Overview
Immune globulin products from human plasma were first used in 1952 to treat primary immune deficiency. Intravenous immunoglobulin (IVIG) contains the pooled immunoglobulin G (IgG) immunoglobulins from the plasma of approximately a thousand or more blood donors.
IVIGs are sterile, purified IgG products manufactured from pooled human plasma and typically contain more than 95% unmodified IgG, which has intact Fc-dependent effector functions and only trace amounts of immunoglobulin A (IgA) or immunoglobulin M (IgM). [1] Initially, immune globulin products were administered by intramuscular injection. One of biggest advances with IVIG in recent years has been the use of sorbitol-based formulations as opposed to sucrose-based formulations, which allow for IV administration with fewer reactions. [2, 3] IVIG was initially shown to be effective in immune thrombocytopenic purpura (ITP) in 1981. [4] There are now multiple alternative IVIG preparations available for intravenous administration that have been approved by the US Food and Drug Administration (FDA) for the treatment of primary humoral immunodeficiencies and chronic immune thrombocytopenic purpura. Also, a liquid, pasteurized, 10% concentrated intravenous gammaglobulin preparation is as effective as a 5% concentrated preparation, making the concentrated versions more convenient to administer.
The image below is a schematic representation of an immunoglobulin G molecule.
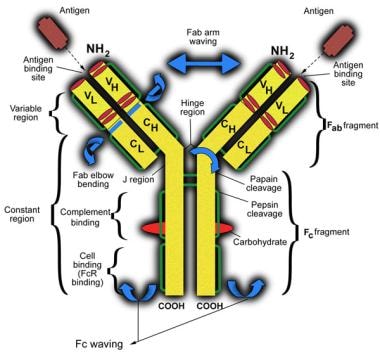 Schematic representation of an immunoglobulin G molecule. CH indicates constant region of heavy chain; CL, constant region of light chain; VH, variable region of heavy chain; and VL, variable region of light chain.
Schematic representation of an immunoglobulin G molecule. CH indicates constant region of heavy chain; CL, constant region of light chain; VH, variable region of heavy chain; and VL, variable region of light chain.
IVIG is an immunomodulating agent that has multiple activities. These include modulation of complement activation; suppression of idiotypic antibodies; saturation of Fc receptors on macrophages; and suppression of various inflammatory mediators, including cytokines, chemokines, and metalloproteinases. [5] Fc receptors are a class of receptors on immune cells that bind to the Fc (constant region) portion of an antibody. The Fc region of IgG facilitates interaction with and signaling through Fc receptors on antigen presenting cells such as phagocytes, B cells, and other cells and with Fc-binding plasma proteins (eg, components of the complement system). [6]
Blockade of macrophage Fc receptors is considered the primary mechanism of action of immune globulin in persons with ITP and other autoantibody-mediated cytopenias. In persons with Kawasaki disease and dermatomyositis, IVIG is thought to inhibit the generation of membrane attack complexes (C5b-C9) and subsequent complement-mediated tissue damage by binding the activated components C3b and C4b, thus preventing their deposition on target surfaces. In persons with dermatomyositis, IVIG induces a decrease in the plasma levels of membrane attack complex and a substantial decrease in the amounts of C3b and membrane attack complex deposited in endomysial capillaries. The high content of anti-idiotypes against autoantibodies in IVIG facilitates its ability to neutralize autoantibodies, as is shown in patients with acquired hemophilia due to autoantibodies against factor VIII. [6]
IVIG also has effects on the clearance of opsonized cells. The results of in vitro C3 uptake studies evaluating the effect of IVIG on the clearance of pre-opsonized cells suggest that IVIG produces a kinetic depression of C3 uptake and modifies the process of complement fragment deposition on erythrocytes.
IVIG also contains natural antibodies. Normal serum contains IgG, IgM, and IgA antibodies, which are referred to as natural antibodies because they are induced without deliberate immunization and are independent of antigenic exposure. They are considered key to the immunoregulatory effects of immune globulin in immune-mediated disorders. [6] Natural autoantibodies appear to be more polyreactive than immune antibodies; natural antibodies can frequently bind to different antigens. [6] Natural autoantibodies can (1) bind to pathogens; (2) help remove senescent or altered molecules, cells, and tumors; (3) induce remyelination; and (4) inhibit the growth of autoreactive B-cell clones. In the multifocal motor neuropathy disease state, IVIG intercedes to stop complement deposition that is triggered by anti-GM1 antibodies. [7] The effect of IVIG could also relate to the presence of natural antibodies. IVIG also contains cytokines, and perhaps neutralizing antibodies; interestingly, antibodies against granulocyte macrophage colony-stimulating factor, interferon, interleukin 1, and interleukin 6 in immune globulin have biologic activity in vivo. [6]
The broad range of applications of IVIG shows the importance of immunoglobulins in the immune homeostasis in healthy people.
