Practice Essentials
Infertility is the failure to conceive (regardless of cause) after 1 year of unprotected intercourse. This condition affects approximately 10-15% of reproductive-aged couples.
Female and male factor infertility
Female factors that affect fertility include the following categories:
-
Cervical: Stenosis or abnormalities of the mucus-sperm interaction
-
Uterine: Congenital or acquired defects; may affect endometrium or myometrium; may be associated with primary infertility or with pregnancy wastage and premature delivery
-
Ovarian: Alteration in the frequency and duration of the menstrual cycle—Failure to ovulate is the most common infertility problem
-
Tubal: Abnormalities or damage to the fallopian tube; may be congenital or acquired
-
Peritoneal: Anatomic defects or physiologic dysfunctions (eg, infection, adhesions, adnexal masses)
Male factors that affect fertility include the following categories:
-
Pretesticular: Congenital or acquired diseases of the hypothalamus, pituitary, or peripheral organs that alter the hypothalamic-pituitary axis
-
Testicular: Genetic or nongenetic
-
Posttesticular: Congenital or acquired factors that disrupt normal transport of sperm through the ductal system
Factors that affect the fertility of both sexes include the following:
-
Environmental/occupational factors
-
Toxic effects related to tobacco, marijuana, or other drugs
-
Excessive exercise
-
Inadequate diet associated with extreme weight loss or gain
-
Advanced age
Evaluation of infertility
Infertility is a problem that involves both partners. Diagnostic testing is unnecessary if the couple has not attempted to conceive for at least 1 year, unless the woman is age 35 years or older, or if they have a history of a male factor infertility, endometriosis, a tubal factor, diethylstilbestrol (DES) exposure, pelvic inflammatory disease, or pelvic surgery. A complete infertility evaluation is performed according to the woman's menstrual cycle and may take up to 2 menstrual cycles before the etiology is determined.
Obtain the following medical history and information from the couple:
-
Copy of previous medical records
-
Completed medical history questionnaire
-
Details regarding the type of infertility (primary or secondary) and its duration
-
History of previous pregnancies and their outcomes; pregnancy intervals; and detailed information about pregnancy loss, pregnancy duration, human chorionic gonadotropin (hCG) level, ultrasonographic data, and presence/absence of fetal heartbeat
-
History of previous infertility evaluation/treatment, including details about frequency of intercourse, use of lubricants (eg, K-Y gel) that could be spermicidal, use of vaginal douches after intercourse, and presence of any sexual dysfunction
-
Female menstrual history, frequency, and patterns since menarche, as well as history of weight changes, hirsutism, frontal balding, and acne
-
Male medical history, including previous semen analysis results, history of impotence, premature ejaculation, change in libido, history of testicular trauma, previous relationships, history of any previous pregnancy in female partners, and the existence of offspring from previous female partners
-
Couple’s history of sexually transmitted diseases (STDs); surgical contraception (eg, vasectomy, tubal ligation); lifestyle; consumption of alcohol, tobacco, and recreational drugs (amount and frequency); occupation; and physical activities
-
Couple’s current medical treatment (if any), reason, and any history of allergies
-
Complete review of systems to identify any endocrinologic or immunologic issue that may be associated with infertility
Examination for infertility should include the following:
-
Routine records of blood pressure, pulse rate, and temperature (if applicable)
-
Height/weight findings to calculate body mass index; measure arm span when indicated
-
Head and neck assessment: (1) The presence of exophthalmos can be associated with hyperthyroidism; (2) the presence of epicanthus, lower implantation of ears and hairline, and webbed neck can be associated with chromosomal abnormalities; (3) exclude thyroid gland enlargement/nodules, which may indicate thyroid dysfunction
-
Breast evaluation: Assess breast development and seek any abnormal masses or secretions, especially galactorrhea
-
Abdominal evaluation: Assess for presence of abnormal masses at hypogastrium level
-
Thorough gynecologic evaluation: Assess for hair distribution, clitoris size, Bartholin glands, labia majora/minora, and any condylomata acuminatum or other lesions that could indicate the existence of venereal disease
-
Speculum examination: Obtain a Papanicolaou test and cultures for gonorrhea, chlamydia, Ureaplasma urealyticum,Mycoplasma hominis; assess for cervical stenosis
-
Bimanual examination: Establish direction of the cervix plus size/position of the uterus to exclude the presence of uterine fibroids, adnexal masses, tenderness, or pelvic nodules indicative of infection or endometriosis; assess for defects (eg, absence of vagina and uterus, vaginal septum)
-
Extremities evaluation: Exclude malformation (eg, shortness of fourth finger, cubitus valgus), which can indicate chromosomal abnormalities and other congenital defects
-
Dermatologic evaluation: Assess for the presence of acne, hypertrichosis, and hirsutism
The urologist usually examines the male partner if the patient's history of his semen analysis produces an abnormal finding. Attention should be directed to the following:
-
Congenital abnormalities of the genital tract (eg, hypospadias, cryptorchid, congenital absence of the vas deferens)
-
Testicular size, urethral stenosis, and presence of any varicocele
-
Any previous inguinal hernia repair: Can indicate accidental ligation of spermatic artery
Laboratory, imaging, and/or surgical evaluation
Laboratory, radiologic, and/or surgical assessment of the female includes the following areas:
-
Uterine and endometrial: Hysterosalpingogram—most frequently used diagnostic tool to assess endometrial cavity (see the image below); pelvic ultrasonograms; saline infusion sonograms; pelvic magnetic resonance imaging; hysteroscopy; endometrial biopsy
-
Tubal and peritoneal: Laparoscopy and hysterosalpingogram
-
Ovarian: Progesterone levels and/or serial ultrasonography to assess ovulation; follicle-stimulating hormone and estradiol levels (or antral follicle counts, ovarian volume, inhibin B level, and antimüllerian hormone level) to assess ovarian reserve; clomiphene citrate challenge test for dynamic ovarian reserve testing
Laboratory evaluation of the male partner includes the following:
-
Semen analysis: Volume, pH level, concentration, motility, morphology, WBC count
Treatment of infertility
Treatment plans are based on the diagnosis, duration of infertility, and the woman's age. Management of any underlying female and/or male factors affecting fertility may include medical treatment (eg, pharmacotherapy), surgical intervention, or both.
Assisted Reproductive Technologies
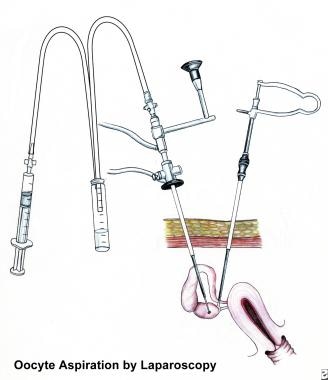
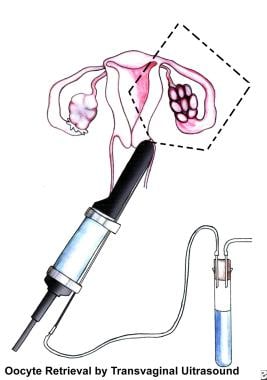
Assisted reproductive technologies used to treat infertility include the following:
-
In vitro fertilization (IVF)
-
Gamete intrafallopian transfer (GIFT)
-
Zygote intrafallopian transfer ZIFT)
-
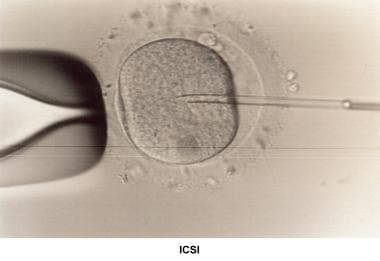
Intracytoplasmic sperm injection (ICSI)
-
Intrauterine insemination (IUI)
-
Sperm, oocyte, or embryo cryopreservation
Assisted fertilization techniques used clinically include ICSI and assisted hatching.
Alternative treatment plans
If pregnancy has not been established within a reasonable time, consider further evaluation and/or an alternative treatment plan, such as use of donor oocyte, sperm, or embryo, or the use of a gestational carrier or surrogate mother.
Overview
Infertility is the failure to conceive (regardless of cause) after 1 year of unprotected intercourse. Infertility affects approximately 10-15% of reproductive-aged couples. Its overall prevalence has been stable during the past 50 years; however, a shift in etiology and patient age has occurred. As a woman's age increases, the incidence of infertility also increases.
Fertility is defined as the capacity to reproduce or the state of being fertile. This term should be differentiated from fecundability, which is the probability of achieving a pregnancy each month, and fecundity, which is the ability to achieve a live birth within 1 menstrual cycle. The fecundability rate in the general reproductive-aged population is fairly constant and is approximately 0.22 per month. [9] The estimated fecundity rate is 0.15-0.18 per month, representing a cumulative pregnancy rate of 90% per year. [10]
In societies where family planning and professional career development are prioritized, some women postpone childbearing until their 30s and beyond. As a result, these women may have more difficulty conceiving and have an increased risk of miscarriage. Because fecundability rates are higher in younger women and lower in older women, counseling a 40-year-old woman to wait a year before seeking fertility services is inappropriate. In women older than 35 years, a complete evaluation after 4-6 months of trying to conceive is prudent because their response to treatment may be suboptimal due to diminished ovarian reserve.
Significant improvements in fertility treatment have made it possible for many patients to conceive with medical assistance. Women with fallopian tube pathology or who have had a prior tubal ligation can conceive with in vitro fertilization (IVF). Men who have very low sperm counts or absence/blockage of the vas deferens but have sperm on a testicular biopsy or epididymal aspiration can have a family using intracytoplasmic sperm injection (ICSI), a technique developed in 1991. Advances in culturing techniques have resulted in improved pregnancy rates using assisted reproductive technologies (ART).
The American Society of Reproductive Medicine (ASRM) has several patient education guides and physician practice guidelines regarding infertility.
For related information, see Medscape's Infertility Resource Center.
Etiology of Infertility
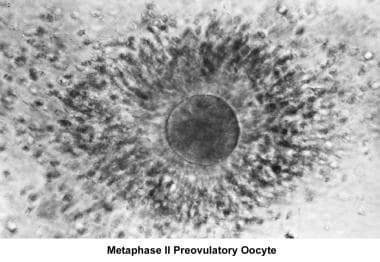
Reproduction requires the interaction and integrity of the female and male reproductive tracts, which involves (1) the release of a normal preovulatory oocyte, (2) the production of adequate spermatozoa, (3) the normal transport of the gametes to the ampullary portion of the fallopian tube (where fertilization occurs), and (4) the subsequent transport of the cleaving embryo into the endometrial cavity for implantation and development.
Infertility is caused by male and/or female factors. Male and female factors each account for approximately 35% of cases. Often, there is more than one factor, with male and female factors combined causing 20% of infertility. In the remaining 10% of cases, the etiology is unknown.
Couples with unknown etiology can be categorized as unexplained infertility or normal infertile couples (NICs), indicating that all findings from standard tests used in the infertility workup are normal. In normal infertile couples, the actual cause for infertility cannot be detected; perhaps there is dysfunctional interaction between the sperm and the oocyte, poor quality of the embryo, or a disruption at the implantation site. In the future, identifying a mutation or the absence of a specific gene as the cause of infertility may be possible in this patient population.
Other lifestyle factors that have been associated with an increased risk of infertility include environmental and occupational factors; toxic effects related to tobacco, marijuana, or other drugs; excessive exercise; inadequate diet associated with extreme weight loss or gain; and advanced age.
Female Factor Infertility
Female factor infertility can be divided into several categories: cervical or uterine, ovarian, tubal, and other. Although stress and distress (anxiety or depression) have been considered factors in reducing pregnancy chances with ART, the number of studies has been limited and considerable between-study heterogeneity is noted. [11]
Cervical factor infertility
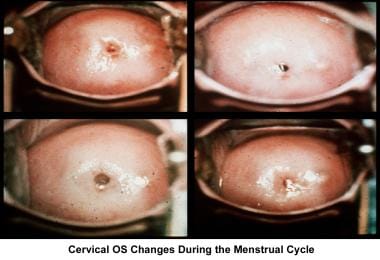
Cervical factor infertility can be caused by stenosis or abnormalities of the mucus-sperm interaction. The uterine cervix plays a pivotal role in the transport and capacitation of the sperm after intercourse. Cervical factors account for 5-10% of infertility. Cervical mucus production and characteristics change according to the estrogen concentration during the late follicular phase.
At the beginning of the menstrual cycle, cervical mucus is scanty, viscous, and very cellular. The mucus forms a netlike structure that does not allow the passage of sperm. Mucus secretion increases during the mid follicular phase and reaches its maximum approximately 24-48 hours before ovulation.
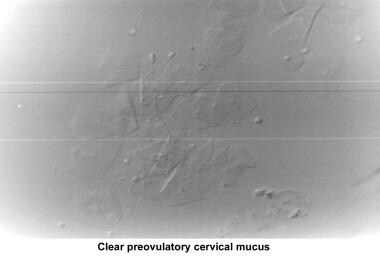
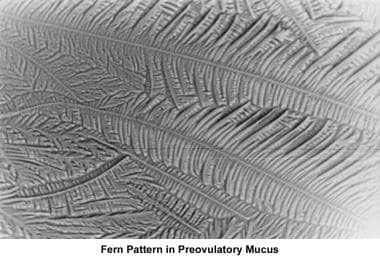
The water and salt concentration increases, changing the physical characteristics of the mucus. The mucus becomes thin, watery, alkaline, acellular, and elastic (spinnbarkheit) because of the increased concentration of sodium chloride, despite a fernlike pattern when the mucus is allowed to dry on a cover slide under the microscope (see the images below).
At this point, the mucus organizes itself, forming multiple microchannels so the spermatozoa can travel through. During this journey, the spermatozoa simultaneously undergo activation and capacitation. [1] In addition, the mucus acts as a filter for abnormal spermatozoa and cellular debris present in the semen.
Mucus secretion may be altered by hormonal changes and medications, especially drugs like clomiphene citrate, which decrease the production. Hypoestrogenism may cause thickened cervical mucus, which impairs the passage of sperm.
Cervical stenosis can cause infertility by blocking the passage of sperm from the cervix to the intrauterine cavity. Cervical stenosis can be congenital or acquired in etiology, resulting from surgical procedures, infections, hypoestrogenism, and radiation therapy.
Uterine factor infertility
The uterus is the final destination for the embryo and the place where the fetus develops until delivery. Therefore, uterine factors may be associated with primary infertility or with pregnancy wastage and premature delivery. Uterine factors can be congenital or acquired. They may affect the endometrium or myometrium and are responsible for 2-5% of infertility cases.
Congenital defects
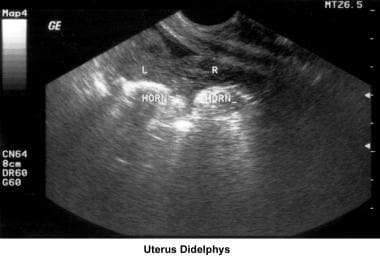
The development of the müllerian ducts accounts for the normal anatomic configuration of the uterus, fallopian tubes, cervix, and upper vagina. The full spectrum of congenital/müllerian abnormalities varies from total absence of the uterus and vagina (Rokitansky-Küster-Hauser syndrome) to minor defects such as arcuate uterus and vaginal septa (transverse or longitudinal).
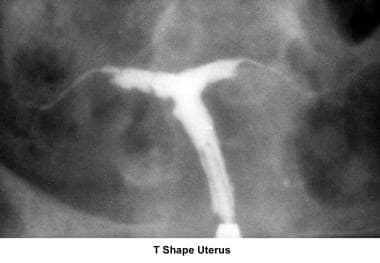
The most common uterine malformations observed during the past 40 years were drug induced. From the late 1950s until the early 1970s, diethylstilbestrol (DES) was used to treat patients with a history of recurrent miscarriages. Years later, DES was found to be responsible for inducing malformations of the uterine cervix, irregularities of the endometrial cavity (eg, T-shaped uterus), malfunction of the fallopian tubes, menstrual irregularities, and the development of clear cell carcinoma of the vagina. [12, 13]
In 1988, the American Fertility Society (AFS) established a new classification of müllerian anomalies. The purpose of this classification was to gather prospective clinical information, to determine its relevance, and to generate future recommendations for patient care. The relationship between müllerian anomalies and infertility is not entirely clear except when absolute absence of the uterus, cervix, vagina, or a combination of these occurs.
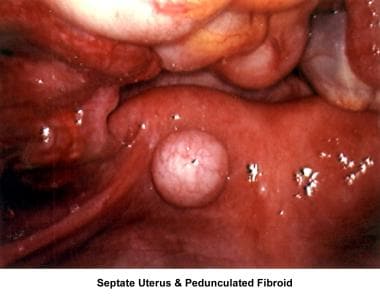
Premature delivery has been associated with cervical incompetence, unicornuate uterus associated with a blind horn, and septate uterus. Septate uterus may also be responsible for implantation problems and first-trimester miscarriages.
Acquired defects
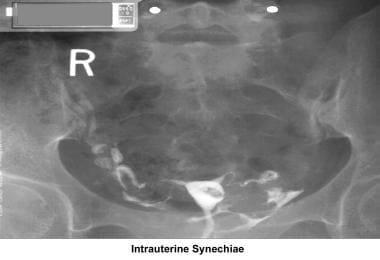
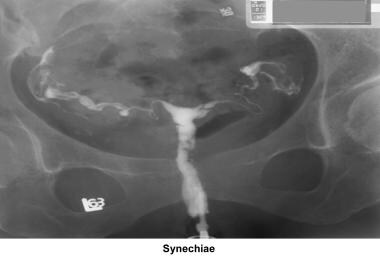
Endometritis associated with a traumatic delivery, dilatation and curettage, intrauterine device, or any instrumentation (eg, myomectomy, hysteroscopy) of the endometrial cavity may create intrauterine adhesions or synechiae (ie, Asherman syndrome), with partial or total obliteration of the endometrial cavity.
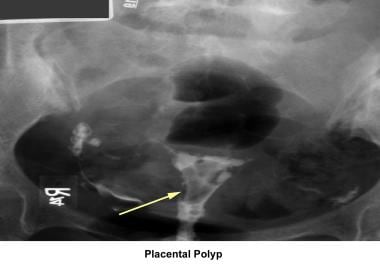
Placental polyps may develop from placental remains.
