch guidelines alone is not sufficient. Electronic drug-ordering systems have been introduced during the past years, enabling a central computer to provide a daily list of all patients who are on intravenous antibiotics for more than 48 hours and are therefore potential candidates for an IV-to-PO switch. The consulting infectious diseases physician can review these patients' charts and contact the attending physician to investigate whether the patient can indeed be switched to oral therapy. Electronic drug-ordering systems might be a more convenient way to streamline antibiotic prescribing methods.
In 1999, Teich et al reported on their study of a computer program to facilitate switch therapy. They found that physicians agreed to change (or had just changed) the patient's medication from intravenous to oral in 31.7% of cases. [61]
Special Concerns
According to Wilcox, less obvious potential benefits of sequential antimicrobial therapy include fewer intravascular catheter infections because of shorter line-dwell times and less endoluminal contamination. [62] Sequential antimicrobial therapy may also be used as part of a policy to reduce the selective pressure, particularly due to cephalosporin use, for endemic hospital pathogens such as C difficile and extended-spectrum–producing gram-negative bacilli.
Caceres et al found that an in-hospital observation period after a patient is changed to oral treatment is of limited usefulness. In this study, only 1% of patients had evidence of clinical relapse within the study period. Four percent of patients had adverse reactions to their oral antibiotic, none of which was serious. Thus, discharging patients after changing to oral antibiotics could result in savings from avoiding an extra day of hospitalization, amounting to millions of dollars annually in the United States. [63]
A 2010 case report noted elevated tacrolimus levels associated with intravenous azithromycin and ceftriaxone. After the ceftriaxone was discontinued and the intravenous azithromycin was switched to an oral dose, the tacrolimus levels returned to previous levels. [64]
Questions & Answers
Overview
What are the benefits of switching from intravenous to oral therapy?
In which conditions is intravenous-to-oral switch therapy beneficial?
What is the role of clarithromycin in intravenous-to-oral switch therapy?
What is the role of metronidazole in intravenous-to-oral switch therapy?
What is the role of trimethoprim-sulfamethoxazole (Bactrim) in intravenous-to-oral switch therapy?
Which antibiotic therapies are options for intravenous-to-oral switch therapy?
What is the role of fluoroquinolones in intravenous-to-oral switch therapy?
What are the roles of levofloxacin and ofloxacin in intravenous-to-oral switch therapy?
What is the efficacy of levofloxacin and ofloxacin in intravenous-to-oral switch therapy?
What is the role of ciprofloxacin in intravenous-to-oral switch therapy?
What is the role of moxifloxacin in intravenous-to-oral switch therapy?
What is the role of delafloxacin in intravenous-to-oral switch therapy?
What is the efficacy of cephalosporins in intravenous-to-oral switch therapy?
Which macrolides are used in intravenous-to-oral switch therapy?
What is the role of azithromycin in intravenous-to-oral switch therapy?
What is the role of clindamycin (Cleocin) in intravenous-to-oral switch therapy?
What is the role of ertapenem in intravenous-to-oral switch therapy?
What is the role of linezolid in intravenous-to-oral switch therapy?
What are antifungals that can be used in intravenous-to-oral switch therapy?
What is the role of citalopram in intravenous-to-oral switch therapy?
What is the role of doxepin in intravenous-to-oral switch therapy?
What is the role of acetaminophen in intravenous-to-oral switch therapy?
What is the role of acyclovir (Zovirax) in intravenous-to-oral switch therapy?
What is the role of famciclovir and valacyclovir in intravenous-to-oral switch therapy?
What is the efficacy of early implementation of intravenous-to-oral switch therapy?
What are special concerns in intravenous-to-oral switch therapy?
-
The Classification of Community-Acquired Pneumonia