Noteworthy to remember is that while IVIG replacement prevents severe and lower respiratory tract infections, it does not prevent upper respiratory tract and non-respiratory infections in persons with common variable immune deficiency. [8] The largest challenge faced in 2018 is identifying those persons with specific diseases who will respond to IVIG and what biomarkers might inform apposite therapy. [9, 10]
Uses of Intravenous Immunoglobulin
IVIG is used to treat various autoimmune, infectious, and idiopathic diseases. IVIG is an approved treatment for multifocal motor neuropathy, chronic lymphocytic lymphoma, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease and ITP. The beneficial effects of an intramuscular injection of immune globulin for the prophylactic treatment of patients with primary immunodeficiency syndromes are well established. The therapeutic effects of IVIG go beyond antibody replacement in those patients with antibody deficiency. The number of inflammatory and autoimmune diseases (especially when it is administered intravenously) for which IVIG is used has expanded enormously. These diverse disorders range from blistering skin diseases to transplant rejection, neurologic diseases. It is widely accepted for use in persons with multiple other diseases including, but not limited to, Guillain-Barré syndrome, multiple myeloma, myasthenia gravis, acquired factor VIII inhibitor syndrome, autoimmune neutropenia, post-transfusion purpura, and polymyositis/dermatomyositis.
However, it does not work for all diseases; for example, a Korean study of 63 patients (ie, folliculitis decalvans hidradenitis suppurativa, folliculitis, furunculosis) with recalcitrant suppurative skin diseases reported that it helped 59% of patients, but with only a 20% success rate in treating hidradenitis. [11]
Diseases that are purely of hematological or clotting factor defects such as Degos disease or paroxysmal nocturnal hemoglobinuria do not respond to IVIG. [12]
The US Food and Drug Administration has approved the use of IVIG for the following conditions [13, 14, 15, 16] :
-
Chronic lymphocytic leukemia
-
Common variable immunodeficiency (CVID) - A group of approximately 150 primary immunodeficiencies (PIDs) that have a common set of features (including hypogammaglobulinemia) but that have different underlying causes
-
Chronic inflammatory demyelinating polyneuropathy (CIDP) - Solely Gamunex
-
Primary immunodeficiency disorders associated with defects in humoral immunity.
-
Immune-mediated thrombocytopenia
-
Kawasaki disease (see the Kawasaki Disease Diagnostic Criteria calculator)
-
Multifocal motor neuropathy
Its reported uses have been outlined by the National Guideline Clearinghouse, including the following (off-label) applications:
-
Hematology
Diamond-Blackfan anemia
Acquired factor VIII inhibitors
Acquired von Willebrand disease
Refractoriness to platelet transfusion
Neonatal alloimmune/autoimmune thrombocytopenia
Posttransfusion purpura
Thrombotic thrombocytopenia purpura/hemolytic uremic syndrome
-
Infectious diseases: Conditions in which acquiring an infectious disease could be deleterious include low birth weight (ie, < 1500 g), solid organ transplantation, surgery, trauma, burns, and HIV infection.
-
Neurology
Myasthenia gravis: IVIG may improves the quality of life in patients; sometimes it is combined with plasmapheresis. [17] A 2014 report noted that IVIG may act as prophylaxis against acute exacerbations. [18]
-
Obstetrics: IVIG may be helpful for recurrent pregnancy loss.