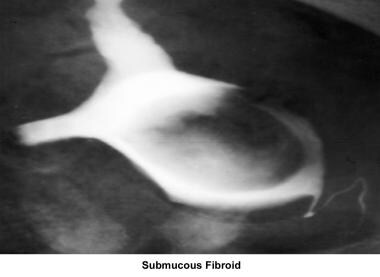
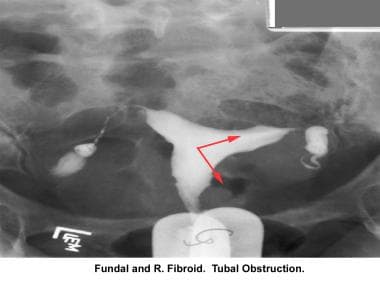
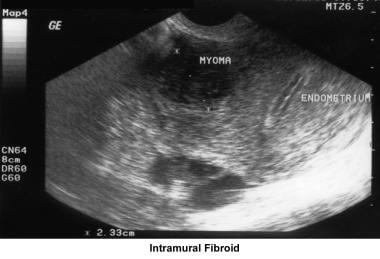
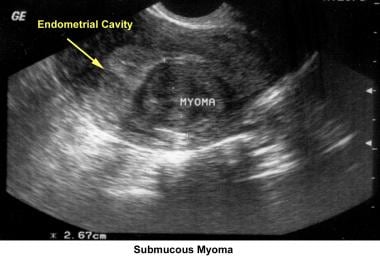
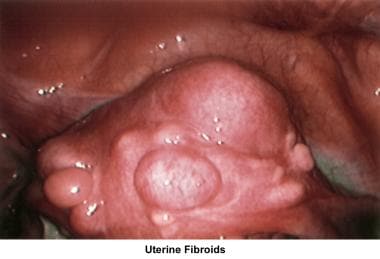
Intrauterine and submucosal fibroids are very common, affecting 25-50% of women. They are more common in women of African descent and can cause distortion of the cavity and compromise the blood supply. They may also be implicated in implantation failure, early miscarriages, premature delivery, and abruptio placentae.
Ovarian factor infertility
Oogenesis occurs in the ovary from the first trimester of embryonic life and is completed by 28-30 weeks of gestation. By then, approximately 7 million oocytes are present. They are arrested at the prophase stage of the first meiosis division. Subsequently, the number of oocytes decreases because of a continuous process of atresia. At birth, the pool of oocytes is reduced to approximately 2 million. By menarche, approximately 500,000 oocytes are present. Those oocytes are used throughout the reproductive years until menopause.
The ovulatory process is initiated once the hypothalamus-pituitary-ovarian axis matures and follicle-stimulating hormone (FSH) and luteinizing hormone (LH), under the regulation of gonadotropin-releasing hormone (GnRH), acquire their normal secretory patterns. From the cohort of follicles available each month, only a single oocyte is selected, establishes dominance, and develops to the preovulatory stage. During follicular development, the granulosa cells secrete increasing amounts of estradiol (E2), initially down-regulating the secretion of FSH. Later, through a positive feedback mechanism, E2 generates the LH surge that triggers the ovulatory process, induces the resumption of meiosis by the oocyte, and stimulates the formation of the corpus luteum and subsequent progesterone secretion.
Ovulatory dysfunction is defined as an alteration in the frequency and duration of the menstrual cycle. A normal menstrual cycle lasts 25-35 days, with an average of 28 days. Failure to ovulate is the most common infertility problem. Absence of ovulation can be associated with primary amenorrhea, secondary amenorrhea, or oligomenorrhea.
Primary amenorrhea is the absence of a spontaneous menstrual period by age 16 years or after 3 years of pubarche and thelarche. [14, 15] Primary amenorrhea in the setting of abnormal puberty can be divided into 2 categories: hypergonadotropic hypogonadism and hypogonadotropic hypogonadism.
Hypergonadotropic hypogonadism is often related to gonadal development failure, as in Turner syndrome, where the karyotype 45,X indicates an absence of an X chromosome. These patients present with sexual infantilism associated with short stature, webbed neck, and cubitus valgus. Streak gonads replace their ovaries, but they have a small uterus and normal fallopian tubes and vagina. This condition is associated with elevated FSH and LH levels and low estrogen levels. Other chromosomal abnormalities include 46,XX, which is associated with partial deletions of the short or long arm of one of the X chromosomes, and mosaicism (eg, X/XXX; X/XX/XXX; pure gonadal dysgenesis; 46,XX; 46,XY). Hypergonadotropic hypogonadism resulting in primary amenorrhea can also be seen in patients with a history of being treated with certain alkylating chemotherapy or pelvic radiation.
Primary amenorrhea also occurs in patients with hypothalamic failure (hypogonadotropic hypogonadism) secondary to inadequate GnRH synthesis, neurotransmitter defects, or isolated gonadotropin insufficiency. Chronic disease conditions, high levels of stress, and starvation or malnutrition are other possible etiologies.
Structural entities associated with primary amenorrhea include congenital absence of the uterus, vagina, or hymen (cryptomenorrhea).
Secondary amenorrhea is the absence of menses for more than 6 months in a woman who has previously menstruated. Pregnancy should always be ruled out first. In the absence of pregnancy, this condition is related to dysfunction of the endocrine system and can be related to thyroid, adrenal, and pituitary disorders, including tumors. One common cause of secondary amenorrhea is premature ovarian failure, which is the loss of ovarian function by the age of 40. For a thorough review of this topic, please see Medscape Reference's article Ovarian Insufficiency.
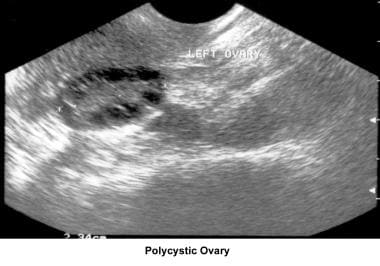
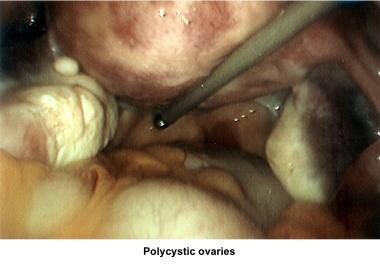
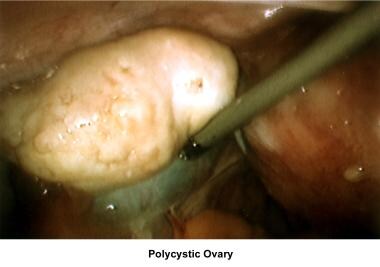
Oligomenorrhea is a dysfunction of the hypothalamus-pituitary-ovarian axis and is the most common ovulatory disorder associated with infertility. Patients with this disorder present with a history of irregular menstrual cycles that fluctuate from 35 days to 2-5 months, sometimes associated with a history of dysfunctional uterine bleeding or prolonged periods of breakthrough bleeding. Patients may have symptoms of hyperandrogenism, acne, hirsutism, and baldness. Obesity is frequently associated and aggravates the prognosis. Although these patients are not sterile, their fertility is decreased, and the obstetrical outcome is poor because of an increased risk of pregnancy loss. Many of these women have polycystic ovarian syndrome (see Medscape Reference article Polycystic Ovarian Syndrome).
Advanced age
The prevalence of infertility rises dramatically as age increases. [16] Furthermore, fertility decreases with marriage duration because of less frequent intercourse and/or the use of contraception. Studies report that among Mormons, fertility appears to be stable until age 36 years, declines slightly until age 40 years, and is followed by a sharp decline after age 42 years. [10, 17] Additionally, in the North American Hutterite population where contraception is condemned, infertility rates are 11% after age 34, 33% after at age 40 and 87% at age 45. [18]
Similar conclusions can be drawn from the experience of many in vitro fertilization (IVF) programs. Chromosomal abnormalities and poor oocyte quality are 2 examples of causes of poor embryonic quality, low implantation rate, increased miscarriage, and low birth rates. [19] Analysis from donor oocyte programs in which the oocytes of younger patients (aged 21-30 y) are used has shown that the pregnancy rate in older recipients is comparable to the pregnancy rate of younger patients undergoing ART. [20]
Tubal factors
The fallopian tubes play an important role in reproduction. After ovulation, the fimbriae pick up the oocyte from the peritoneal fluid that has accumulated in the cul-de-sac. The epithelial cilia transport the oocyte up to the ampulla. The capacitated spermatozoa are transported from the endometrium through the cornual section and advanced through the fallopian tube down into the ampulla, where fertilization occurs. The embryo initiates its early cleaving stages and is propelled upward to arrive at the endometrial cavity at the blastocyst stage (ie, 96-120 h after ovulation).
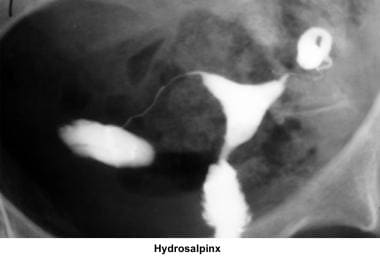
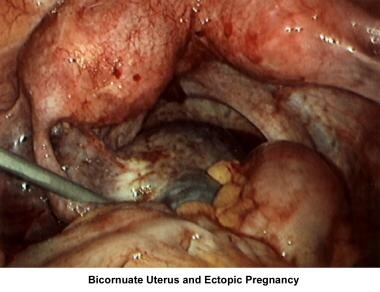
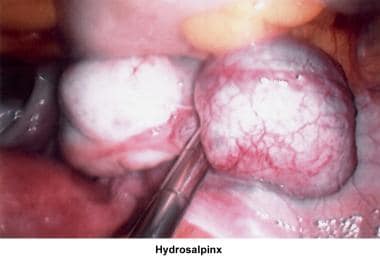
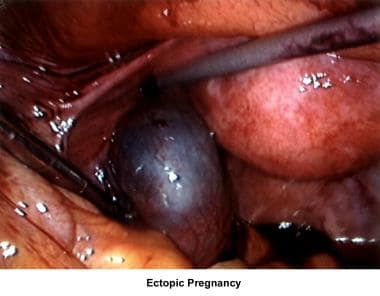
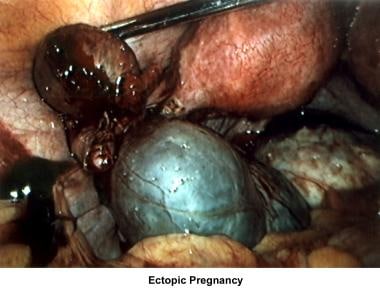
Abnormalities or damage to the fallopian tube interferes with fertility and is responsible for abnormal implantation (eg, ectopic pregnancy). Obstruction of the distal end of the fallopian tubes results in accumulation of the normally secreted tubal fluid, creating distention of the tube with subsequent damage of the epithelial cilia (hydrosalpinx).
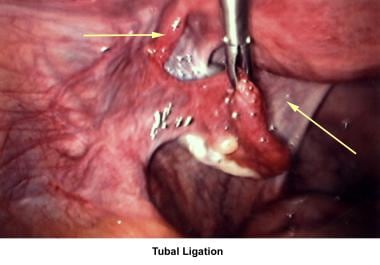
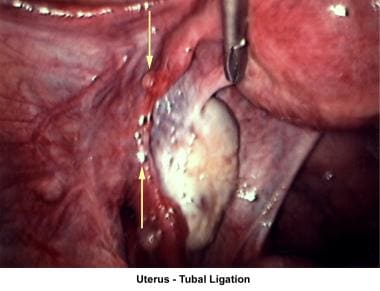
Other tubal factors associated with infertility are either congenital or acquired. Congenital absence of the fallopian tubes can be due to spontaneous torsion in utero followed by necrosis and reabsorption. Elective tubal ligation and salpingectomy are acquired causes.
Peritoneal factors
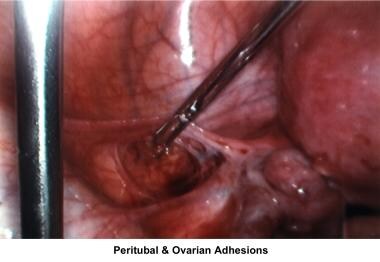
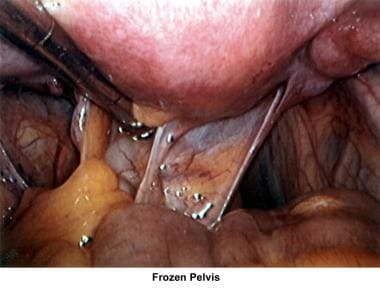
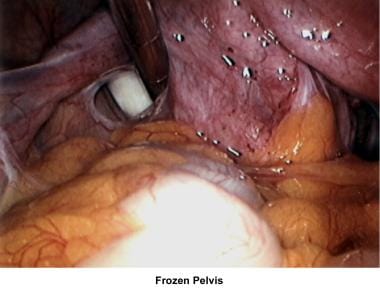
The uterus, ovaries, and fallopian tubes share the same space within the peritoneal cavity. Anatomical defects or physiologic dysfunctions of the peritoneal cavity, including infection, adhesions, and adnexal masses, may cause infertility. Pelvic inflammatory disease, peritoneal adhesions secondary to previous pelvic surgery, endometriosis, and ovarian cyst rupture all compromise the motility of the fallopian tubes or produce blockage of the fimbriae with development of hydrosalpinx. Large myomas, pelvic masses, or blockage of the cul-de-sac interferes with the accumulation of peritoneal fluid and interferes with the normal oocyte pickup mechanism. Peri-ovarian adhesions that encapsulate the ovary interfere with the normal oocyte release at ovulation, becoming a mechanical factor for infertility.
Pelvic inflammatory disease
Pelvic inflammatory disease (PID) has been associated with gonorrhea infection for more than a century. [21] While gonorrhea still plays an important role in tubal disease, it has been surpassed by chlamydia.
Westrom reported a 21% incidence of infertility in a group of Swedish women who were diagnosed with PID, which was confirmed by laparoscopy findings. [22]
The rate of damage to the fallopian tubes increases with subsequent PID episodes, from 34% for the first episode to 54% in women with second and third episodes. [23]
PID can be diagnosed clinically and confirmed by results from cervical culture and serologic antibody assays for gonorrhea and chlamydia. [24, 25, 26]
In many instances, a patient never recalls having had an acute PID episode; however, years later, the incidental finding of tubal obstruction after hysterosalpingogram (HSG) or laparoscopy may be the only indication of previous disease.
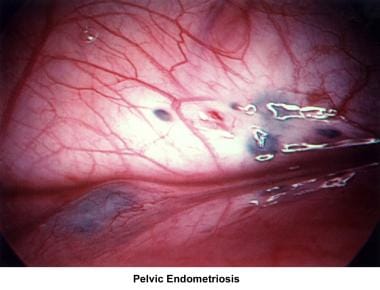
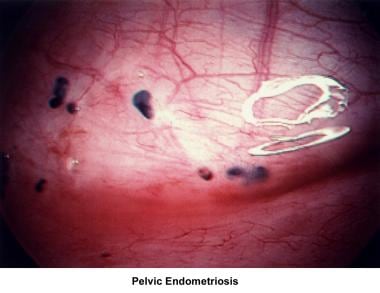
Endometriosis
Endometriosis remains an enigmatic disease that affects women during their reproductive years. The incidence increases with patient age and low parity.
The evolution of the disease is unpredictable. Pelvic pain and reproductive failure are the 2 major complaints of patients with endometriosis. [27, 28]
Although a gene defect has not yet been identified for endometriosis, a genetic link seems probable based on the observation of chromosomal defects in endometriotic tissue and the observation of a 7-fold increased risk of endometriosis in patients with a family history of the disease. [29, 30, 31, 32]
Endometriotic lesions vary from microscopic to macroscopic. Classic endometriosis appears as bluish-black pigments, (ie, "powder-burn lesions") that affect the peritoneal surfaces of the bladder, ovary, fallopian tubes, cul-de-sac, and bowel. Nonclassic endometriosis may appear as red, tan, or white lesions and vesicles. The final diagnosis should be confirmed by demonstrating endometrial stroma and glands in biopsy tissue. [33, 34]
The incidence of endometriosis in primary and secondary infertility varies according to the population studied. Mahmood reported an incidence rate of 26% and 13%, respectively. [35]
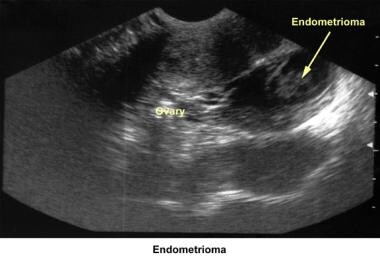
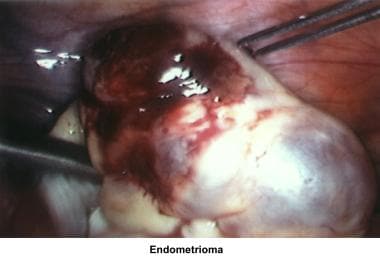
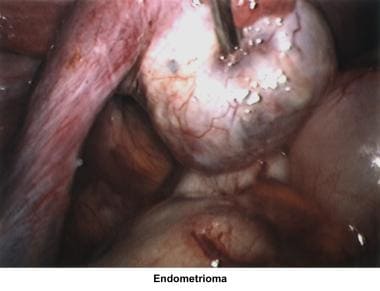
Severe endometriosis with damage to the fallopian tubes and ovaries due to adhesions or the presence of endometriomas is an obvious cause of infertility. The hypothesis that minimal and mild endometriosis cause infertility is controversial. Several studies failed to prove an increased pregnancy rate after treatment or expectant therapy. [36, 37, 38, 39]
Minimal and mild endometriosis is hypothesized to reduce fertility by the following mechanisms: [40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53]
-
Decreased sperm binding to the zona pellucida
-
Proliferation of peritoneal lymphocytes
-
Increased cytokinin levels
-
Increased immunoglobulin production
-
Embryo toxic serum
-
Defective natural killer activity
Endometriosis has been associated with ovulatory disorders such as luteal phase deficiency (LPD), oligo-ovulation, and luteinized unruptured follicle (LUF) syndrome. [54] Although pelvic pain appears to be a common symptom of endometriosis, in some patients, endometriosis is an incidental finding discovered during diagnostic laparoscopy for evaluation of infertility. [55, 56, 57, 58]
Male Factor Infertility
Male factor infertility can be divided into pretesticular, testicular, and posttesticular etiologies. For a more thorough review of male factor infertility, see Medscape Reference’s article Male Infertility.
In a prospective study of 105 couples undergoing in vitro fertilization (IVF), pregnancy rates were lower among couples in which the male had been exposed to high levels of the environmental pollutant bisphenol A (BPA). BPA levels above 3 ng/mL were labeled as high, while levels under 3 ng/mL were categorized as low. [59] Mean BPA levels were 3.9 ng/mL in men and 3.1 ng/mL in women. The implantation rate for men was significantly higher in the low BPA group than in the group with high BPA (48.4% vs 24.4%, respectively). [59]
In women, no differences in fertility parameters, including in the number of retrieved oocytes, the fertilization rate, and the implantation rate, were seen between BPA groups, but peak estradiol was higher in the low BPA group than in the group with high BPA (1412.7 vs 1150.1 pg/mL, respectively). [59]
Pretesticular factors
Pretesticular causes of infertility include congenital or acquired diseases of the hypothalamus, pituitary, or peripheral organs that alter the hypothalamic-pituitary axis. Such disorders include idiopathic hypogonadotrophic hypogonadism, prolactinomas, gonadotropin deficiencies, and Cushing syndrome.
Testicular factors
Testicular factors can be genetic or nongenetic in nature. Klinefelter syndrome is the most common chromosomal cause of male infertility and results in primary testicular failure. Nongenetic etiologies include drugs, radiation, infections, trauma, and varicoceles.
Aging also affects male fertility. As a man ages, testosterone levels decrease, gonadotropin levels increase, sperm concentration and semen volume change, and libido decreases. In addition, the incidence of birth defects increases. While age affects female fertility dramatically, males are not affected as much; anecdotal reports exist of men fathering children well into their 80s.
Posttesticular factors
Posttesticular factors are those that do not allow the normal transport of sperm through the ductal system. Such factors can be congenital or acquired. Men who were exposed to DES in utero may have ductal obstruction. Congenital bilateral absence of the vas deferens is seen in men with cystic fibrosis. Additionally, infections, surgical procedures, and trauma may cause ductal blockage.
Factors Affecting Both Sexes
Environmental and occupational factors
Concern regarding the impact of environmental factors on fertility is increasing. Published semen analysis reports from 1985 confirm a 20% decrease of sperm concentration compared with reports published in the 1960s. [60]
Excessive radiation damages the germinal cells. Exposure to lead, other heavy metals, and pesticides has also been associated with male infertility. [61, 62] Many other factors, such as excessive heat exposure, microwave radiation, ultrasonography, and other health hazards are controversial as infertility-inducing factors. [63]
Toxic effects related to tobacco, marijuana, and other drugs
Smoking has been associated with infertility in both males and females. [64, 65, 66] In experimental animals, nicotine and polycyclic aromatic hydrocarbons block spermatogenesis and decrease testicular size. In women, tobacco alters the cervical mucus and the cilial epithelium and affects gamete transport. [67, 68]
Marijuana and its metabolite, delta-9-tetrahydrocannabinol, inhibit the secretion of LH and FSH, thus inducing ovulatory disorders and luteal phase dysfunction in women. [69] Marijuana use affects males by decreasing the sperm count and the quality of the sperm. Heroin, cocaine, and crack cocaine use induces similar effects but places the user at increased risk for pelvic inflammatory disease and HIV infection.
Chronic alcoholism may induce ovulatory dysfunction, therefore impacting fertility. Alcohol use by males interferes with the synthesis of testosterone and has an impact on sperm concentration. Alcoholism may inhibit sexual response and cause impotence.
Exercise
Exercise should be encouraged as part of normal activity. However, compulsive exercise is deleterious, especially for long-distance runners. Jogging stimulates the secretion of endorphins; excessive secretion of endorphins interferes with the normal production of FSH and LH, in turn inducing ovulatory disorders and luteal phase dysfunction, which accounts for lack of embryo implantation and first-trimester miscarriages. [70, 71] In males, exercise has been associated with oligospermia.
Inadequate diet associated with extreme weight loss or gain
Obesity is a major health issue in the United States. Weight has an impact on fertility at either extreme. Although weight loss associated with anorexia nervosa or bulimia induces hypothalamic amenorrhea, obesity may be associated with anovulation and oligomenorrhea. In men, obesity has been associated with decreased sperm quality. [72]
General Guidance on Evaluation of Infertility
Infertility is a problem that involves both partners. The consultation is incomplete if only the woman is evaluated. Anxiety is very common, and many couples seek consultation after a few months of unprotected intercourse. Diagnostic testing is unnecessary if the couple has not attempted to conceive for at least 1 year, unless the woman is 35 years old or older, or they have a history of a male factor infertility, endometriosis, a tubal factor, DES exposure, pelvic inflammatory disease, or pelvic surgery. A brief explanation of the physiology of reproduction and reassurance are usually enough to lessen the anxiety of the couple.
History
The couple should provide a copy of their previous medical records and complete a medical history questionnaire. Ideally, this should be submitted in advance of the initial consultation. During the consultation, the following actions should be taken:
-
Obtain a detailed medical history regarding the type of infertility (primary or secondary) and its duration.
-
Obtain a history of previous pregnancies and their outcomes; interval between pregnancies; and detailed information about pregnancy loss, duration of pregnancy, human chorionic gonadotropin (hCG) level, ultrasonographic data, and the presence or absence of a fetal heartbeat.
-
During the history of previous infertility evaluation and treatment, specific questions should address the issues of frequency of intercourse, use of lubricants (eg, K-Y gel) that could be spermicidal, use of vaginal douches after intercourse, and the presence of any sexual dysfunction such as anorgasmia or dyspareunia. (See Medscape Reference article Male Anorgasmia and Female Anorgasmia.)
-
Question female patients about their menstrual history, frequency, and patterns since menarche. A history of weight changes, hirsutism, frontal balding, and acne should also be addressed.
-
Ask male patients about previous semen analysis results, history of impotence, premature ejaculation, change in libido, history of testicular trauma, previous relationships, history of any previous pregnancy, and the existence of offspring from previous partners.
-
Ask the couple about their history of sexually transmitted diseases (STDs); surgical contraception (eg, vasectomy, tubal ligation); lifestyle; consumption of alcohol, tobacco, and recreational drugs (amount and frequency); occupation; and physical activities.
-
Ask the couple whether they are currently under medical treatment, the reason, and whether they have a history of allergies.
-
A complete review of systems may be helpful to identify any endocrinological or immunological problem that may be associated with infertility.
Physical
A physical examination should be completed if one has not been recently performed by a gynecologist. Some health insurance carriers designate or include an obstetrician/gynecologist as a patient's primary care physician. Note the following:
-
Routine records of blood pressure, pulse rate, and temperature (if applicable) are needed.
-
Measure height and weight to calculate the body mass index, and measure arm span when indicated.
-
Perform an eye examination to establish the presence of exophthalmos, which can be associated with hyperthyroidism.
-
The presence of epicanthus, lower implantation of the ears and hairline, and webbed neck can be associated with chromosomic abnormalities.
-
Carefully evaluate the thyroid gland to exclude gland enlargement or thyroid nodules.
-
Perform a breast examination to evaluate breast development and to seek abnormal masses or secretions, especially galactorrhea. Take the opportunity to educate patients about breast self-examination during the early days of their menstrual cycles.
-
The abdominal examination should be directed to the presence of abnormal masses at the hypogastrium level.
-
A thorough gynecologic examination should include an evaluation of hair distribution, clitoris size, Bartholin glands, labia majora and minora, and any condylomata acuminatum or other lesions that could indicate the existence of venereal disease. The inspection of the vaginal mucosa may indicate a deficiency of estrogens or the presence of infection. The evaluation of the cervix should include a Papanicolaou test and cultures for gonorrhea, chlamydia, Ureaplasma urealyticum, and Mycoplasma hominis.
-
Bimanual examination should be performed to establish the direction of the cervix and the size and position of the uterus to exclude the presence of uterine fibroids, adnexal masses, tenderness, or pelvic nodules indicative of infection or endometriosis.
-
The examination of the extremities is important to rule out malformation, such as shortness of the fourth finger or cubitus valgus, which can be associated with chromosomal abnormalities and other congenital defects. Examine the skin to establish the presence of acne, hypertrichosis, and hirsutism.
The urologist usually examines the male partner if the patient's history of his semen analysis produces an abnormal finding. Note the following:
-
Attention should be directed to congenital abnormalities of the genital tract (eg, hypospadias, cryptorchid, congenital absence of the vas deferens).
-
Testicular size, urethral stenosis, and presence of varicocele are also determined.
-
A history of previous inguinal hernia repair can indicate an accidental ligation of the spermatic artery.
Comprehensive Evaluation of Infertility
Evaluation of infertile couples should be organized and thorough. Diagnostic tests should progress from the simplest (eg, pelvic ultrasonography) to the more complex and invasive (eg, laparoscopy). The couple may be stressed by their need to seek medical intervention; therefore, to relieve anxiety, emphasize that a complete infertility evaluation is performed according to the woman's menstrual cycle and may take up to 2 menstrual cycles before the etiology is determined.
Evaluation of the Female Partner
Several congenital or acquired conditions affect female reproductive function. These conditions alter the anatomy and/or physiology of reproduction, thereby impairing the transport of the gametes or embryos and/or disrupting implantation and embryo/fetal development.
A complete evaluation of the female reproductive tract must include cervical, uterine, endometrial, tubal, peritoneal, and ovarian factors.
Cervical factors
The postcoital test (PCT), also known as the Sims-Huhner test, [1, 2, 3, 4] consists of evaluating the amount of spermatozoa and its motility within the cervical mucus during the preovulatory period. While of historical interest, this test is no longer routinely performed in the standard infertility workup because it has been shown to have limited diagnostic potential and poor predictive value. Its use has been associated with increased testing without improvement in pregnancy rates. Furthermore, cervical factor infertility is easily addressed by performing intrauterine inseminations.
Cervical stenosis can be diagnosed during a speculum examination. Complete cervical stenosis is confirmed by the inability to pass a 1-2 mm probe into the uterine cavity.
Uterine factors
Many defects can be detected during the pelvic examination. These include absence of the vagina and uterus, vaginal septum, and the presence of fibroids. Detection of most defects requires ancillary studies such as HSG, pelvic ultrasonography, hysterosonogram, and MRI. Operative procedures such as laparoscopy and hysteroscopy are often necessary for confirmation of the final diagnosis.
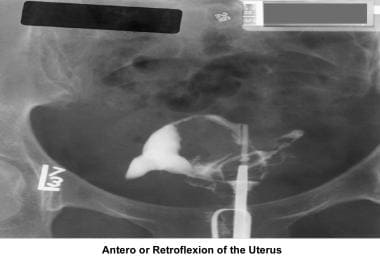
Hysterosalpingogram
The HSG is the most frequently used diagnostic tool to evaluate the endometrial cavity. Some have tried to displace the role of HSG in the evaluation of infertility; however, a meticulous and well-executed procedure, performed under fluoroscopy, provides accurate information about the (1) endocervical canal; (2) diameter and configuration of the internal os; (3) endometrial cavity; (4) uterine/tubal junction (cornual ostium); (5) diameter, location, and direction of the fallopian tubes; (6) status of the fimbriae; and (7) spill into the endometrial cavity. Furthermore, the HSG provides indirect evidence of pelvic adhesions and uterine, ovarian, or adnexal masses. [73]
The HSG should be performed during the early follicular phase. At this time, the endometrium is thin and the HSG provides better delineation of minor defects. In addition, the possibility of accidental irradiation to the fetus in an undiagnosed pregnancy is eliminated.
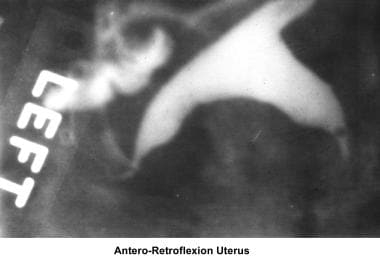
The cervix is cleansed with a povidone-iodine solution (Betadine) to avoid the transfer of bacteria to the endometrial cavity during the procedure. A breakaway vaginal speculum is used so it can be removed before injection of the radiopaque medium. A single-tooth tenaculum is used to apply traction of the uterus and to correct any anteroflexion or retroflexion that yields suboptimal images. A Jarcho-type metal cannula with a plastic adjustable acorn or a balloon HSG catheter is used for the injection of radiocontrast media. The use of water-based contrast media is preferable to oil-based media to avoid the risks of oil embolism and granuloma formation. The images below provide further information.
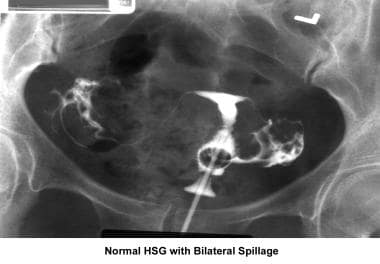 Infertility. Hysterosalpingogram image demonstrating normal findings with bilateral spillage. Image courtesy of Jairo E. Garcia, MD.
Infertility. Hysterosalpingogram image demonstrating normal findings with bilateral spillage. Image courtesy of Jairo E. Garcia, MD.
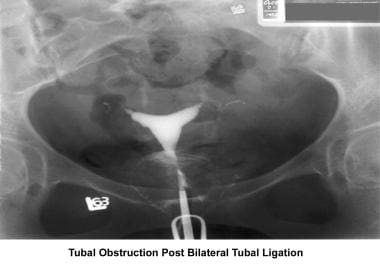 Infertility. Tubal obstruction post–bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
Infertility. Tubal obstruction post–bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
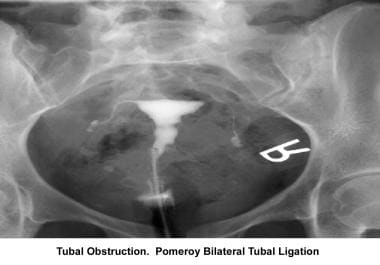 Infertility. Tubal obstruction: Pomeroy bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
Infertility. Tubal obstruction: Pomeroy bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
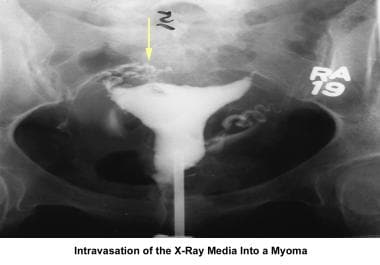 Infertility. Intravasation of the contrast medium due to myoma. Image courtesy of Jairo E. Garcia, MD.
Infertility. Intravasation of the contrast medium due to myoma. Image courtesy of Jairo E. Garcia, MD.
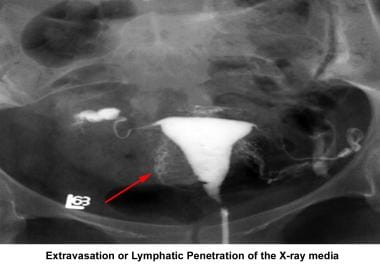 Infertility. Extravasation or lymphatic penetration of the contrast medium. Image courtesy of Jairo E. Garcia, MD.
Infertility. Extravasation or lymphatic penetration of the contrast medium. Image courtesy of Jairo E. Garcia, MD.
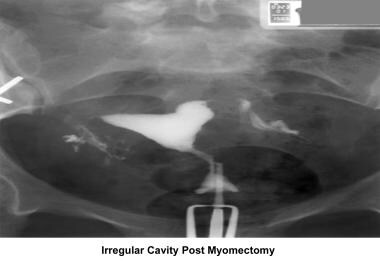
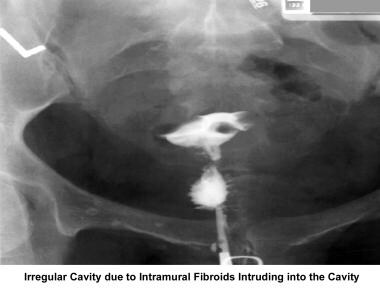 Infertility. Irregular cavity due to intramural fibroids intruding into the cavity. Image courtesy of Jairo E. Garcia, MD.
Infertility. Irregular cavity due to intramural fibroids intruding into the cavity. Image courtesy of Jairo E. Garcia, MD.
Ultrasonography
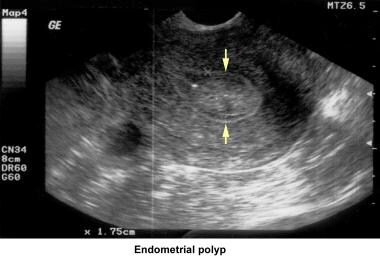
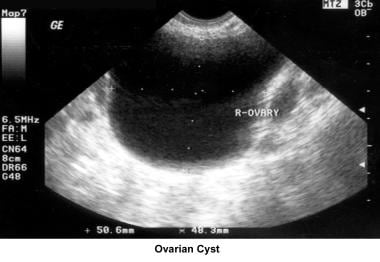
In the 1980s, pelvic ultrasonography became an important tool in the evaluation and monitoring of infertile patients, especially during ovulation induction. Pelvic ultrasonography should be part of the routine gynecologic evaluation because it allows a more precise evaluation of the position of the uterus within the pelvis and provides more information about its size and irregularities. Pelvic sonograms also help in the early detection of uterine fibroids, endometrial polyps, ovarian cysts, adnexal masses, and endometriomas
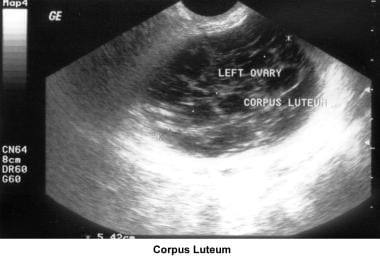
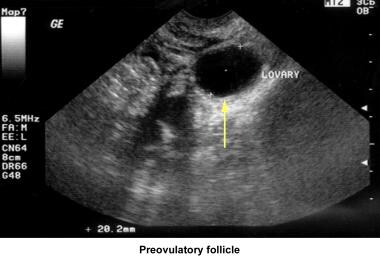
Ultrasonography can also assist in the diagnosis of anovulation, polycystic ovaries, and persistent corpus luteum cysts. Some common ultrasonographic findings are depicted in the images below.
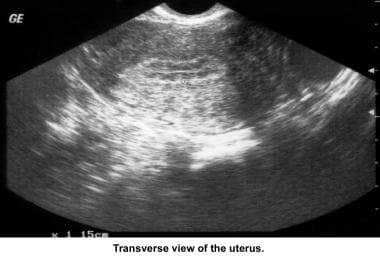
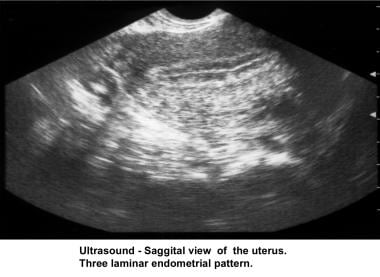 Infertility. Sonogram: Sagittal view of the uterus. Three-laminar endometrial pattern. Image courtesy of Jairo E. Garcia, MD.
Infertility. Sonogram: Sagittal view of the uterus. Three-laminar endometrial pattern. Image courtesy of Jairo E. Garcia, MD.
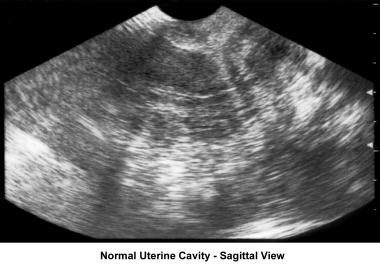 Infertility. Sonogram: Sagittal view of normal uterine cavity. Image courtesy of Jairo E. Garcia, MD.
Infertility. Sonogram: Sagittal view of normal uterine cavity. Image courtesy of Jairo E. Garcia, MD.
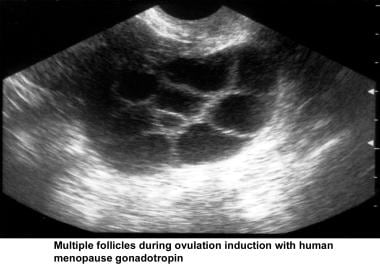 Infertility. Multiple follicles during ovulation induction with human menopause gonadotropin. Image courtesy of Jairo E. Garcia, MD.