-
Pulmonology
Chronic chest symptoms
-
Rheumatology
Rheumatoid arthritis (adult and juvenile)
Lupus nephritis [19]
Systemic vasculitides
-
Granulomatosis with polyangiitis (formerly known as Wegener granulomatosis): Successful remission induction with the use of IVIG and steroids alone has been described in a woman diagnosed with de novo granulomatosis with polyangiitis during the first trimester of pregnancy. Another report noted that high-dose IVIG could treat severe, corticosteroid-resistant extensive, GCSF-induced Sweet syndrome. [20]
-
Miscellaneous
Adrenoleukodystrophy
Acute cardiomyopathy
Congenital heart block
Autoimmune blistering dermatosis
Acute idiopathic dysautonomia
Endotoxemia
Hemophagocytic syndrome
Lower motor neuron syndrome
Human T-cell lymphotrophic virus-1–associated myelopathy
Nephritic syndrome
Membranous nephropathy
Euthyroid ophthalmopathy
Opsoclonus-myoclonus
Recurrent otitis media
Paraneoplastic cerebellar degeneration
Paraproteinemic neuropathy
Parvovirus infection (general)
Polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome
Progressive lumbosacral plexopathy
Rasmussen syndrome
Reiter syndrome
Thrombocytopenia (nonimmune)
Streptococcal toxic shock syndrome
Additional investigations include the following:
-
Monoclonal gammopathy-associated systemic capillary-leak syndrome, also known as Clarkson disease, is a rare condition characterized by recurrent life-threatening episodes of capillary hyper-permeability in the context of a monoclonal gammopathy. A study by de Chambrun et al was conducted to better describe the clinical characteristics, natural history, and long-term outcome of monoclonal gammopathy-associated systemic capillary-leak syndrome. Since this condition is unpredictable when life threatening attacks occur, a cohort analysis of 69 patients was performed comparing those who received IVIG (48; 74%) and those that did not in a 5-year period. Multivariate analysis found preventive treatment with IVIg [hazard ratio (HR) 0.27 (0.10-0.70), p=0.007] and terbutaline [HR 0.35 (0.13-0.96), p=0.041] to be independent predictors of mortality. This study indicated a possible survival benefit in those who received IVIG as a preventive measure. [21]
-
Mori et al (2007) have found that intravenous immunoglobulin therapy does not change the course of Miller Fisher syndrome when used as treatment. [22]
-
Korber et al (2007) have reported successful therapy of scleromyxedema with low-dose intravenous immunoglobulin. [23]
-
Zinman et al (2007), in a randomized trial of 51 patients with myasthenia gravis, found that IVIG had a positive effect in patients with worsening weakness due to myasthenia gravis. [24]
-
Suchak et al (2007) found that intravenous immunoglobulin is effective as a sole immunomodulatory agent for treating pyoderma gangrenosum unresponsive to systemic corticosteroids. [25]
-
Intravenous immunoglobulins are not recommended as a treatment of atopic dermatitis based on published data.
-
Kerr and Ferguson (2007) have noted type II adult-onset pityriasis rubra pilaris successfully treated with intravenous immunoglobulin. [26]
-
In febrile ulceronecrotic pityriasis lichenoides, high-dose IVIG combined with extracorporeal photochemotherapy can be an effective treatment. [27]
-
Peripheral polyneuropathy linked to Churg-Strauss syndrome was improved by 6 rounds of high-dose IVIG. [28]
-
A clinical trial sponsored by the National Institutes of Health found that a combination of anti-coronavirus hyperimmune IVIG and remdesivir did not result in better outcomes than the use of remdesivir alone in the treatment of hospitalized adults with COVID-19. [29]
Pharmacology and Monitoring
The IVIG that is available contains complete IgG molecules. The IgG subclasses match those in normal human serum. Most preparations contain trace amounts of IgA, which can sensitize IgA-deficient persons during long-term treatment. Immune globulin also contains trace amounts of cytokines, soluble CD4, CD8, and HLA molecules.
Immune globulin is prepared from pooled plasma from 3000-10,000 healthy blood donors. In some instances, blood from as many as 100,000 donors is used. The entire array of variable (antigen-binding) regions of antibodies in normal serum is contained in IVIG. The large number of donors in the pool increases the number of individual antibody activities in the preparation but risks diluting any useful rare activity. Cold alcohol fractionation is used to isolate the immunoglobulin-containing fraction. This is followed by further purification techniques, including additional precipitation procedures to remove the non-immunoglobulin G proteins and ion exchange chromatography to further separate out the desire IgG. Most immune globulin preparations also undergo several specific treatments to inactivate or remove bloodborne pathogens that may be present.
All preparations of IgG have traces of IgA. IgA-deficient patients with severe recurrent viral or bacterial respiratory tract infections or with isolated IgA deficiency (and additional IgG2 and IgG4 deficiency) who may develop severe anaphylactic reactions after an IVIG infusion should receive the first infusion in the hospital under medical supervision.