Infertility. Multiple follicles during ovulation induction with human menopause gonadotropin. Image courtesy of Jairo E. Garcia, MD.
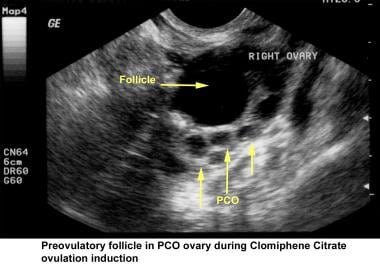 Infertility. Preovulatory follicle in polycystic ovary during clomiphene citrate ovulation induction. Image courtesy of Jairo E. Garcia, MD.
Infertility. Preovulatory follicle in polycystic ovary during clomiphene citrate ovulation induction. Image courtesy of Jairo E. Garcia, MD.
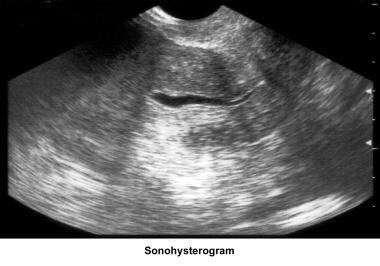
Saline infusion sonography
Saline infusion sonography (SIS) provides a simple and inexpensive means by which to evaluate the uterine cavity and assess tubal patency. It is well-tolerated by patients and can be done in the office. Additionally, it eliminates the risks associated with the use of dye and radiation required by the HSG. SIS has been shown to reveal a substantial percentage of infertile patients with intracavitary abnormalities and uterine anomalies. [74] See the image below.
The SIS should be performed during cycle days 6-12 so that the endometrium is thin, allowing better detection of intrauterine lesions. In addition, this ensures that an undiagnosed pregnancy is not disrupted.
A breakaway speculum is placed and the cervix is cleansed with Betadine solution. A transcervical catheter with acorn or balloon is placed. The speculum is removed and saline is injected under ultrasonographic visualization. Longitudinal and transverse views of the cavity are evaluated for filling defects. Finally, a small amount of air bubbles are injected to assess tubal patency.
If the patient has a history of genital tract infection or pelvic inflammatory disease, antibiotics may be given before the procedure. If hydrosalpinges are noted, antibiotics are given after the procedure.
While the SIS can confirm tubal patency, it does not provide information about the contour of the tubes. Thus, if a patient has a history of endometriosis or other tubal disease, an HSG would be preferred.
Magnetic resonance imaging
The use of MRI has increased in recent years, although it should be limited to those patients in whom a definitive diagnosis cannot be ascertained by conventional HSG, ultrasonography, and hysteroscopy findings.
MRI is useful for delineating complex pelvic masses and for assisting in the diagnosis of such conditions as congenital malformations related to cryptomenorrhea and absence of the cervix. [75]
Hysteroscopy is a method of direct visualization of the endometrial cavity. The instrument used has evolved from the historical cystoscope and is based on the same principles. [76, 77, 78, 79] The technology has changed substantially and now uses optical devices, video camera-enhanced images, and television monitors, which allow more efficient participation and coordination of other members of the operating room team.
The use of glycine and sorbitol solutions, different from the classic Hyskon, administered under constant pressure using an automatic pump, improves imaging resolution and is less risky to the patient. The diameter of the device has become smaller, making it more user friendly; thus, the procedure can be performed in the physician's office using local anesthesia (ie, paracervical block).
Carbon dioxide hysteroscopy is for diagnostic purposes only and requires a constant flow of carbon dioxide. It does not require cervical dilation and allows a rather easy evaluation of the endometrial cavity. [80]
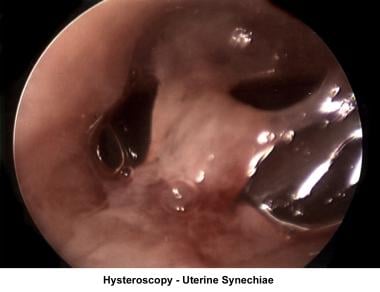
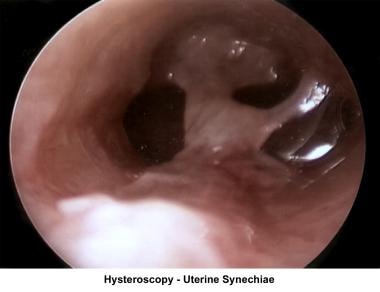
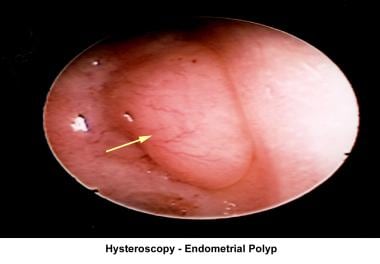
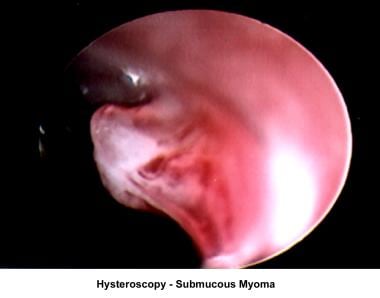
The operative hysteroscope has been designed based on the resectoscope principle. [81] It allows both the diagnosis and treatment of endometrial pathology. The design of refined instruments (eg, scissors, cautery loops, lasers) facilitated the treatment of pathologies such as uterine synechiae, endometrial polyps, submucous myomas, and the removal of foreign bodies (eg, intrauterine devices). In combination with specially designed catheters, it can be used to perform tubal cannulation. [82, 83, 84] The images below show some of the common hysteroscopy findings.
Endometrial biopsy
The endometrial lining constantly responds to the different hormonal secretions that occur during the menstrual cycle or to the exogenous administration of estrogen and progesterone. In the 1950s, Novack and Noyes published their findings on the microscopic changes of the endometrium throughout the menstrual cycle and established the criteria for endometrial dating. [85]
Jones first described the luteal phase dysfunction and its association with recurrent pregnancy loss. A luteal phase dysfunction diagnosis is based on the lack of correlation between (1) endometrial development, diagnosed using premenstrual endometrial biopsy, and (2) the onset of the immediate menstrual cycle. [86] To fulfill the diagnostic criteria, more than 2 days' difference must exist between the endometrial date and the beginning of the next menstrual period; furthermore, the same findings should be repeated in 2 consecutive menstrual cycles. [87]
A large, multicenter prospective study showed that out-of-phase biopsy results poorly discriminated between women from fertile and infertile couples in either the midluteal or late luteal phase. Therefore, histological dating of the endometrium does not discriminate between women of fertile and infertile couples and should not be used in the routine evaluation of infertility. [88]
Tubal and peritoneal factors
The 2 most frequent tests used for diagnosis of tubal pathology are laparoscopy and hysterosalpingogram.
Laparoscopy
The laparoscope is one of the greatest developments in gynecologic instrumentation. Its origin dates to the pioneering work of Jacobaeus in 1910. [89] The laparoscope was first used to visualize the pelvic cavity. The procedure was abandoned in the 1930s because of fatal complications.
In the 1950s, a new generation of laparoscope was developed using a fiberoptic technique; later, safer electrocautery techniques resurrected the application and use of operative laparoscopy, especially for sterilization purposes and for diagnosis of ectopic pregnancy. [90] In 1970, Semm advanced the field of operative laparoscopy with the development of numerous accessory instruments. [91, 92] Semm opened the doors to new surgical applications and forever changed the traditional way of practicing gynecologic surgery. [93]
Laparoscopy is not part of the routine infertility evaluation. It is used when abnormalities are found on ultrasonography, HSG, or suspected by symptomology. Because of the added risks of surgery, need for anesthesia, and operative cost, it is only used when clearly indicated.
Laparoscopy is contraindicated in patients with probable bowel obstruction (ileus) and bowel distention, cardiopulmonary disease, or shock due to internal bleeding. Because of the risk of bowel perforation, uterine and pelvic vessel injury, and bladder trauma, a skilled and experienced surgeon must perform the procedure. Relative contraindications include massive obesity, large abdominal mass or advanced pregnancy, severe pelvic adhesions, and peritonitis.
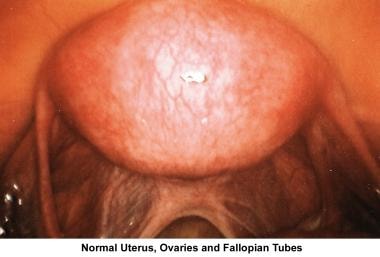
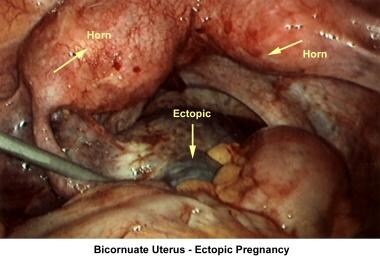
The images below show laparoscopic findings associated with infertility.
Ovarian factors
Ovulation
Ovulation is usually inferred when a woman reports regular cycles. If there is doubt, a progesterone greater than 4 ng/mL is indicative of ovulation. Sonographic confirmation of follicle rupture with serial ultrasonography can also be performed.
Basal body temperature charts can be used to predict ovulation. A basal body thermometer measures the slight rise in temperature that occurs immediately after ovulation. However, most patients and physicians prefer to use urinary ovulation predictor kits as they are more accurate and easier to administer.
Ovarian reserve
The level of ovarian reserve and the age of the female partner are the most important prognostic factors in the fertility workup.
Ovarian reserve is most commonly evaluated by checking a cycle day 3 FSH and estradiol level. Normal ovarian function is indicated when the FSH level is less than 10 mIU/mL and the estradiol level is less than 65 pg/mL. [94]
In cases where the patient is 35 years or older, dynamic ovarian reserve testing may be indicated. The most common test used is the clomiphene citrate challenge test (CCCT). A serum FSH and estradiol level is drawn on cycle day 3. Clomiphene citrate 100 mg by mouth is administered on cycle days 5-9 and a serum FSH level is drawn again on day 10. An FSH level greater than 10 is associated with decreased fertility and lower pregnancy rates.
Other tests of ovarian reserve include antral follicle counts, ovarian volume, inhibin B, and antimüllerian hormone. One large prospective observational trial suggests that age, AMH, inhibin B, and FSH combined have significant predictor of poor oocyte yield. However, most of these have not been found to be of adequate sensitivity, specificity, or positive predictive value when applying cutoffs across all age groups for pregnancy. They are predictive of response to ovulation induction medications. Thus, any result must be interpreted within the clinical context of the patient. [95, 96]
A serum AMH assay could be used to identify patients with decreasing ovarian reserves and polycystic ovarian insufficiency. The results of a study by Kallio et al support the idea that AMH is mainly secreted by small nonselected follicles because follicular granulosa cells were AMH-positive and serum AMH levels were normal/low-normal among women with FSH-resistant ovaries who lacked follicle development past the small antral stage. [97]
Because thyroid disease and hyperprolactinemia can cause menstrual abnormalities and infertility, a serum TSH and prolactin should always be checked and corrected prior to instituting therapy.
Evaluation of the Male Partner
The male partner must submit a semen sample for a comprehensive semen analysis. Previous paternity does not guarantee current fertility status. The comprehensive semen analysis must be performed in a certified andrology laboratory. The semen sample should be collected at the same andrology laboratory that will conduct the test. However, if the sample must be collected at home, it must be collected in a sterile plastic container and delivered to the andrology laboratory at body temperature no later than 30 minutes after ejaculation.
Some patients cannot produce a semen sample through masturbation. In these cases, the sample can be collected through intercourse, using a special nonspermicidal condom provided by the andrology laboratory. To optimize results, the semen sample should be collected after a period of 3 days but no more than 5 days of sexual abstinence.
Semen analysis
The basic semen analysis assesses sperm concentration, motility, morphology, and viability. The World Health Organization's semen analysis parameters (with the variable and the corresponding reference range) are as follows [98] :
-
Volume - 2-5 mL
-
pH level - 7.2-7.8
-
Sperm concentration - 20 million or greater
-
Motility - 50%, forward progression
-
Morphology - Normal sperm (>4%)
-
White blood cells - Fewer than 1 million cells/µL
Morphology has become an important parameter to evaluate the quality of sperm and fertilization capability. Kruger reported a new classification based on strict sperm morphology after fixing and staining the sperm. [99] Using the Kruger criteria, sperm morphology must be greater than 14% to be considered normal. Morphology of less than 4% is associated with severe infertility and is an indication for assisted reproduction technology/intracytoplasmic sperm injection.
Specific biochemical analyses relevant to accessory sex gland function can be performed using the semen sample. These include fructose from the seminal vesicles, zinc and acid phosphatase from the prostate gland, and α-glucosidases and carnitine from the epididymis. [100]
Sperm agglutination is an indirect indicator of the presence of sperm antibodies. The immunobead test can be performed either directly on the sperm or indirectly on sperm and blood. Surface antibodies against immunoglobulin A (IgA) or immunoglobulin G (IgG) may be present. The antibodies can be specific for the head or for the tail of the sperm. [101, 102, 103] IgA sperm antibodies interfere with the sperm-oocyte interaction and account for decreased fertilization, whereas IgG sperm antibodies are more responsible for impaired sperm motility. Sperm antibodies are associated with infection (ie, orchitis), testicular trauma, and a history of vasectomy.
Interpretation of semen analysis
Spermatogenesis takes approximately 72 days. Abnormal semen analysis results can be attributed to various unknown reasons (eg, short period of sexual abstinence, incomplete collection, poor sexual stimulus); therefore, repeating the semen analysis at least 1 month later is important before a diagnosis is made. The patient should be informed of the normal fluctuation that can occur between semen samples.
Azoospermia indicates absence of sperm that could result from congenital absence or bilateral obstruction of the vas deferens or ejaculatory ducts, spermatogenesis arrest, Sertoli cell syndrome, or postvasectomy.
Oligozoospermia indicates a concentration of fewer than 20 million sperm/mL and may be associated with ejaculatory dysfunction such as retrograde ejaculation, genetic conditions, or hormonal disturbances.
Asthenozoospermia indicates sperm motility of less than 50%. This can be caused by extreme temperatures and delayed analysis after sperm collection.
Teratospermia indicates an increased number of abnormal sperm morphology at the head, neck, or tail level.
Hypospermia indicates a decrease of semen volume to less than 2 mL per ejaculation.
Hyperspermia indicates an increase of semen volume to more than 8 mL per ejaculation.
Sperm function tests
A proliferation of different sperm tests have been developed to evaluate and predict the sperm fecundability, including (1) the acrosome reaction test with fluorescent lectins or antibodies, (2) computer assessment of the sperm head, (3) computer motility assessment, (4) hemizona-binding assay, (5) hamster penetration test, and (6) human sperm-zona penetration assay. [5, 6, 7, 8] Numerous publications describe the positive and negative aspects of these tests. They are subject to variation in interpretation, which render them more of academic interest than of practical therapeutic value.
Infertility Treatment
A consultation once the evaluation has been completed is imperative. A treatment plan should be generated according to the diagnosis, duration of infertility, and the woman's age. If pregnancy has not been established within a reasonable time, further evaluation and/or an alternative treatment plan should be considered.
Treatment of Cervical Factors
Chronic cervicitis may be treated with antibiotics. Reduced secretion of cervical mucus due to destruction of the endocervical glands by previous cervical conization, freezing, or laser vaporization responds poorly to low-dose estrogen therapy. The easiest and most successful treatment is intrauterine insemination (IUI). [104] Similar treatments apply when oligospermia, hypospermia, and ejaculatory disorders such as impotence, hypospadias, or retrograde ejaculation are present. [105, 106, 107] Patients with azoospermia that is not amenable to in vitro fertilization/intracytoplasmic sperm injection treatment benefit from artificial insemination (AI) with donor sperm. [108]
Artificial insemination can be performed by depositing the sperm at the cervical level (cervical insemination) [106] or inside the endometrial cavity (intrauterine insemination). Cervical insemination has almost been abandoned because of its low success and has been relegated only to cases in which the sperm count is normal, such as in artificial insemination using donor sperm or if the sample has elevated white cells.
For intrauterine insemination, in vitro fertilization, and intracytoplasmic sperm injection procedures, the removal of certain components of the ejaculate (ie, seminal fluid, excess cellular debris, leukocytes, morphologically abnormal sperm) with the retention of the motile fraction of sperm is desirable.
For most specimens, the greatest recovery of the motile portion results from separation via centrifugal filtration through a discontinuous density gradient system. However, for certain very poor specimens with low original concentrations of motile sperm, the use of the gradient system results in such a negligible recovery as to render it useless. The recourse for these specimens is to remove the seminal fluid by successive media washes.
A small number of specimens have acceptable original concentrations of motile sperm but poor recoveries with the gradient system. These specimens benefit most from layering a washed pellet of sperm with nutrient media and allowing the motile fraction to swim up into the media before being separated. [109]
After sperm preparation, the spermatozoa are enhanced in motility and become activated and ready to fertilize an oocyte. Intrauterine insemination is performed during a natural cycle or after ovulation induction with CC or gonadotropins. The procedure is performed 30-34 hours after the spontaneous LH surge or 36 hours after the administration of 10,000 U of hCG (human chorionic gonadotropin). [110] The sperm is delivered into the endometrial cavity using an intrauterine insemination catheter. After injection of the sperm, the patient remains in the recumbent position for 10-15 minutes.
The average pregnancy rate achieved after a natural-cycle intrauterine insemination is 8%. The rate increases to 10-12% after CC ovulation induction and to 12-15% per cycle after hMG/hCG ovulation induction. Of the successful pregnancies, 85% are achieved within the first 4 cycles of intrauterine inseminations.
Homologous insemination refers to the use of sperm from the patient's partner. Heterologous or therapeutic insemination, formerly called artificial insemination by donor sperm, refers to the use of frozen sperm that has been quarantined for at least 6 months. [108] Thereafter, the specimen is ready to use once the donor has undergone the necessary screening tests required by the tissue bank, the US Food and Drug Administration (FDA), and the American Society for Reproductive Medicine (ASRM). [111] The source of the sperm can be either anonymous or from a donor designated by the couple. A cumulative pregnancy rate of 80% is achieved during the first 6 cycles of therapeutic insemination.
Treatment of Uterine Factors
Until in vitro fertilization became available, a patient with congenital absence of the uterus and vagina (Rokitansky-Küster-Hauser syndrome) had no chance to have a biologic child. Today, it is feasible by using a gestational carrier. Once patients desire to have children, they proceed with stimulation of the ovaries, oocyte aspiration, and in vitro fertilization, but the embryos are transferred to a gestational carrier (see In Vitro Fertilization).