The World Health Organization has published minimum standards for manufacturing IVIG preparations [30]
-
IVIG should be extracted from a pool of at least 1000 individual donors
-
It should contain as little IgA as possible
-
It should be free from preservatives or stabilizers that might accumulate in vivo
-
The IgG molecules should have as little biochemical modification as possible an possess opsonizing and complement-fixing activities
The following IVIG preparations are marketed in the United States:
-
Gammagard S/D (Baxter/Hyland; Deerfield, Ill)
Lyophilized powder in 5% and 10% concentrations
Sodium content in mEq/mL of 0.85%
Contains IgM in trace amounts
Manufactured by the Cohn-Oncley cold ethanol fractionation process followed by ultrafiltration and ion exchange chromatography; solvent detergent treated; prepared from "a large number of donors"
Additives in 5% solution include 0.3% albumin, 2.25% glycine, and 2% glucose
pH of 6.8
IgA content of 1.6 mcg/mL in 5% solution
-
Gammar-IV (Armour; Blue Bell, Pa)
Lyophilized powder in 5% concentration
Sodium content in mEq/mL of 0.5%
Prepared by cold alcohol fractionation of pooled plasma; not chemically altered or enzymatically degraded; prepared from a large pool of at least 1000 donors
IgA content of less than 25 mcg/mL
-
Gamimune-N (Miles; Elkhart, Ind; Bayer)
Sterile solution in 5% and 10% concentrations
Sodium content in mEq/mL considered trace amount (incompatible in saline)
Not sugar-glycine based
Advanced viral removal and inactivation technologies used in manufacturing; solvent detergent treated
Contraindicated in patients with history of prior systemic allergic reaction to IVIG products or a history of IgA deficiency
pH of 4-4.5
Additives in 5% solution include 9-11% maltose; in 10% solution, 0.16-0.24 M glycine
IgA content of 270 mcg/mL
-
Iveegam (Immuno-US; Rochester, NY)
Lyophilized power in 5% concentration
Sodium content in mEq/mL of 0.3%
Prepared using modified Cohn-fractionation process combined with hydrolase treatment and polyethylene glycol precipitation
Additives in 5% solution include 5% glucose and 0.3% NaCl
pH of 7.0
IgA content of less than 10 mcg/mL
-
Polygam S/D (Baxter/Hyland for American Red Cross; Washington, DC)
Lyophilized powder in 5% and 10% concentrations
Sodium content in mEq/mL of 0.85%
Manufactured by the Cohn-Oncley cold ethanol fractionation process followed by ultrafiltration and ion exchange chromatography; solvent detergent treated; sterile, freeze-dried preparation of highly purified IgG derived from large pools of human plasma
Additives in 5% concentration include 0.3% albumin, 2.25% glycine, and 2% glucose
pH of 6.8
IgA content less than 1.2 mcg/mL
-
Sandoglobulin (Sandoz; Vienna, Austria)
Lyophilized powder in 3%, 6%, 9%, and 12% concentrations
Sterile, highly purified polyvalent antibody product that contains, in concentrated form, all IgG antibodies regularly occurring in donor population
Produced by cold alcohol fractionation from the plasma of volunteer US donors; fractionation process includes several filtration steps performed in presence of filter aids; some filtration steps used for separation of cold ethanol precipitate
Additives per gram of IgG include 1.67 g sucrose and less than 20 mg NaCl
pH of 6.6
IgA content of 720 mcg/mL
-
Venoglobulin-I or Venoglobulin-S (Alpha Therapeutic; Los Angeles, Calif)
Sterile solution in 5% and 10% concentrations
Sodium content in mEq/mL is less than 1
Prepared using cold alcohol fraction, polyethylene glycol bentonite fraction, and ion exchange chromatography; solvent detergent treated
Additives in 5% solution include 5% sorbitol and 0.13% albumin; in 10% solution, 5% sorbitol and 0.26% albumin
pH of 5.2-5.8
IgA content of 15-50 mcg/mL
-
Carimune/Panglobulin (ZLB Bioplasma/ARC Swiss Red Cross)
Lyophilized powder in 3%, 6%, 9%, and 12% concentrations
Sodium content in mEq/mL 0-0.9% depending on preparation
Viral inactivation and preparation involves Kistler-Nitschmann; cold alcohol fractionation using pH of 4.0, trace pepsin, and nanofiltration
pH of Panglobulin is 6.6
IgA content of 720 mcg/mL
-
Gamunex (Bayer)
Sterile solution
Sodium content in mEq considered trace amount (incompatible in saline)
Not sugar-glycine based
Viral inactivation and preparation uses pH 4.25, caprylate, ion exchange chromatography, and low salt
IgA content of 46 mcg/mL
Baxter AG [31] created a new liquid intravenous immunoglobulin product (Immune Globulin Intravenous [IGIV] 10%) using a new manufacturing procedure. Baxter AG used a modified Cohn fractionation and ion exchange chromatography to produce an IgG solution with no alterations to the Fc region.