The treatment of uterine malformations depends on the severity of the problem. Fertility is not an issue for some patients affected by DES, and they remain undiagnosed until they have an abnormal Papanicolaou test result. Those who do have fertility problems are treated according to the following guidelines: [112, 113, 114]
-
Chronic cervical factor of absence of mucus - Intrauterine insemination
-
Cervical incompetence - Cerclage
-
Damage/absence of fallopian tubes (ectopic) - In vitro fertilization
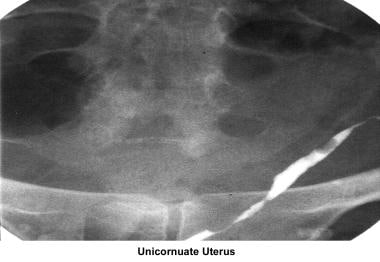
Unicornuate uterus
A unicornuate uterus remains undetected unless fertility is compromised. Patients with this type of uterus can have a normal term pregnancy. Most problems are related to premature labor and pregnancy loss. Unicornuate uterus is associated with renal abnormalities including absence of a kidney or presence of a pelvic kidney; this occurs in 15% of cases. Thus, an intravenous pyelogram must be performed once this diagnosis is made. Whether interventions before conception or early in pregnancy, such as resection of the rudimentary horn and prophylactic cervical cerclage, decidedly improve obstetrical outcomes is uncertain; however, current practice suggests that such interventions may be helpful. Women presenting with a history of this anomaly should be considered high-risk obstetrical patients. [115]
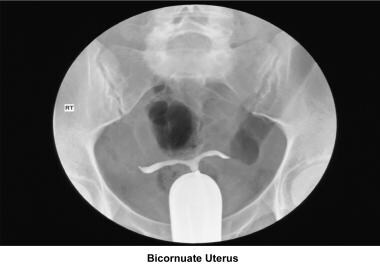
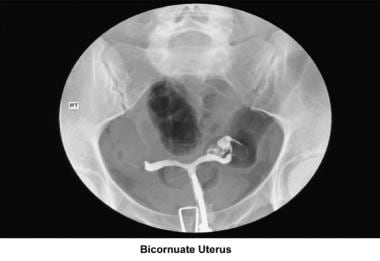
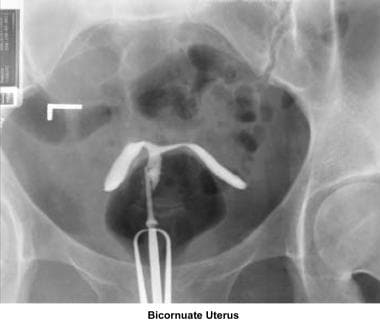
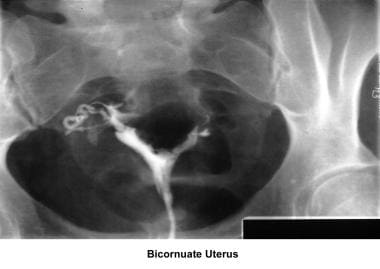
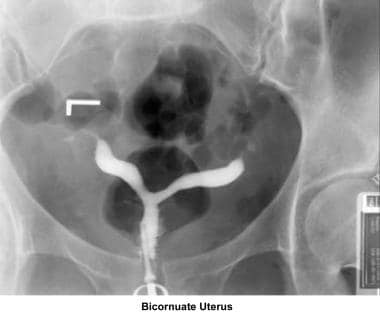
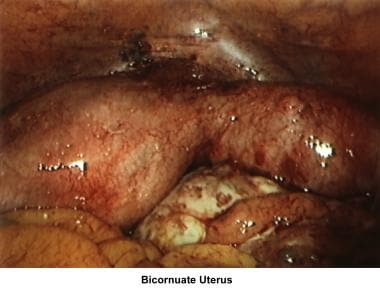
Bicornuate uterus
A bicornuate uterus causes only minimal problems with infertility (if any). A bicornuate uterus can be associated with a history of recurrent miscarriages, and its repair is indicated only if other etiologies for the miscarriage have been excluded (see Surgical intervention below).
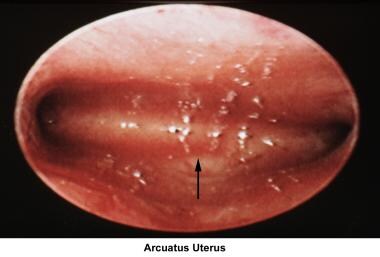
Arcuate uterus
In general, an arcuate uterus does not cause infertility. Whether it should be corrected in cases of primary infertility is controversial.
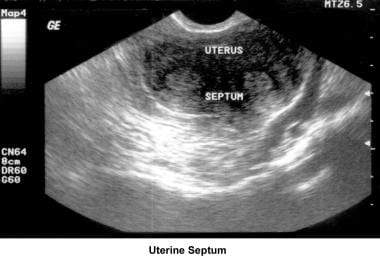
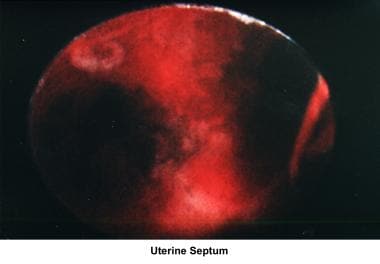
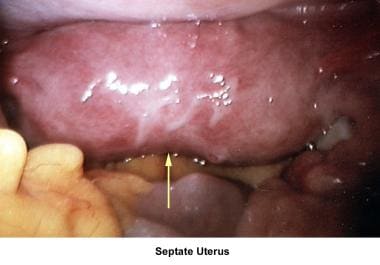
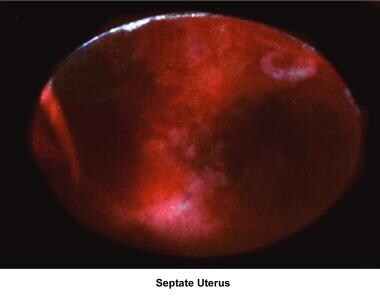
Septate uterus
The hypothesis that a uterine septum can cause infertility is controversial. Advising surgery in cases of primary infertility is difficult. The avascular nature of the septum is theorized to interfere with implantation and maintenance of the embryo.
Surgical Intervention
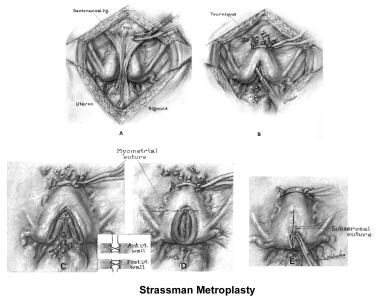
Uterine anomalies can be corrected through operative hysteroscopy under general anesthesia or conscious sedation. [116] Ideally, the procedure should be performed during the early follicular phase and under laparoscopic surveillance to decrease the risk of uterine perforation. Furthermore, laparoscopy assists in the differential diagnosis between a septate and a bicornuate uterus. A bicornuate uterus is characterized by the presence of an indentation at the fundus.
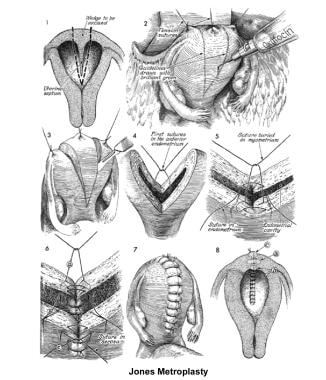
The 2 techniques are the Strassman metroplasty and the Jones metroplasty. The Strassman metroplasty consists of performing an incision at the fundus of the uterus between both cornual areas and closing the defect with an anteroposterior suture. The Jones metroplasty consists of resecting the septum using an anteroposterior wedge incision and closing the defect in the same direction (see the images below). [117, 118, 119]
Uterine synechiae
Uterine synechiae are corrected using operative hysteroscopy. The surgery is performed during the early follicular phase. Once the synechiae have been resected, leaving an intrauterine balloon for 7 days is advisable to prevent a recurrence of adhesions. The patient should receive prophylactic antibiotics and uterine relaxants (eg, ibuprofen) during these 7 days to prevent infection and balloon expulsion, respectively. The patient should be prescribed high-dose estradiol (5 mg qd for 21 d) followed by medroxyprogesterone (10 mg for 10 d). A postoperative HSG should be performed 2 months later. In many instances, more than one hysteroscopy is required for total resection.
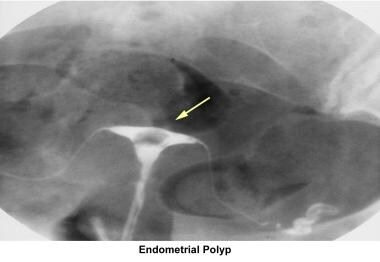
Endometrial polyps
Endometrial polyps are removed through operative hysteroscopy associated with a dilatation and curettage, if necessary. An HSG follow-up procedure is not necessary. To prevent further polyp development associated with anovulation, the patient should have withdrawal bleeding at least every 6 weeks.
Myoma treatment
In general, small and asymptomatic myomas do not require treatment, but the patient should be periodically monitored. Fibroids should be treated if they are associated with abnormal uterine bleeding or if they are thought to be the cause of infertility. Three modalities are used to treat myomas: medical treatment, surgical treatment, and embolization.
Medical treatment is a temporary treatment, ideally used for patients who are close to menopause or who are risky surgical candidates. However, medical treatment can be used to reduce the myoma size prior to removal. GnRH analog ([GnRHa], leuprolide acetate, nafarelin acetate, goserelin acetate) causes down-regulation of the pituitary, inducing chemical menopause after injections of 3.75 mg intramuscularly every 4 weeks for a period of up to 6 months. [117, 120, 121, 122] Disadvantages of this treatment include symptoms of menopause, osteoporosis, and recurrence of the myomas after discontinuation of the treatment.
Surgical treatment of myomas is indicated in cases of abnormal uterine bleeding, when the myoma is implicated in recurrent miscarriages or when it is thought to interfere with embryo implantation. The 3 classes of surgical techniques are conventional laparotomy, operative laparoscopy, and operative hysteroscopy, as follows:
-
Laparotomy: This technique is indicated for large myomas, for submucous myomas larger than 3 cm in diameter, or for myomas that, regardless of being submucous, have a portion of the myoma that compromises the myometrium so that a complete resection through the hysteroscopy is not feasible.
-
Operative laparoscopy: This technique is indicated for pedunculated and superficial intramural myomas. This technique should be reserved for myomas with a diameter less than 6 cm. [123] Several cases have been reported of uterine rupture during pregnancy because the reconstruction of the uterus after laparoscopic myomectomy was not as good as a myomectomy performed using laparotomy. [124]
-
Operative hysteroscopy: The removal of a submucous fibroid using hysteroscopy should be limited to small fibroids (≤3 cm) with minimal compromise of the myometrium. [81] This is important to decrease the risk of excessive bleeding and to decrease the risk of electrolyte imbalance, water intoxication, and pulmonary edema from excessive intravasation of Hyskon, glycine, or sorbitol used during the procedure. To avoid this complication, the circulating nurse must record the amount of distention fluid injected and the amount recovered in the suction device. If a deficit of greater than 1 L is recorded, the procedure should be terminated, and, preferably, the myomectomy should be completed in a second hysteroscopic attempt. The patient's electrolyte levels must be checked to consider the need for diuretics. Uterine synechiae development is a potential complication after the surgery; therefore, a postoperative HSG should be part of follow-up care.
Uterine fibroid embolization (UFE) consists of catheterization of the uterine artery and the injection of microbeads of polyvinyl alcohol to selectively occlude the circulation of the fibroid. The procedure is performed by interventional radiology and requires overnight admission for the patient. [125] It is NOT intended for patients who desire fertility.
Treatment of Tubal and Peritoneal Factors
The treatment of tubal-factor infertility underwent major changes, especially during the last quarter of the century when microsurgery became available. [126, 127] Tubal reconstruction was the only hope for those patients before assisted reproductive therapy became available.
Because of the intimate relationship between the fallopian tubes and the other pelvic organs and because, in the great majority of the cases, peritoneal pathology involves tubal pathology, the treatments of these factors are discussed together.
Tubal and peritoneal factor infertility treatment requires a good surgeon who is skilled in currently available techniques. [128] The patient's age and the severity of the tubal pathology play important roles in the selection of patients, as do any other infertility issues such as the presence of endometriosis and severe pelvic adhesions. Before surgery, the HSG films and results of previous laparoscopies should be thoroughly reviewed to decide on the type of surgical technique that is required and to explain to the patient the expected degree of success and risks involved with the procedure.
Tubal obstruction and lysis of adhesions can be corrected through laparotomy, operative laparoscopy, and, in special circumstances, through operative hysteroscopy and tubal cannulation.
Laparotomy is indicated in patients with severe pelvic adhesions that compromise the bowel, ovaries, and tubes, with obliteration of the cul-de-sac. [129] The aim of the procedure is to correct what is necessary to allow the normal transport of the gametes; complete restoration of the anatomy is not intended. [130] Lysis of adhesions should be meticulous, using hydrodissection and fine instruments. Blunt dissection should be avoided. Constant irrigation with Ringer lactate solution and heparin prevents fibrin formation. Meticulous hemostasis is imperative. [131, 132, 133]
Operative laparoscopy was reintroduced into the surgical armamentarium in the 1950s; however, in the 1970s, Semm developed different procedures and operative instruments that currently allow for the outpatient laparoscopic surgical treatment of multiple tuboperitoneal pathologies, [91] electrocautery, endocoagulation, lasers, and ultrasonography scalpels facilitate the performance of operations that otherwise used to require a laparotomy.
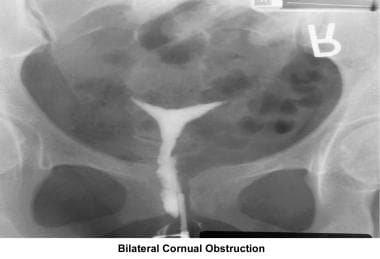
Operative hysteroscopy associated with tubal cannulation is helpful to treat cornual obstruction.
Fimbrial phimosis and periadnexal disease can be treated with laparoscopy. [134, 135, 136, 137, 138] The pregnancy rate after salpingolysis is 50-60% during the first year after treatment. Fimbrioplasty for fimbria agglutination or phimosis without destruction of the cilial epithelium is equally successful. The incidence rate of ectopic pregnancy after surgery is in the range of 5%.
Treatment of hydrosalpinx (distal tubal obstruction) with salpingostomy can be performed through microsurgery or operative laparoscopy. No difference in the pregnancy rate occurs if a skillful microsurgeon or laparoscopist performs the salpingostomy. The success of the procedure is related to the diameter of the hydrosalpinx and to the damage to the cilial epithelium. If the cilial epithelium has been destroyed, the outcome of the procedure is poor, and it is better to perform a salpingectomy in preparation for future IVF. The pregnancy rate fluctuates from 20-35%, and the expected ectopic pregnancy rate is as high as 20%.
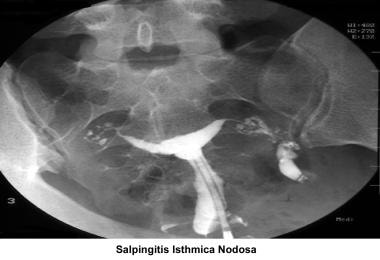
Before treating cornual obstruction, the diagnosis should be confirmed. In many cases, cornual obstruction diagnosed on HSG represents simple cornual spasm. Before performing a tubocornual anastomosis, the patient should have a diagnostic laparoscopy associated with tubal cannulation by hysteroscopy. [139, 140, 141] If one tube remains open, anastomosis is not needed because pregnancy can be achieved in 50% of cases. The success rate of tubocornual anastomosis ranges from 20-58%. The ectopic pregnancy rate is 5-7%. If the obstruction is caused by salpingitis isthmica nodosa or fibrosis, the best results are achieved through IVF. [142]
Surgical preparation for IVF
While the surgeon should be as conservative as possible, he or she should consider that the patient is better served with a single well-functioning fallopian tube than with 2 defective tubes, which elicits an increased risk for ectopic pregnancy or recurrence of pelvic adhesions. If the fallopian tubes are beyond repair, bilateral salpingectomy with destruction of the cornual area is recommended in preparation for IVF.
Tubal obstruction due to elective sterilization is better repaired with microsurgery, although the modern tendency is to perform the anastomosis using operative laparoscopy. [33, 143, 144] In either event, knowing in advance what type of tubal ligation technique was used is important. Unfortunately, tubal cauterization destroys a large amount of tissue, so the amount of fallopian tube remaining is often not long enough to facilitate a successful reanastomosis.
Before anastomosis, evaluate the patient using HSG and laparoscopy findings to measure how far proximal and distal fragments of the fallopian tubes remain from the tubal ligation. To have a successful reanastomosis, the final tube should measure at least 4.5 cm. If fimbriectomy was performed, no treatment is available other than IVF. The best candidates for tubal reanastomosis are patients who had tubal ligation by the method of fallopian ring, Filshie clip, or Pomeroy. [144] The pregnancy rate following a tubal reanastomosis performed by a skilled surgeon fluctuates from 70-80%. The ectopic pregnancy rate is approximately 7%.
Treatment of Endometriosis
Endometriosis treatment may be divided according to the severity of the disease and patient needs. Four alternatives are currently available to treat endometriosis: expectant therapy, surgical intervention, medical treatment, and combined therapy.
Expectant therapy
Expectant therapy should be based on a complete workup with diagnosis of very early stages of the disease (minimal) in patients without clinical symptoms, ie, an incidental finding. [145]
A second-look laparoscopy is required for follow-up observation within 6-18 months.
Surgical treatment
Surgical treatment should be directed at destroying the disease using electrocoagulation, laser vaporization, endocoagulation, or excision. [84] Removal of endometriomas and lysis of adhesions complete the treatment.
Most surgical treatment for endometriosis is currently performed through operative laparoscopy. [145, 146, 147] Laparotomy has been relegated to the treatment of severe disease or if a need for hysterectomy arises. [145]
Medical treatment
Medical treatment is directed toward suppressing estrogen production by the ovary. Different modalities of treatment are available. Depending on the therapeutic agent and the duration of treatment, endometriosis can be treated with oral contraceptives, progestins, androgens, or GnRH agonists.
The progestins that can be used and the doses are as follows:
-
Medroxyprogesterone acetate (eg, Depo-SubQ Provera 104; administer 104 mg SC q3mo or q12-14wk)
-
Megestrol acetate (eg, Megace 20-40 mg PO qd for up to 2 months)
The androgens used are 17-ethinyl testosterone derivatives (eg, danazol 400-800 mg PO divided BID; not to exceed 9 months) [151, 152, 153]
The GnRH agonists used are as follows:
-
Leuprolide acetate (eg, Lupron 3.75 mg IM q4wk or 11.25 mg Lupron Depot IM q3mo for up to 6 months)
-
Nafarelin acetate (eg, Synarel 400 mcg intranasal qd; not to exceed 6 months)
-
Goserelin acetate (eg, Zoladex 3.6 mg SC q4wk for 6 months)
Combined therapy
Medical and surgical treatments are usually combined for the treatment of severe endometriosis. No consensus exists as to whether the medical treatment should precede surgery or vice versa. [154, 155, 156] Those who prefer medical treatment first argue that the size of the endometriosis decreases; therefore, surgery will be easier and shorter. Those who prefer surgery first argue that because the size of endometriosis decreases, lesions that cannot be observed during surgery may be present; therefore, the operation is less than ideal and is associated with an increased chance for early recurrence.
Regardless of the treatment approach, establish a 6- to 12-month interval during which a spontaneous pregnancy is expected to occur.
For patients wishing to conceive, the medical approach is not indicated, as it delays treatment for infertility. Medical treatment for minimal to mild disease has not been shown to be of benefit. However, for women with moderate to severe endometriosis, surgical treatment then assisted reproductive therapy can be offered.
A more proactive approach now exists. Ovulation induction and intrauterine insemination are used after completion of the treatment in hopes of expediting the establishment of a pregnancy before relapse of the disease. [39, 157, 158]
Treatment of Ovarian Factors
Ovulation induction is the appropriate treatment for infertile patients who have dysfunction of the hypothalamic-pituitary-ovarian axis. The ovulation induction agents used include clomiphene citrate, hMG, hCG, recombinant FSH, and recombinant LH.
Clomiphene citrate (Clomid, Serophene)
The chemical formula for clomiphene citrate (CC) is 2-[p -(2-chloro-1,2-difhenylvinyl) phenoxy] triethylamine dihydrogen citrate. CC is a nonsteroidal estrogen capable of interacting with estrogen receptor–binding proteins in a manner similar to estrogen but in a more prolonged way. [159, 160] Therefore, CC behaves similar to an antiestrogen.
CC has been in clinical use since the early 1960s. Its mechanism of action is still not well understood, but it competes for the estrogen receptor at the hypothalamus, pituitary, and ovarian levels. Because of the action at the estrogen-receptor level within the hypothalamus, CC alleviates the negative feedback effect exerted by endogenous estrogens. [161, 162, 163] As a result, CC normalizes the GnRH release; therefore, the secretion of FSH and LH is capable of normalized follicular recruitment, selection, and development to reestablish the normal process of ovulation. [161, 164]
The standard dose of CC is 50 mg PO qd for 5 days, starting on the menstrual cycle day 3-5 or after progestin-induced bleeding. As an antiestrogen, CC requires that the patient have some circulating estrogen levels; otherwise, the patient will not respond to the treatment. The CC response is monitored using pelvic ultrasonography starting on menstrual cycle day 12. The follicle should develop to a diameter of 23-24 mm before a spontaneous LH surge occurs.
Basal body temperature (BBT) can be used to observe the thermogenic shift (temperature rises 0.5°F above the basal level) induced by the early secretion of progesterone. The only disadvantage with BBT is that in many instances, the shift does not occur in a clear way, and the patient misses the time of ovulation. While BBT is an inexpensive way to monitor ovulation, it is often impractical.
Urinary monitoring of the LH surge (eg, with an LH Predictor Kit) can be a substitute for BBT. The patient should start monitoring the urinary LH secretion daily starting on menstrual cycle day 12. Ovulation usually occurs within the 32-40 hours after the indicative color change. Serum LH determination is more precise, especially when performed in combination with pelvic ultrasonography.
Because of the antiestrogenic effect, CC may thicken the cervical mucus, creating an iatrogenic cervical factor that can be responsible for the lack of pregnancy in a patient who has otherwise ovulated. [165] Other adverse effects associated with CC are hot flashes, scotomas, dryness of the vagina, headache, and ovarian hyperstimulation, which, although rare, has been reported in patients who are sensitive to CC. [166, 167] Whether the use of CC increases the risk of ovarian cancer is unknown, although 2 articles illustrate a potential risk. [168] Other authorities disagree with this assumption.
The principal indications for CC use are oligomenorrhea, especially polycystic ovarian syndrome (PCOS), and for patients with slight menstrual irregularities. Its use has been extended to assisted reproduction technologies.
The use of CC is contraindicated in cases of ovarian cyst, pregnancy, and liver disease. Its use is controversial in patients with a history of breast cancer.
Aromatase inhibitors
Aromatase inhibitors (letrozole, anastrozole) inhibit the action of the enzyme aromatase, which converts androgens into estrogens by a process called aromatization. As a result, estrogen levels are dramatically reduced, releasing the hypothalamic-pituitary axis from its negative feedback. Aromatase inhibitors are available for clinical use and FDA approved for treatment of postmenopausal breast cancer, but not for ovulation induction.
When used in the early follicular phase, letrozole inhibits estrogen synthesis, thereby causing enhanced GnRH pulsatility and consequent FSH and inhibin stimulation. This results in normal or enhanced follicular recruitment without the risk of multiple ovulation and ovarian hyperstimulation syndrome. Letrozole has a very short half-life (45 hours) and, therefore, is quickly cleared from the body. For this reason, it is less likely to adversely affect the endometrium and cervical mucus. In a recent meta-analysis, letrozole was found to be as effective as other methods of ovulation induction. [169]
The usual dose for letrozole ovulation induction is 2.5 mg on cycle days 3-7. However, the optimal dosage and length of administration is under investigation. [170, 171] Aromatase inhibitors are generally well tolerated. The main side effects are hot flushes, gastrointestinal events (nausea and vomiting), headache, back pain, and leg cramps. These adverse effects were reported in older women with advanced breast cancer who were given the drugs on a daily basis over several months. In younger women taking them at lower doses for a short period of time, fewer adverse effects are noted.
The use of aromatase inhibitors for ovulation induction in premenopausal women is controversial due to the possibility of fetal toxicity and fetal malformations raised by one abstract. [172] However, 2 subsequent publications have shown no evidence of fetal malformations with the letrozole and no difference in birthweight compared with spontaneous conceptions. [173, 174] Furthermore, based on the half-life of each drug, administration in the early follicular phase should result in clearance of the aromatase inhibitors before implantation takes place.
Human menopausal gonadotropins
Crowe discovered that the gonads were under the control of the anterior hypophysis. [175] Zondek and Aschheim discovered that FSH and LH were responsible for the development of the gonads in immature animals and confirmed Crowe's work. [135, 136] In the 1930s, ovulation induction was attempted by using gonadotropins from a mare, but its use was discontinued because of the development of antibodies. [176, 177]
Borth et al demonstrated the effect of FSH and LH extracted from menopausal urine. [178, 179] Gemzell reported the first ovulation induction using human pituitary gonadotropin in 1958, and the first pregnancy was reported in 1960. [180, 181] Lunenfeld reported preliminary results using hMG; however, in 1963, it was definitely established as a real ovulation induction agent. [182, 183, 184]
Human menopausal gonadotropin (hMG [eg, Repronex, Menopur]) contains 75 U of FSH and 75 U of LH per mL, although the concentration may vary among batches (ranges from FSH at 60-90 U and LH at 60-120 U). In the 1980s, a pure form of FSH became available. Urofollitropin (eg, Bravelle) contains 75 U of FSH. The new generations of available gonadotropins are produced by genetically engineered mammalian cells (ie, Chinese hamster ovary cells), in which the gene coding for the alpha and beta FSH subunits has been inserted (follitropin alfa [eg, Gonal F] and follitropin beta [eg, Follistim AQ]). [185] Recombinant LH may beaddedtorecombinantFSHprotocolsasanalternative,particularlyuseful in patients with hypothalamic amenorrhea.
The administration of hMG and its derivatives should be under the direct supervision of a reproductive endocrinologist. An ultrasonography unit and an endocrine laboratory capable of performing daily determinations of E2, FSH, and LH are necessary. [186, 187, 188, 189]
Multiple adverse effects and complications may occur during the use of the gonadotropins, including (1) multiple pregnancy (24-33%), (2) ectopic pregnancy (5-8%), (3) miscarriages (15-21%), (4) ovarian torsion and rupture, and (5) ovarian hyperstimulation syndrome, which is the most severe. [190, 191] Whittemore et al, using a large combined data set derived from case-controlled studies in the United States, showed that the increase of ovarian cancer associated with infertility might be due to the use of fertility drugs. [192]
Ovarian hyperstimulation syndrome is an iatrogenic condition that occurs in patients undergoing ovulation induction with hMG or controlled ovarian hyperstimulation (COH) for assisted reproductive technologies. The incidence rate fluctuates from 0.1-30%. The pathophysiology of the disease is not well understood, but a massive extravascular accumulation of fluid occurs that is associated with a severe depletion of the intravascular volume responsible for dehydration, hemoconcentration, and electrolyte imbalance (ie, hyponatremia, hyperkalemia). [193] Ovarian hyperstimulation syndrome can be mild, moderate, or severe. [194]
Mild ovarian hyperstimulation syndrome is characterized by ovarian enlargement (up to 5-12 cm in diameter), minimal ascites, and weight gain of less than 10 lb. Moderate ovarian hyperstimulation syndrome is characterized by ovarian enlargement (5-12 cm in diameter) moderate ascites, nausea, vomiting, abdominal discomfort, and weight gain greater than 10 lb. Severe ovarian hyperstimulation syndrome is characterized by easily palpable ovaries, severe ascites, nausea, vomiting, diarrhea, shortness of breath, hydrothorax, peripheral edema, oliguria, hemoconcentration (eg, hematocrit level >48% and hemoglobin level >16 g), and creatinine level greater than 1.6 mg/dL. Renal failure and thrombosis can occur if the patient is not treated correctly. [195]
Some patients have a greater risk of developing ovarian hyperstimulation syndrome. They are usually young patients with a history of polycystic ovarian syndrome or oligo-ovulation who responded with elevated E2 levels (3000 pc/mL) and multiple follicles (>15) and patients in whom the ovulation has been triggered by the administration of exogenous hCG. [196, 197]
Ovarian hyperstimulation syndrome usually has 2 phases. The first phase develops between the second and seventh day after ovulation, and the second phase only occurs if the patient becomes pregnant. Ovarian hyperstimulation syndrome is self-limited, and the symptoms subside within 2-6 weeks. [198]
Patients with mild and moderate ovarian hyperstimulation syndrome are treated at home with bedrest and strict control of fluid intake and output. If a weight gain greater than 2 lb occurs, the patient should be evaluated to determine if hospitalization is required.
Patients with severe ovarian hyperstimulation syndrome are often hospitalized and confined to bed, with strict control of fluid intake and output. Intravenous fluids (ie, isotonic sodium chloride solution) must be administered until hemodilution is achieved. If the urinary output remains low, albumin 25% (50 mL/h IV for 4 h) has been effective in promoting diuresis. Transvaginal or abdominal paracentesis should be performed if the patient becomes uncomfortable. Thoracentesis is rarely indicated in cases of pleural effusion. Because of the risk of thrombosis, heparin (5000 U SC q12h) is recommended. [195] Some have had success treating severe ovarian hyperstimulation syndrome on an outpatient basis by performing aggressive transvaginal paracentesis with good outcomes. [199]
hMG and its derivatives are indicated for ovulation induction in patients with primary amenorrhea due to hypopituitarism and in patients with secondary amenorrhea who did not respond to CC ovulation induction. For the past 20 years, hMG and its derivatives have been the first choice for controlled ovarian hyperstimulation in assisted reproductive technologies.
Gonadotropin-releasing hormone
Synthetic GnRH (gonadorelin) has a chemical composition similar to native GnRH and is indicated for patients with hypothalamic dysfunction, especially those who do not respond to CC. [200, 201] Although quite effective, it is not available in the United States due to lack of use and low demand. This drug is administered in a pulsatile fashion every 60-120 minutes, intravenously or subcutaneously using a delivery pump. The starting dose is 5 mcg per pulse intravenously or 5-25 mcg subcutaneously. The administration on GnRH should be extended throughout the luteal phase, or this should be supplemented with the administration of exogenous hCG. [202]
Monitoring folliculogenesis is simpler than using hMG. Because ovarian hyperstimulation syndrome does not occur, the patient's response is slow. In most cases, only 1 follicle is recruited and develops until ovulation. A urinary LH kit is a practical way to monitor these patients. Pelvic ultrasonography can be used once a week until the dominant follicle is detected; once this occurs, ultrasonography can be used more frequently until ovulation occurs. Determination of serum E2 and LH levels can also be performed. [203]
Treatment of Primary Amenorrhea
hMG is the treatment of choice for patients with primary amenorrhea due to hypopituitarism. Generally, their response to hMG ovulation induction is too brisk or delayed, and predicting whether the patient will respond easily is not possible. The risk of ovarian hyperstimulation syndrome and multiple pregnancy is heightened; therefore, hMG should be started at the minimal dose (75 IU SC qd for 7 days). On the seventh day, E2 measurement and ultrasonography are performed. If the E2 level is below 100 pc/mL and the sonogram shows small follicular development, hMG is increased to 150 IU/day for an additional 5 days. However, if the E2 level is greater than 100 pc/mL and the follicles are 10 mm in diameter, hMG should be continued at the same dose. Once the follicular diameter reaches 18 mm and the E2 level is below 2000 pc/mL, ovulation is triggered by the administration of hCG (10,000 IU IM).
The ideal response is one in which only 2-3 follicles develop. If the response is exaggerated, with more than 5 sizable follicles (18 mm in diameter), and the E2 level is greater than 2500 pc/mL, cancelling the ovulation is better to avoid the risk of ovarian hyperstimulation syndrome and a high order of multiple pregnancy. In current practice, an alternative for patients with more than 5 sizable follicles is to convert the treatment to IVF.
Treatment of Secondary Amenorrhea and Oligo-ovulation
Once the diagnosis is established and any other endocrinopathy has been excluded, the ovulation induction agent of choice depends on a functioning hypothalamic-pituitary-ovarian axis. In patients with low gonadotropins and low estrogen, the treatment of choice is hMG, and the protocol is similar to that for patients with primary amenorrhea. If the E2 and FSH levels are in the normal range, clomiphene citrate is the drug of choice, as previously described.
Polycystic ovarian syndrome is the most frequent indication for ovulation induction. CC is the drug of choice. Restrict the treatment to 4 ovulatory cycles because 85% of patients conceive by the fourth ovulatory cycle. [204] If pregnancy is not achieved, further evaluation is required to exclude other factors that may be associated with infertility and may interfere with the success of CC therapy. If ovulation does not occur with the 50-mg dose, the CC dose must be increased in subsequent cycles to 100 mg for 5 days. The maximum recommended dosage is 150 mg/day for 5 days. Most recommend dosing CC on cycle days 3-7 to improve response and ovulation around cycle day 14.
Lack of ovulation
Patients with anovulation who did not ovulate after several cycles of CC at different doses of treatment are deemed clomiphene resistant. This situation can be related to the presence of other endocrine disorders such as hyperprolactinemia, congenital adrenal hyperplasia, adrenal tumors, Cushing syndrome, thyroid dysfunction, and extreme obesity. Therefore, this problem must be corrected first or concomitantly to obtain an ovulatory response. Although obesity is well known to be associated with insulin resistance, women with PCOS are even more resistant to insulin than other patients who are obese.
A subgroup of patients has PCOS with hyperinsulinism, hyperandrogenism associated with acanthosis nigricans, and resistance to CC. [205, 206] This group is amenable to metformin treatment. [207, 208] Metformin improves insulin sensitivity and decreases hepatic gluconeogenesis and, therefore, reduces hyperinsulinism, basal and stimulated LH levels, and free testosterone concentration. Consequently, the patient with PCOS becomes responsive to CC ovulation induction.
Adverse effects of metformin include GI intolerance, nausea, vomiting, and abdominal cramps. Weight loss has also been observed. Therefore, patients must build up tolerance. The initial dose is 500 mg PO qd for 7 days, then 500 mg bid for another 7 days, and, finally, 500 mg tid. Start CC at the initial dose of 50 mg/day for 5 days. In many instances, patients can ovulate while on metformin treatment. Therefore, pelvic ultrasonography is required before CC is initiated. [207]
Pure FSH treatment for ovulation induction is another alternative for patients with PCOS who are clomiphene resistant. Start pure FSH at 37.5 IU/day subcutaneously. The dosage is increased slowly (ie, by 37.5 IU q5d) until follicle development is detectable based on an elevation of the E2 levels and the presence of follicle development on sonograms. Using this small amount of FSH, the patient generally develops 1-2 follicles, decreasing the risk for multiple pregnancy and eliminating the risk of ovarian hyperstimulation syndrome.
Lack of pregnancy
Lack of pregnancy can be related to disruption of the cervical mucus, inadequate follicular development, presence of luteinized unruptured follicle syndrome, progesterone deficiency, and premature administration of hCG to trigger ovulation.
Some authorities believe that ovulation occurs 5 days after the last dose of CC. In the authors' experience, ovulation may occur any time from the menstrual cycle day 10-23 or between day 1 and day 14 after the last tablet of CC is administered. Thus, pelvic ultrasonography has become an important tool for monitoring ovulation induction.
The quality of the cervical mucus can be improved with the administration of a small dose of estrogens, or the problem can be bypassed by intrauterine insemination. [163, 209] If the follicle is smaller than 23-24 mm at the time of ovulation, a better size can be obtained by starting the CC therapy on the menstrual cycle day 2.
Luteinized unruptured follicle syndrome can be prevented by the administration of hCG (10,000 IU IM) once the follicle reaches 23-24 mm in diameter. Progesterone deficiency can be corrected by the administration of progesterone during the luteal phase, starting 48 hours after ovulation. [204]
Patients with hyperprolactinemia need a thorough evaluation to exclude a pituitary microadenoma. The patient must be treated with bromocriptine (eg, Parlodel) at an initial dose of 2.5 mg PO qd and increased to 5 mg once tolerance is built. If the GI symptoms persist, the medication can be administered intravaginally. Alternatively, cabergoline can be used orally or vaginally weekly at a starting dose of 0.5 mg/wk. Cabergoline is associated with fewer side effects but is more expensive. Patients with adrenal hyperplasia and other disorders must be treated with prednisone. Patients with hyperthyroidism or hypothyroidism must be treated accordingly. [210, 211] Weight reduction should be part of the treatment because it helps the patient's response to ovulation induction. [212]
Treatment of Male Factors
Asthenospermia associated with varicocele is treated surgically with varicocelectomy or with embolization of the spermatic veins. The initial result of the procedure is not detected before 3 months because spermatogenesis takes 72 days. If no improvement occurs, depending on the amount of functional sperm recovery after the sperm wash, the decision must be made to proceed with either intrauterine insemination or in vitro fertilization. Alternatively, the patient may bypass surgical treatment and proceed directly to intrauterine insemination or in vitro fertilization, depending on the severity and the female partner's age.
Oligospermia is the most frequent cause of male infertility. Its treatment depends on the etiologic factor, but, in many instances, the underlying cause remains unknown. CI or intrauterine insemination is the treatment of choice if more than 2 million sperm are recovered after the sperm wash. (See Treatment of Cervical Factors). [106, 109]
Patients whose reproductive tract, FSH, LH, and testosterone levels are determined to be normal or those who have low testosterone in the absence of any other hormonal abnormalities can be treated empirically with cycles of CC (25 mg PO qd for at least 6-12 mo). Supplementation with acetyl L carnitine and antioxidants such as Vitamins C or E are encouraged to enhance sperm maturation and function. A spermiogram control study is performed after 3 cycles of therapy. Improvement in the sperm count is a good sign, and the treatment should be continued. Checking testosterone levels is advisable because an elevation above the reference range has a negative feedback effect on sperm production. Depending on the sperm count, the couple is advised to have intercourse near the time of ovulation or to proceed with intrauterine insemination.