KIOVIG™ (Immune Globulin Intravenous [Human]) is one name for this product. KIOVIG has obtained marketing authorization from the European Medicines Agency (EMEA). KIOVIG is indicated in the European Union for replacement therapy in primary immunodeficiency disorders (PID), myeloma or chronic lymphocytic leukemia (CLL) with severe secondary hypogammaglobulinemia and recurrent infections, children with congenital AIDS and recurrent infections, Kawasaki syndrome, allogeneic bone marrow transplantation, Guillain-Barré Syndrome, and idiopathic thrombocytopenic purpura (ITP) in children or adults at high risk of bleeding or prior to surgery to correct the platelet count. [32]
The significance of IgA content in these preparations for patients with IgA deficiency is unclear.
Dosing and Administration
IVIG liquid products that have been stored in refrigerators should be allowed to come to room temperature before administration to minimize adverse effects.
IVIG is usually administered at an infusion center or healthcare facility. However IVIG may be infused in the home setting, usually by an experienced infusion nurse.
Patients receiving IVIG should be well hydrated prior to infusion. This is particularly important for patients with risk factors for thrombosis and/or renal complications of IVIG therapy.
Dosing varies upon whether IVIG is administered for the purpose of reducing infections or suppression of an inflammatory of autoimmune process. IVIG doses range from 400 to 800 mg/kg/month are used for immune deficiencies. Doses may be given every three to four weeks. In patients who require auto-inflammatory effects of IVIG, high doses are typically required. This will usually range from 1-2 g/kg as a single dose.
The pharmacokinetic properties of IVIG in healthy persons are well defined and last approximately 22 days; however, in persons with certain illnesses, they can last as few as 6 days.
IVIG therapy should be monitored, and obtaining a history and performing a physical examination, with an emphasis on obtaining information regarding hepatic or kidney disease or a history of reactions to blood products or transfusion reactions, is prudent.
Laboratory tests may include the following:
-
Liver function tests
-
Renal function tests
-
CBC count with differential
-
Hepatitis screen to assess for possible disease transmission by IVIG
-
Immunoglobulin levels to exclude IgA deficiency: If no IgA antibodies are found, then anti-IgA antibody titers should be obtained.
-
Rheumatoid and cryoglobulin levels, because IVIG can cause hematological complications
Finally, store a small amount of serum used before each infusion for analysis in the event of infectious disease transmission.
Intravenous Immunoglobulin and Skin Disease
Because most skin disease has an immunological effect, the fact that IVIG is helpful for treating these conditions is not surprising. It has been reported useful in the treatment of autoimmune blistering diseases, lupus erythematosus, dermatomyositis, scleroderma, toxic epidermal necrolysis, atopic dermatitis, pyoderma gangrenosum, epidermolysis bullosa acquisita, herpes gestationis, erythema multiforme, and chronic autoimmune urticaria. Other uses include treatment for pemphigus foliaceus, mucous membrane pemphigoid, and mixed connective-tissue disease.
IVIG has both agonistic and blocking antibodies against Fas (CD95), the receptor for the Fas ligand, which triggers apoptotic signals into cells. This is likely what underlies its efficacy for treating toxic epidermal necrolysis.
In persons with dermatomyositis, IVIG decreases plasma levels of membrane attack complex and substantially decreases the amounts of C3b and membrane attack complex deposited in endomysial capillaries.
Immune Thrombocytopenia
A prime use of IVIG is in the treatment of hematological diseases. The first description of the treatment of individuals with ITP with IVIG was by Imbach et al in 1981. [4] They noted that dose administration of IVIG promoted a rapid recovery for children with ITP.
Platelet destruction occurs in the spleen. The spleen contains large numbers of Fc receptor (Fc-gamma-R)–bearing phagocytic cells, such as monocytes and macrophages. These cells can bind and destroy opsonized platelets. Although platelets are destroyed in many different organs, splenectomy is a successful treatment for many cases of ITP. In 1982, Fehr et al demonstrated that in cases of ITP without splenectomy, the infusion of IVIG prolonged the clearance of radiolabeled, antibody-sensitized RBCs in vivo. [33]
In 1983, Salama et al suggested that the success of IVIG in the treatment of ITP was due to competitive inhibition of Fc receptors on phagocytic cells within the reticuloendothelial system by sensitized erythrocytes. [34] Possible mechanisms that underlie this are (1) IVIG dimers and multimers, which are present in low but significant levels in preparations of IVIG, bind to Fc receptors and block platelet clearance and (2) IVIG contains IgG molecules that have a multitude of host antigenic reactivities. These IgG molecules likely bind to host antigens, form immune complexes, and compete with antibody-sensitized platelets for Fc receptors in the reticuloendothelial system, resulting in prolonged platelet survival. [35]
Other suggested mechanisms for IVIG include the regulatory properties of an antibody type referred to as antiidiotypic antibodies, ie, antibodies that interact with the antigen-combining region of other antibodies. Others have suggested that competitive inhibition by IVIG-induced immune complexes and opsonized platelets for occupancy of activating Fc-gamma-R in the reticuloendothelial system affects the course of ITP. IVIG also has its own immunological effects on the cellular immune response. These involve expression of interleukins and cytokines and growth arrest of lymphocytes.
The standard dose of IVIG is 400 mg/kg daily for 5 days. [36, 37] A new dosing level is 1 g/kg/d for 2 days, may be more effective. [37]
Kawasaki Disease and Graft Versus Host Disease
In the United States, the recommended treatment for Kawasaki disease (see the Kawasaki Disease Diagnostic Criteria calculator) in the acute phase is a single, high dose of intravenous gammaglobulin (2 g/kg) and a high dose of aspirin (80-100 mg/kg/d).
The therapeutic mechanism of IVIG in Kawasaki disease may be partially due to the reversal of the inhibited lymphocyte apoptosis. [38] Intravenous gammaglobulin has inhibitory effects on platelet adhesion and thrombus formation. Some competitive inhibition between intact IgG and adhesive protein (eg, von Willebrand factor) is suggested, and Fc receptors of the platelet membrane and Fab and Fc receptors of the subendothelium of the vessel wall may have some role in the interaction. IVIG therapy induces neutrophil apoptosis in persons with Kawasaki disease.
High-dose IVIG down-regulates the activated levels of inflammatory indices (except erythrocyte sedimentation rate) in the acute stage of Kawasaki disease.
IVIG has been shown to decrease the severity of acute graft versus host disease in recipients of allogeneic bone marrow transplants. The dosage is 250 or 500 mg/kg/wk. Prevention against acute graft versus host disease with IVIG might be mediated by the induction of apoptosis of activated alloreactive CD4+ CD134+ donor T cells. [39]
Other Uses
Other uses of IVIG are as follows:
-
The effectiveness of IVIG as a treatment for recurrent spontaneous pregnancy loss remains unproven. IVIG does not prevent further losses among women with primary, recurrent, spontaneous pregnancy loss.
-
IVIG is a useful treatment for immunodeficiencies with a greater role than simple replacement therapy. [40]
-
One study suggests that IVIGs are effective in the treatment of pretibial myxedema and may have immunomodulatory action in patients with Graves disease and related disorders.
-
Patients with ataxic sensory neuronopathy with Sjögren syndrome and stiff man syndrome have benefited from IVIG therapy.
-
Selected patients with chronic lymphocytic leukemia who are at risk of bacterial infection can be substantially protected from this complication by regular therapy with IVIG.
-
Chronic idiopathic pericarditis is a chronic disease of unknown origin characterized by recurrent episodes of pericardial inflammation. The cause of the recurrence is unknown, although in some cases it may be traced to a viral infection and to the presence of antimyocardial antibodies. Because a viral infection can induce an autoimmune process through a mechanism of molecular mimicry and because the optimal therapy to prevent recurrences has not been established, Peterlana et al reasoned that treatment with human IVIG could be beneficial for patients who did not respond to previous immunosuppressive therapies. Four patients affected by chronic idiopathic pericarditis benefited from 5 treatments of monthly high-dose human IVIG (0.4 g/kg/d for 5 d) followed by administration every 2 months. Three of 4 patients could permanently discontinue steroid therapy and were still in remission after years of follow-up. [41]
-
The beneficial effects on cardiac function from IVIG treatment in patients with dilated cardiomyopathy is not due to neutralization of antireceptor autoantibody. [42]
-
IVIG and interferon have been reported as a successful treatment for optic neuritis in patients with pediatric multiple sclerosis.