Azoospermia treatment depends on the etiology. In patients with obstructive azoospermia and normal gonadotropin levels, sperm can be obtained through microsurgical epididymal sperm aspiration or testicular biopsy. [213] Fertilization of the oocytes is performed using IVF/intracytoplasmic sperm injection. In patients with nonobstructive azoospermia, retrograde ejaculation can be the etiologic factor. [107] The treatment consists of recovering sperm from a urine sample collected immediately after ejaculation.
Alkalinizing the urine before the procedure is necessary. The night before the procedure, the patient must take 2 tablespoons of sodium bicarbonate. The bladder must be emptied 1 hour prior to sperm collection, and a second dose of sodium bicarbonate is taken along with 16 ounces of fluid. A urine sample must be collected immediately after ejaculation. The urine specimen must be centrifuged immediately. The sediment is suspended in a buffer solution, and the recovered sperm is processed using the sperm-wash technique before it can be used for intrauterine insemination.
Treatment of the Normal Infertile Couple
The prognosis for the normal infertile couple is poor and unpredictable. Only 5-10% of these couples eventually achieve a pregnancy within 5 years. Empirical treatment with controlled ovarian hyperstimulation followed by intrauterine insemination has improved the pregnancy rate in those patients. [214] If pregnancy does not occur during the first 4 intrauterine insemination cycles, other alternatives include IVF or any of the associated assisted reproductive technologies procedures.
Assisted Reproductive Technologies
The first successful human IVF attempt resulted in the 1978 delivery of Louise Brown in England and is considered the beginning of a new era for the treatment of infertility. [215]
Since 1981, the understanding of the ovulatory process has been revolutionized, hopelessly infertile patients have seen their dreams fulfilled, and success has shown that patients who were considered sterile in the past are now capable of having children.
IVF indications have departed from the narrow scope of tubal infertility to other indications that were almost impossible to overcome, including infertility related to oligospermia and obstructive azoospermia.
In May 2013, the American Society of Clinical Oncology published an update of its 2006 guidelines on fertility preservation for adults and children with cancer. [216, 217] One of the key recommendations of the new guidelines is that fertility preservation should be discussed with and documented for all patients of reproductive age as early as possible in the treatment process. In addition, refer patients interested in fertility preservation to reproductive specialists. Oocyte cryopreservation is now considered standard practice, and the word “oncologist” has been replaced with “healthcare provider.” More flexible options are available for ovarian-stimulation protocols, as is information on the relationship between the BRCA mutation and fertility [216, 217]
In Vitro Fertilization
IVF consists of retrieving a preovulatory oocyte from the ovary and fertilizing it with sperm in the laboratory, with subsequent embryo transfer (replacement) within the endometrial cavity. Biologists and veterinarians have used IVF for several decades in the laboratory for applications such as animal husbandry and cattle breeding. Lack of understanding of human embryo development and special metabolic needs accounted for the delay in achieving success. The pioneering work of Edwards and Steptoe has been duplicated worldwide, and IVF is now recognized as an established treatment for infertility.
Indications
Absence of the fallopian tubes and severe pelvic adhesions were the absolute indications for IVF, but they have been broadened. Patients with a history of endometriosis unsuccessfully treated medically or surgically can undergo IVF. Patients with some malformation of the uterus related to DES exposure during pregnancy are candidates. Patients with husbands who have severe oligospermia or a history of obstructive azoospermia are also candidates for IVF. Finally, patients who have failed more conservative therapies or with an unknown etiology of infertility (ie, NICs) may undergo IVF.
Procedure
IVF consists of retrieving preovulatory oocytes from the ovary and fertilizing them with sperm in the laboratory, with subsequent embryo transfer (replacement) within the endometrial cavity.
The following steps are required during an IVF cycle:
-
Ovarian stimulation
-
Follicular aspiration
-
Oocyte classification
-
Sperm preparation
-
Oocyte insemination
-
Embryo culture
-
Embryo transfer
Ovarian stimulation for IVF
Although the world's first IVF pregnancy, which occurred in England, followed the fertilization of a single oocyte obtained during a spontaneous menstrual cycle, IVF success is related to the patient's age and the number of embryos transferred into the endometrial cavity, among other factors. [19, 218, 219] The number of embryos obtained in any given IVF cycle depends on the number of oocytes obtained after ovulation induction, follicular aspiration, and fertilization.
Several protocols are available for ovarian stimulation, and all are based on the principles of follicular recruitment, selection, and dominance.
Clomiphene citrate
Currently, CC is used infrequently in IVF. A clomiphene-only protocol consists of 5-7 days of treatment, with doses of 50-150 mg starting on the second menstrual cycle day. [220] The ovarian response is monitored using pelvic ultrasonography and determinations of serum E2 and LH levels. When the follicle diameter reaches 18 mm, LH must be measured every 3-4 hours to detect a premature LH surge that generally occurs when the follicle reaches 23-24 mm in diameter. [221] Oocyte retrieval must be performed within 24-26 hours after the LH surge.
The advantages of the CC protocol include low cost and an almost nonexistent risk of ovarian hyperstimulation syndrome.
The major disadvantages include (1) low oocyte yield (1-2 per cycle), (2) frequent LH surges that lead to oocyte retrieval at any time of the day, (3) high cancellation rate (25-50%), and (4) low pregnancy rate.
Clomiphene citrate and human menopausal gonadotropins
This combination has the advantage of increasing the number of recruited follicles. The doses of CC are similar, but the hMG (150 IU qd) is administered for a period of 2-7 days after the CC. [222] Frequent pelvic ultrasonography and daily determinations of E2 levels are required, as are frequent determinations of LH once the follicular diameter reaches 15 mm. Remember that the follicle is smaller with this protocol. The follicle completes its development at 17-18 mm; therefore, hCGs (10,000 IU IM) must be administered at this time to complete the oocyte maturation. Oocyte aspiration should be performed 35 hours after the hCG injection.
The advantage of the CC/hMG protocol is an increase in the number of recruited follicles. The disadvantages of the protocol are premature luteinization, spontaneous LH surge (20-50%), and high cancellation rate (15-50%).
Human menopausal gonadotropins
The administration of hMG for ovarian stimulation in a healthy anovulatory patient has been one of the groundbreaking discoveries in IVF. [187] The use of hMG has evolved with the introduction of new technologies and the generation of pure FSH gonadotropins that can be used subcutaneously. [223] Furthermore, recombinant FSH and LH gonadotropins are currently available. [224] hMGs are as follows:
-
Human menopausal gonadotropin (Repronex, Menopur) - FSH of 75 IU and LH of 75 IU
-
Purified FSH (Bravelle) - FSH of 75 IU and LH of less than 1 IU
-
Recombinant FSH (Follistim) - FSH of 75 IU and LH of 0 IU
-
Recombinant LH (in clinical trials) - FSH of 0 IU and LH of 75 IU
The doses of gonadotropins (based on FSH amounts) vary from 150-450 IU/day, depending on the patient's age and history of previous ovulatory response. In general, patients are started on the second or third menstrual cycle day. The response is monitored using daily serum E2 determination and later using pelvic ultrasonography. [218]
Once most of the follicles reach 17-18 mm in diameter, the gonadotropins are discontinued. hCG (10,000 IU) is administered that evening, and oocyte retrieval is performed 35 hours later. With the use of gonadotropins, ovulation induction appears to be more predictable. However, a spontaneous LH surge occurs in approximately 25% of cycles, which is one of the reasons for cancellation.
Regardless of the use of gonadotropins, a group of patients, known as poor responders, develops only 2-3 follicles, and their oocytes are of poor quality. [225] The possibility of a successful pregnancy in this group of patients is seriously compromised. In general, these patient responses are related to poor ovarian reserve (POR). [226]
Patient response, which is defined by an elevated baseline FSH level (>10 mIU/mL), a low E2 level (< 20 pg) associated with an absence of follicle development on sonograms, or elevated E2 levels (>100 pg) associated with early follicle recruitment, characterize poor ovarian reserve. Some patients respond with premature luteinization due to elevated tonic LH levels and subsequent premature elevation of progesterone that compromise oocyte quality. Analysis of each ovarian stimulation cycle is important to modify future stimulations and to increase the patient's response.
GnRHa agonists
Initially, GnRHa agonists were used in IVF patients who had a history of premature luteinization, spontaneous LH surge, and an ovarian stimulation response that was less than acceptable or ideal. [227] The GnRHa agonists can be used in 2 protocols known as the flare-up protocol and the luteal-phase protocol. [228, 229, 230, 231]
The flare-up protocol has the advantage of using the transitory elevation of FSH (agonist effect) that occurs during the first 4 days of the follicular phase. This elevation helps in the follicular recruitment process; after 5 days of GnRHa administration, the pituitary gland undergoes down-regulation, which prevents premature luteinization and the spontaneous LH surge. The administration of gonadotropins must be initiated on the fifth menstrual cycle day. [228]
The modified flare-up protocol is the administration of birth control pills for 4-21 days during the preceding IVF menstrual cycle. A microdose of GnRHa agonist leuprolide acetate is administered twice daily subcutaneously starting on day 1 of the menstrual cycle, and the hMG (recombinant FSH) begins on day 4 of the menstrual cycle.
In the luteal-phase protocol, GnRHa is started on the 17th or 21st menstrual cycle day. By the onset of the menstrual cycle, the phenomenon of pituitary down-regulation is in effect, so the administration of the gonadotropins begins on the second day of bleeding. [230, 231]
The adjunctive use of GnRHa in ovarian stimulation leads to an overall improvement in IVF. This accounts for the increase in the number, quality, and synchronization of the oocytes recovered per cycle and improves the fertilization rate, the number of embryos, and the pregnancy rate. [228] Furthermore, because more embryos are available than the number used in fresh embryo transfer, an opportunity exists to cryopreserve the excessive number of embryos for future embryo transfer(s).
Potential risks and disadvantages with the use of GnRHa include (1) increased requirements of gonadotropins, (2) increased costs due to additional days of therapy, (3) the risk of ovarian hyperstimulation syndrome due to excessively high E2 levels, and (4) an increased rate of multiple pregnancies.
GnRHa antagonists
The GnRHa antagonists are the latest generation of GnRHa that block LH secretion without a flare-up effect. [232] The GnRHa antagonists (eg, cetrorelix [Cetrotide], ganirelix) are administered (1) as a single dose on the eighth menstrual cycle day; (2) in small amounts over several days, starting on a given menstrual cycle day; (3) when the largest follicle reaches a diameter of 14 mm; or (5) when the LH levels in serum are greater than 10 mIU/mL. [233]
GnRHa antagonists have the advantage of blocking the LH surge at the periovulatory period; therefore, premature luteinization or spontaneous LH surge does not occur. Because the pituitary gland is not down-regulated at the beginning of the menstrual cycle, smaller amounts of gonadotropins are required to stimulate ovulation. Another advantage with this protocol is the prevention of ovarian hyperstimulation syndrome, especially in patients with elevated E2 levels (>3000 pg/mL) or more than 15 follicles during the stimulation. Because the half-life of the GnRHa antagonist is short, it is possible to elicit the preovulatory LH surge by the administration of leuprolide acetate and avoid the long-term effects of the hCG injection that are responsible for triggering the ovarian hyperstimulation.
Follicular aspiration
Oocyte aspiration is undertaken 35-36 hours after hCG administration. Originally, all aspirations were performed using laparoscopy. [234] This requires general anesthesia, a skillful laparoscopist, major discomfort for the patient, and the risks involved with the laparoscopy. Oocyte aspiration in patients with severe pelvic adhesions is extremely difficult with most patients and virtually impossible with others (see the image below).
Follicular aspirations under ultrasonographic guidance were first performed transabdominally, then transurethrally, and, finally, transvaginally. [235] The transvaginal route became the preferred procedure for most IVF programs. Anesthesia is given after the patient empties her bladder. She is placed in the dorsal lithotomy position for the transvaginal oocyte aspiration. The vaginal wall is washed with saline. A 5- to 9-MHz ultrasonographic probe with a sterile cover and attached needle guide is inserted in the vagina to localize the ovaries and the follicles. A 17-gauge needle is subsequently passed via the needle guide through the vaginal fornix into the ovaries. The image below illustrates how the follicular fluid is aspirated. The fluid is delivered to the IVF laboratory, which, ideally, is located adjacent to the operating room.
General anesthesia is not required for ultrasonographic-guided oocyte aspiration. Heavy sedation is suitable for this procedure. The risk of bowel injury is almost zero and allows for oocyte retrieval in patients with a frozen pelvis in whom laparoscopic retrieval is not possible. Furthermore, it avoids the use of carbon dioxide pneumoperitoneum. The major risks are infection and damage to the pelvic vessel, but they should not be a problem if the procedure is carefully performed. [236]
Oocyte classification
The classification of the oocyte is a crucial step for success with IVF. [237] The follicular fluid is scanned under either a dissecting microscope or an inverted microscope. The oocytes are graded according to the appearance of the corona-cumulus complex. The presence of a polar body (metaphase II stage) and/or germinal vesicle (prophase stage) is a determining factor for the preincubation time prior to the insemination. Other oocytes are degenerated (atretic and fractured zona). The last category constitutes fewer than 15% of the total oocytes obtained (see the images below).
Sperm preparation and oocyte insemination
A semen sample is obtained after a 3- to 5-day period of sexual abstinence immediately prior to the oocyte retrieval. For intrauterine insemination (IUI), IVF, and intracytoplasmic sperm injection (ICSI) procedures, the removal of certain components of the ejaculate (ie, seminal fluid, excess cellular debris, leukocytes, morphologically abnormal sperm) with the retention of the motile fraction of sperm is desirable.
For most specimens, the greatest recovery of the motile portion results from separation via centrifugation through a discontinuous density gradient system. However, for certain very poor specimens with low original concentrations of motile sperm, the use of the gradient system results in such a negligible recovery that it is rendered useless. The recourse for these specimens is to simply remove the seminal fluid through successive media washes.
Furthermore, a small number of specimens have acceptable original concentrations of motile sperm but poor recoveries with the gradient system. These specimens benefit most by layering a washed pellet of sperm with nutrient media and allowing the motile fraction to swim up into the media before being separated. [109]
The sperm are incubated for 60 minutes in an atmosphere of 5% carbon dioxide in air. Finally, the supernatant containing the motile fraction of sperm is removed. Sperm concentration and motility are determined. A final number of 200,000 motile sperm in a small volume of media with a layer of mineral oil on top is added to the oocytes.
Embryo culture
The inseminated oocytes are incubated in an atmosphere of 5% carbon dioxide in air with 98% humidity. Ideally, the presence of 2 pronuclei and the extrusion of a second polar body are the criteria required to ascertain fertilization, which should occur approximately 18 hours after insemination (see the image below).
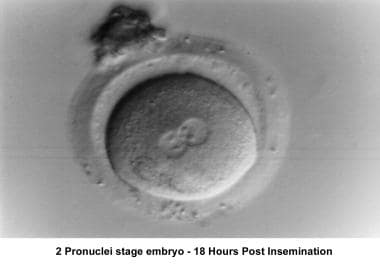 Infertility. Two-pronuclei stage embryo - Eighteen hours postinsemination. Image courtesy of Jairo E. Garcia, MD.
Infertility. Two-pronuclei stage embryo - Eighteen hours postinsemination. Image courtesy of Jairo E. Garcia, MD.
The presence of 3 or more pronuclei can be related to polyspermia or to the retention of the second polar body. This occurs in 9% of all fertilized oocytes (see the image below).
Mechanical dispersion of the cumulus is required when the oocytes are heavily covered by the cumulus; with this, the number of pronuclei can be visualized.
The fertilized oocytes (embryos) are transferred into growth media and placed in the incubator. No further evaluation is performed over the next 24 hours. A 4- to 8-cell stage pre-embryo is observed approximately 36-48 hours after insemination. A 10- to 16-cell embryo is observed after 48-72 hours. The morula or blastocyst stage is observed after 96-120 hours. Although the goal is to observe the embryos as described, pregnancy has been achieved from embryos with slow or delayed cleavage. Embryos are classified according to symmetry, presence of fragments, clarity, and number of blastomeres (see the images below). [237]
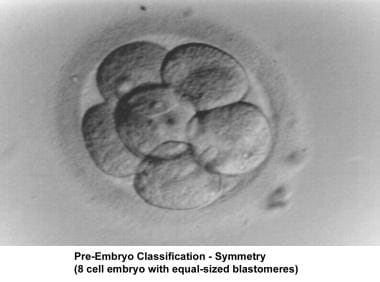 Infertility. Preembryo classification - Symmetry (8-cell embryo with equal-sized blastomeres). Image courtesy of Jairo E. Garcia, MD.
Infertility. Preembryo classification - Symmetry (8-cell embryo with equal-sized blastomeres). Image courtesy of Jairo E. Garcia, MD.
A study by Kleijkers et al sought to determine if embryo culture medium influence pregnancy and perinatal outcome in IVF. The study compared the use of HTF and G5 embryo culture media in IVF and found that the number of utilizable embryos per cycle (2.8 ± 2.3 versus 2.3 ± 1.8), implantation rate after fresh embryo transfer (20.2% versus 15.3%) and clinical pregnancy rate (47.7% versus 40.1%) were higher for couples assigned to G5 compared with those assigned to HTF. The study also found that birthweight was lower in the G5 group compared with the HTF group with a mean difference of 158 g. [238, 239]
Embryo transfer
Transcervical transfer under transabdominal ultrasonography is the most common method used for embryo transfer. The procedure is usually performed within 72 hours after oocyte insemination. However, pregnancies have been reported after transfer performed at the 2-pronuclei stage. Some centers delay the transfer until the embryo reaches the blastocyst stage on day 5. [240, 241, 242, 243]
Several catheters have been designed for embryo transfer (see the first image below). All are equally suitable for embryo transfer, and their use is a matter of physician preference rather than a specific catheter being synonymous with a greater pregnancy rate. The embryos should be loaded with 15-20 µL of culture media. The catheter is advanced up to the fundus of the endometrial cavity, then withdrawn slightly. The embryos are ejected into the miduterine cavity, approximately 1-2 cm from the fundus (see the second image below). Subsequent to the embryo transfer, the patient must be on bedrest for 30-60 minutes.