-
Successful management of cataplexy with IVIGs at narcolepsy onset has been reported. [43]
-
IVIG therapy reportedly has been successful in the treatment of acute disseminated encephalomyelitis.
-
IVIG therapy for Alzheimer disease has had mixed success.
-
Patients with Churg-Strauss syndrome have achieved complete clinical and functional recovery with a long-term stable remission with IVIG therapy and plasmapheresis, and the incidence of adverse effects was low. [44]
-
IVIG treatment for the first year from onset of the first neurological event suggestive of demyelinative disease significantly lowers the incidence of a second attack and reduces disease activity as measured by brain magnetic resonance imaging. [45]
-
IVIG was well tolerated and therapy was completed in 9 patients with livedoid vasculitis who were treated with IVIG. In all patients, the clinical evaluation revealed a marked improvement of erythema, pain, and healing of areas of active ulceration. Although this was an open noncontrolled study, the authors propose that IVIG is a promising therapeutic option for livedoid vasculitis refractory to other treatment modalities. [46]
-
High-dose immunoglobulins combined with extracorporeal photochemotherapy were reported in the treatment of one case of febrile ulceronecrotic Mucha-Habermann disease. [27]
-
Although case reports and case series have described dramatic responses to IVIG in people with presumed viral myocarditis, a Cochrane Review concluded that until higher-quality studies have demonstrated benefit in a particular group of patients, IVIG for presumed viral myocarditis should not be provided as routine practice in any situation. [47]
Adverse Effects
Undesirable effects from IVIG are reported to occur in up to 5-15% of all IVIG infusions and to affect 20-50% of individuals receiving IVIG. Most of these are mild, reversible, and transient. The most common adverse effects occur soon after infusions and can include headache, flushing, chills, myalgia, wheezing, tachycardia, lower back pain, nausea, and hypotension. If this happens during an infusion, the infusion should be slowed or stopped. If symptoms are anticipated, a patient can be premedicated with antihistamines and intravenous hydrocortisone. The risk of adverse reactions generally correlates with the dose of IVIG and the rate of the infusion.
-
IVIG can induce reactions in patients with IgA deficiency. This occurs in 1 in 500-1000 patients. Serious anaphylactoid reactions occur soon after the administration of IVIG. Anaphylaxis associated with sensitization to IgA in patients with IgA deficiency can be prevented by using IgA-depleted immune globulin. The presence of IgG anti-IgA antibodies is not always associated with severe adverse reactions to IVIG. [6]
-
Pompholyx (dyshidrotic eczema) and eczematous reactions have been linked to IVIG therapy. [48]
-
An uncommon but potentially irreversible adverse event is acute renal failure. From June 1985 to November 1998, the US Food and Drug Administration received 120 reports worldwide, 88 in the United States, of renal injury. Acute renal failure with IVIG therapy occurs with the sucrose-stabilized formulation, but not with the D-sorbitol–stabilized formulation.
-
IVIG is associated with rare cases of thrombosis. It has caused disseminated intravascular coagulation, transient serum sickness, and transient neutropenia.
-
One study reported 7 patients who had thromboembolic events while being treated with IVIG. [49] Four patients had strokes or transient ischemic attacks, 1 had an inferior wall myocardial infarction, 1 developed deep venous thrombosis, and 1 had a retinal artery infarct. The age range of the patients was 57-81 years, and most had underlying risk factors such as hypertension, hypercholesterolemia, atrial fibrillation, history of vascular disease and stroke, and deep venous thrombosis. Three patients received multiple IVIG infusions before developing the thromboembolic complications. Therefore, clinicians should be vigilant about the possibility of thromboembolic complications with each IVIG infusion and should be especially judicious with the use of IVIG in patients with underlying risk factors.
-
Life-threatening human parvovirus B19 infection and hepatitis C have been transmitted by IVIG.
-
Severe cutaneous vasculitis has been reported following an intravenous infusion of gammaglobulin in a patient with type II mixed cryoglobulinemia.
-
IVIG can precipitate acute myocardial infarction.
-
Aseptic meningitis is a rare but well-recognized complication of IVIG therapy. It manifests as fever, neck stiffness, headache, confusion, nausea, and vomiting.
-
IVIG therapy can result in postinfusion hyperproteinemia, increased serum viscosity, and pseudohyponatremia.
-
IVIG should not be given to patients with sensitivity to thimerosal.
-
IVIG has caused eczematous dermatitis and alopecia.
-
Complement consumption associated with an eczematous cutaneous reaction has been noted during infusions of high doses of IVIG.
-
Orbach et al noted encouraging reports on the efficacy of IVIG in different types of glomerulonephritis (mainly lupus nephritis) resistant to conventional therapy, but the exact success rate and clinical indications remain undetermined. However, the issue of IVIG treatment and renal function is a 2-edged sword because nephrotoxicity can be a serious rare complication of IVIG therapy. Products containing sucrose as a stabilizer are mainly associated with such injury through the mechanism of osmotic nephrosis. Preexisting renal disease, volume depletion, and old age are risk factors for such toxicity. [50]
-
Use of IVIG has been linked to two cases of stroke. [51]
-
Fukuzono et al reported the case of a patient with dermatomyositis who had two episodes of IVIG-induced thrombocytopenia, without hemorrhage. Spontaneous remission occurred in both episodes. [52]
-
Cedars Sinai Medical Center developed a protocol using high-dose (2 mg/kg) IVIG to desensitize transplantation patients who are broadly sensitized to HLA antigens.
Since 2000, 57 broadly sensitized patients (19 with cadaver donor kidneys and 38 with living donor kidneys) have been evaluated and subsequently undergone transplantation following IVIG treatment. The incidence of allograft rejection was 38.5%, and 4-year patient and graft survival rates were 96.5% and 82.5%, respectively.
IVIG has also been used in combination with pulse steroids to treat antibody-mediated rejection episodes in 18 patients with C4d deposition in rejection biopsy specimens. Thirteen responded to treatment and 5 grafts were lost in this group with severe antibody-mediated rejections.
These results suggest that in many cases, high-dose IVIG treatment can neutralize or mitigate antibody responses to eliminate positive donor-specific crossmatches and permit transplantation of broadly sensitized patients, and they suggest a means to successfully treat antibody-mediated rejection. [53]
Questions & Answers
Overview
What is intravenous immunoglobulin (IVIG) used to treat?
What are the formulations of intravenous immunoglobulin (IVIG)?
What is the efficacy of intravenous immunoglobulin (IVIG) for the treatment of ITP?
What is the physiology of intravenous immunoglobulin (IVIG) activation?
What are the specific physiologic effects of intravenous immunoglobulin (IVIG)?
What are the uses of intravenous immunoglobulin (IVIG)?
Which diseases do not respond to intravenous immunoglobulin (IVIG)?
For treatment of which conditions has the FDA approved intravenous immunoglobulin (IVIG)?
What are the ”off-label” uses of intravenous immunoglobulin (IVIG) in hematology?
What are the “off-label” uses of intravenous immunoglobulin (IVIG) in neurology?
What are the “off-label” uses of intravenous immunoglobulin (IVIG) in obstetrics?
What are the “off-label” uses of intravenous immunoglobulin (IVIG) in pulmonology?
What are the “off-label” uses of intravenous immunoglobulin (IVIG) in rheumatology?
What is the efficacy of intravenous immunoglobulin (IVIG) for Wegener granulomatosis?
What are “off-label” uses of intravenous immunoglobulin (IVIG)?
What is the evidence of efficacy of intravenous immunoglobulin (IVIG)?
How is intravenous immunoglobulin (IVIG) prepared?
Which intravenous immunoglobulin (IVIG) preparations are marketed in the US?
How is the liquid intravenous immunoglobulin (IVIG) product KIOVIG prepared?
How is the intravenous immunoglobulin (IVIG) administered to patients?
Which lab tests are indicated in the monitoring of intravenous immunoglobulin (IVIG) therapy?
What is the role of intravenous immunoglobulin (IVIG) in the treatment of skin disease?
What is the role of intravenous immunoglobulin (IVIG) in the treatment of Kawasaki disease?
What is evidence of efficacy for the use of intravenous immunoglobulin (IVIG)?
What are common adverse effects from intravenous immunoglobulin (IVIG) and how are they prevented?
What are less common adverse effects of intravenous immunoglobulin (IVIG)?
What is the role of intravenous immunoglobulin (IVIG) in the treatment of hematologic diseases?
What is the role of intravenous immunoglobulin (IVIG) in the treatment of ITP?
What is the therapeutic action of intravenous immunoglobulin (IVIG) in the treatment of ITP?
What is the standard dose of intravenous immunoglobulin (IVIG) for the treatment of ITP?
-
Schematic representation of an immunoglobulin G molecule. CH indicates constant region of heavy chain; CL, constant region of light chain; VH, variable region of heavy chain; and VL, variable region of light chain.