A novel single blastocyst transfer algorithm reduced multiple gestation rates and improved cryopreservation rates without compromising clinical pregnancy rates in patients with good prognosis. [244]
Management of the luteal phase
Progesterone supplementation during the luteal phase is started 36-72 hours after oocyte retrieval. [218] The exogenous progesterone is administered because of concerns that superovulation and the aspiration of granulosa cells at the time of oocyte retrieval may induce an abnormal endocrine milieu. [245] Relatively recent publications support the benefits of supporting the luteal phase with exogenous progesterone. Several preparations are available; for example, natural progesterone in oil base is administered intramuscularly; vaginal progesterone suppositories, gels and capsules of micronized progesterone are used vaginally or sublingually. [246]
Normally, progesterone supplementation is continued for approximately 2 weeks. If the pregnancy test result is positive, progesterone is continued until the twelfth week of gestation.
Assisted fertilization techniques
Assisted fertilization techniques historically include partial zona dissection (PZD), subzonal sperm injection (SUZI), ICSI, and assisted hatching (AH). Currently, only ICSI and AH are used clinically.
PZD consists of creating a small opening at the zona pellucida level either mechanically or using low-pH solutions (Tyrode) to digest a small portion of the zona. [247] The intent is for the sperm to take advantage of this weak spot to make contact with the oocyte membrane so that fertilization will occur. The rate of fertilization per oocyte is approximately 20-25%, the incidence rate of polyspermia is elevated (50%), and the pregnancy rate is only 5% per cycle. To succeed with PZD requires the recovery of approximately 500,000 sperm after the sperm wash.
The SUZI procedure is indicated in patients for whom conventional IVF or PZD does not work. [248] The procedure consists of suspending the oocyte in a sucrose medium. By osmosis, the oocyte is dehydrated; therefore, the perivitelline space is enlarged. Next, 3-5 spermatozoa are injected into the perivitelline space using a microneedle. Fertilization occurs in approximately 15-20% of the oocytes, the pregnancy rate is less than 3%, and the incidence of polyspermia is 50%.
PZD and SUZI are obsolete and have been replaced by the ICSI procedure. Palermo et al developed the ICSI procedure in Belgium. [249] ICSI has revolutionized the treatment of severe male factor infertility because only a single live sperm is required per oocyte. The sperm can be obtained through masturbation, epididymal aspiration, testicular biopsy, or needle puncture of the testes. [213] ICSI gives the opportunity to males with a history of obstructive azoospermia (perhaps due to congenital absence of the vas deferens) to have a biological child.
The sperm is paralyzed by stroking the distal portion of its tail. The oocyte is stripped from the cumulus using a solution of hyaluronidase. To inject the sperm, first the oocyte is stabilized with a micropipette, then the sperm is loaded, tail first, into a microneedle. The oocyte membrane is pierced with the microneedle, and the oolemma is entered. The spermatozoon is released inside the oolemma, and the microinjected oocyte is kept in the incubator (see the images below).
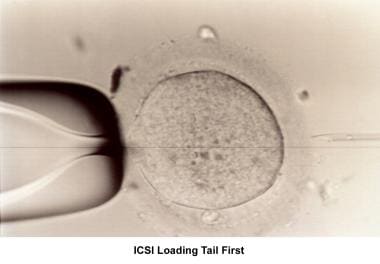 Infertility. Intracytoplasmic sperm injection loading tail first. Image courtesy of Jairo E. Garcia, MD.
Infertility. Intracytoplasmic sperm injection loading tail first. Image courtesy of Jairo E. Garcia, MD.
The fertilization rate varies from 50-75% per oocyte. The current fertilization rate is 95% per patient, and the pregnancy rate is comparable to that of IVF. The risks involved with the procedure are few and include oocyte rupture and damage and/or retention of the second polar body with the presence of 3 pronuclei. [250] Currently, an increased risk of congenital malformation and chromosome abnormalities exists with ICSI. However, a few reports claim that no significant difference exists between IVF and ICSI outcome. [249]
Embryo hatching is an obligatory step in the process of embryo implantation. Cohen et al reported that some of the IVF embryos demonstrated a rather thick zona pellucida; this zona thickness may represent an obstacle for the normal embryo hatching, therefore interfering with the implantation. They suggested the use of selective AH for embryos that show a thick zona pellucida. [251] The procedure is performed a couple of hours before the embryo transfer. AH can be performed mechanically, creating a microrent at the zona level by laser, or by chemical digestion of the zona using a Tyrode solution (low pH). Either of the procedures creates a weak spot within the zona, facilitating the break of the zona and the hatching of the embryo. AH is recommended for patients undergoing IVF who are older than 38 years, patients with multiple ART failures, and in all cryopreserved embryos.
Embryo cryopreservation
The use of GnRHa-COH protocols to prevent the LH surge not only decreased the number of canceled cycles but also increased the number of follicles and their synchronization; therefore, more embryos were available than the ideal number to be transferred. Furthermore, the incidence of multiple pregnancy increased, especially the incidence of triplets, quadruplets, and so on. Embryo cryopreservation became an important part of ART to prevent multiple pregnancies, to prevent maternal and fetal complications, and to decrease the cost of ART because patients have the opportunity to achieve a pregnancy from the same IVF cycle. [252, 253, 254]
Embryo cryopreservation can be performed at the 2-pronuclei stage, on cleaved embryos, and at the blastocyst stage. [255, 256] The embryos are stored in liquid nitrogen for a period previously agreed upon by the IVF program policy and the patient. Most of the IVF programs store the embryos for a period of 3-5 years, and their subsequent disposition should be outlined in the consent form, to include (1) embryo donation to another couple, (2) donating the embryos for research, or (3) disposition of the embryo after thawing.
Frozen embryo transfer
The transfer of cryopreserved embryos can be performed during a natural menstrual cycle or during an artificial endometrium-stimulated cycle. No differences in the pregnancy rate occur, provided that the endometrium, during a natural menstrual cycle, demonstrates a 10-mm thick triple-line pattern. The embryos are thawed 24, 48, or 96 hours after ovulation, depending on the stage at which cryopreservation was performed. The thawed embryos are incubated 24 hours before embryo transfer.
The artificially stimulated endometrium requires detection of ovulation during a previous menstrual cycle and down-regulation of the pituitary gland with GnRHa starting on the 21st menstrual cycle day and is followed by increasing doses of estrogen starting with the onset of the menstrual bleeding. [257] Estrogen is administered for 2 weeks. At that time, pelvic ultrasonography is performed to determine endometrial thickness. If the above-mentioned criteria are fulfilled, progesterone is added to the estrogen for 48 hours, then the embryos are thawed, cultured, and transferred as previously described. The administration of estrogen and progesterone is continued until the day of the pregnancy test. If the pregnancy test result is positive, the hormonal support must be continued until the 10th week of pregnancy.
IVF-Related Procedures
Though rarely performed, gamete and zygote intrafallopian transfer have been used as alternatives to IVF.
Gamete intrafallopian transfer
In 1985, Asch et al described the gamete intrafallopian transfer (GIFT) procedure. [258] The procedure consists of ovarian stimulation, monitored follicular development, and oocyte aspiration similar to IVF. It differs in that the patient must have at least 1 normal-appearing and patent fallopian tube.
On the day of oocyte retrieval, the male provides a semen sample 2 hours before the procedure to perform the sperm wash and capacitation. Once the oocytes have been classified, a laparoscopy or a minilaparotomy is performed. The oocytes, along with 150,000 sperm, are loaded into a special catheter in the laboratory under a microscope. The loaded catheter is brought to the operating room, where the laparoscopist is waiting. The injection of the gametes into the fallopian tube requires that the fimbria be gently picked up with an atraumatic grasping forceps. The ostium has already been identified, so in a minimal amount of time, the tip of the catheter is passed through the ostium and advanced up to the ampulla of the fallopian tube(s), where the gametes are released. Fertilization should occur inside the fallopian tube.
It appears that this procedure is more physiologic. Nevertheless, a major drawback of the GIFT procedure is that it does not allow for visual confirmation of fertilization because it occurs inside the body. Also, if pregnancy does not occur, no method exists to determine whether the cause was lack of fertilization or lack of implantation. Additionally, general anesthesia is required. Finally, with the GIFT procedure, a laparoscopy or minilaparotomy is required, which implies additional expense.
Zygote intrafallopian transfer
The zygote intrafallopian transfer (ZIFT) procedure is a combination of IVF and GIFT. [259] Fertilization occurs in the IVF laboratory. However, the pre-embryo is transferred into the fallopian tube via laparoscopy at the 2-pronuclei stage or 24 hours after oocyte retrieval. One variant of the ZIFT procedure is the transfer of the cleaved pre-embryos at 48 hours (tubal embryo transfer). [260] This procedure requires that the patient be taken to the operating room twice, which represents additional expense and doubles the risk for complications.
Outcomes of ART
A normal term pregnancy is the ultimate goal of IVF; thus, the pregnancy rate is the best indicator for evaluation of a program. Its efficiency can also be determined based on the following parameters:
-
The number of patients who canceled because of deficient response to stimulation or spontaneous LH surge
-
The number and quality of oocytes obtained at aspiration
-
The number of oocytes that fertilized and cleaved properly
-
The number of cryopreserved embryos
-
The number of embryos transferred
The success of a program is also established by the patient population enrolled in the program, the indication(s) for the procedure, and the patient's age at the time of ovarian stimulation. Some IVF programs enroll only patients with good prognoses in order to maintain a higher pregnancy rate.
In 2006, 126,726 ART procedures were performed in the United States as follows [261] :
-
In vitro fertilization - >99%
-
Gamete intrafallopian transfer - < 1.0%
-
Zygote intrafallopian transfer - < 1.0%
-
Cryopreserved embryos - 14%
-
Donor oocyte - 11%
The primary diagnosis or indications for ART were as follows:
-
Tubal factor - 9.0%
-
Male factor - 17.0%
-
Endometriosis - 5.0%
-
Uterine - 1.0%
-
Ovulatory dysfunction - 6.0%
-
Diminished ovarian reserve - 13.0%
-
Unknown factor - 11.0%
-
Other factors - 9.0%
-
Multiple factors, female only - 12.0%
-
Multiple factors, male and female - 17.0%
Success rates for fresh cycles were as follows:
-
Overall pregnancy rate per initiated cycle - 35.0%
-
Live birth rate per initiated cycle - 28.6%
-
Live births per oocyte retrieval - 31.9%
-
Live births per embryo transfer - 35.7%
The incidence of miscarriage (spontaneous abortion, 15.0%), stillbirths (0.5%), congenital malformation, or chromosome abnormality is similar to that of the general population. Ectopic pregnancy has been reported after IVF due to migration of the embryo through the cornual ostium. Ectopic pregnancy occurs in approximately 0.7% of cases. In some instances, ectopic pregnancy is associated with heterotopic pregnancy.
The pregnancy rate for fresh cycles by patient age was approximately 44.7% among women younger than 35 years and declined to 37.2% for the group aged 35-37 years. Women aged 38-40 years yielded a pregnancy rate of 27.6%. The success rate was approximately 17.7% in those older than 40 years. Pregnancy for those aged 43-44 years is the exception (9.2%).
The live birth rate by primary diagnosis was similar in all groups, as follows:
-
Male factors - 35.7%
-
Endometriosis - 33.2%
-
Ovarian factors - 14.4%
-
Tubal factors - 29.7%
-
Uterine factors -26.6%
-
Other causes - 24.8%
-
Unexplained - 31.9%
-
Ovulatory dysfunction - 36.5%
-
Multiple factors, female only - 22.4%
-
Multiple factors, female and male - 27.7%
The percentage of ART births that were multiple births was 30.8% (twins, 28.8%; triplets or more, approximately 2.1%). This compares with a multiple live birth rate of 3% in the general US population.
The pregnancy rate is also related to the number of embryos transferred, as follows:
Table. Live Birth Rates (Open Table in a new window)
Number of Embryos |
Percentage of Cycles |
Live Birth Rate, % |
Multiple Births Rate, % |
1 |
12.8 |
98.0 |
2.0* |
2 |
39.5 |
66.7 |
32.6 |
3 |
37.7 |
61.7 |
32.6 |
4 |
32.8 |
62.3 |
32.1 |
5+ |
28.9 |
63.2 |
31.3 |
* Identical twins |
|||
The live birth rate from frozen pre-embryos is 28.9%, which is less than the 35.7% rate achieved with a fresh embryo transfer.
In a follow-up study of 946 couples by Donckers et al, spontaneous pregnancy occurred in 28% of all couples, and treatment-dependent pregnancies occurred in 32%. IVF (51% live births in couples treated) and no treatment/expectant management (50%) were the most effective treatments. [262]
Alternatives
Donor oocyte
Patients with poor ovarian reserve have a rather low chance of overcoming infertility; yet, some of them, along with patients with premature menopause and patients with physiologic menopause, are interested in having a child. The only alternative for these patients is adoption or an oocyte donation. [263, 264] Donor oocyte is the counterpart of donor sperm. The source of the oocyte can be anonymous or known (ie, younger relative, designated donor). Ideally, the donor should be aged 21-30 years, although the age can extend to 35 years.
The donor undergoes ovulation induction according to the standard IVF protocol. Meanwhile, the recipient takes increasing doses of estrogens to synchronize her endometrium in preparation for a fresh embryo transfer. This technique is similar to the one described under Frozen embryo transfer. [257]
Because oocyte cryopreservation is still in a preliminary stage of development, only fresh oocytes without quarantine are used. However, the donor must be screened for numerous transmissible diseases (eg, HIV, syphilis, hepatitis, gonorrhea, chlamydia) according to FDA regulations, and a complete physical and gynecological evaluation is performed. The donor patient also undergoes a psychological evaluation. The recipient patient also has a psychological evaluation. The oocyte recipient and her partner are required to have the same kinds of screening tests as the oocyte donor. The legal aspects of the procedure and future offspring must be discussed. A thorough consent form must be signed by all parties involved. [265]
The success of donor oocyte programs surpasses the success of conventional IVF. The risk of multiple pregnancy is very high; thus, the conservative approach of transferring only 2 embryos is highly recommended to the recipient couple.
Donor sperm
Men who cannot produce sperm or women who do not have a partner and wish to become pregnant may opt for donor sperm. Many opt to use sperm banks, which require strict and rigorous infectious testing. The sperm donor may choose to be known or anonymous. After deposition, the samples are frozen and quarantined for at least 6 months. Once repeat infectious testing is confirmed to be negative, the sample is available for selection and intrauterine insemination or IVF is performed in sync with the patient's cycle.
Donor embryo
As stated in the embryo cryopreservation consent form, the couple must sign an advance directive regarding embryo ownership and disposition. Those directives should include statements regarding (1) embryo donation to another couple, (2) donation of the embryos for research, or (3) disposition of the embryos after thawing.
Donor embryo is one option the patient can choose; therefore, those embryos can be donated according to the IVF program policy. Embryo donation programs must follow the regulations established for tissue donors, which require that all screening tests must be performed before embryo cryopreservation, with the tests being repeated at least 6 months later. The same screening tests are required for the recipient couple. [266]
The donor embryo transfer protocol is identical to the protocol described for cryopreserved embryo transfer. [257] The embryos must not be sold as a commodity. This concept has been endorsed by the Society of Assisted Reproductive Technology and the ASRM. [267]
Gestational carriers
Patients who were unable to have a biological child because of absence of the uterus (congenital or acquired) or patients in whom a pregnancy is contraindicated are now able to have a biological child by the use of a gestational carrier. A gestational carrier is a woman who is carrying a pregnancy resulting from embryos created by IVF, using the gametes of the intended parents. The gestational carrier program must be designed under the strictest policies because of the medicolegal implications.
The selection or approval of the gestational carrier should be based on the premise that the pregnancy does not imply a risk for the carrier and that all the consent forms have been signed once the carrier and the genetic parents have completed the psychological evaluations and screening tests. The endometrium of the gestational carrier is artificially stimulated through the administration of estrogen and progesterone as described under Frozen embryo transfer. [257] A national consensus does not exist regarding the birth certificate for children born through gestational carrier treatment. Some states have no requirement to mention the gestational carrier; in others, a complete adoption process is required.
Guidelines
The German Society of Gynecology and Obstetrics (DGGG), in cooperation with the Swiss Society of Gynecology and Obstetrics (SGGG) and the Austrian Society of Gynecology and Obstetrics (OEGGG), have developed guidelines for counselling, diagnostic workup, and treatment of infertility. [268]
Infertility consultation and workup
The initial history should include an inquiry concerning risk factors relevant to infertility, such as age, smoking status, alcohol intake, eating disorders, drug use, and intensive physical exercise. The patient must understand that such factors may not only adversely affect the treatment outcome but also potentially damage gametes and embryos. Patients must also understand that a body mass index (BMI) over 30 kg/m2 or under 19 kg/m2 may predispose to ovulation disorders, which may cause infertility.
Before fertility treatment is initiated, women must be informed that folic acid substitution is required.
Appropriate psychotherapy or counseling should be recommended to patients whose fertility disorder is related to behavior (eg, eating disorder, drug addiction).
Sexuality and sexual disorders
Patients should be asked about sexual issues in the couple’s relationship. Sexual therapy should be recommended to couples who feel that their sexual behavior and experience require treatment.
Psychological factors
Screening tools for psychological vulnerability may be considered, if relevant. In these cases, a psychosomatic diagnosis should be offered; routine psychopathological diagnosis is unnecessary. Psychosocial counseling or psychotherapy is generally not recommended in these cases unless the fertility disorder has a behavioral etiology or the patient has a mental illness that requires treatment.
Diagnosis and treatment of congenital and acquired genital anomalies
Following the gynecological examination, vaginal ultrasonography must be performed to rule out congenital malformation. Three-dimensional vaginal ultrasonography with or without hysteroscopy, possibly combined with laparoscopy, should be performed if a congenital malformation is suspected.
Fibroids must be diagnosed with vaginal ultrasonography. Before fertility treatment is initiated, submucosal fibroids (Federation of Gynecology and Obstetrics [FIGO] type 0 and 1) must be removed hysteroscopically. Laparoscopy may be performed for intramural and subserous fibroids.
Suspected intrauterine polyps and/or adhesions should be evaluated with hysteroscopy. Hysteroscopy should be used to remove intrauterine polyps and adhesions.
If tubal patency evaluation is indicated, either laparoscopy with chromopertubation or hysterosalpingo contrast ultrasonography must be performed. Laparoscopy used to investigate tubal patency must be combined with hysteroscopy.
Women with a septate or subseptate uterus should undergo hysteroscopic septum dissection before fertility treatment is initiated. Bicornuate uterus, duplex uterus, and unicornuate unicollis uteri should not be corrected surgically in women with primary infertility.
Hydrosalpinx must be treated with laparoscopic salpingectomy or laparoscopic proximal tubal occlusion before assisted reproductive treatment (ART) is initiated.
Diagnosis and treatment of endometriosis
Infertile women with suspected endometriosis should undergo laparoscopic diagnostic workup with histological confirmation, chromopertubation, and hysteroscopy.
Peritoneal foci of endometriosis should be removed surgically.
Patients with ovarian endometriosis should be counseled regarding the procedural risks (reduced ovarian reserve) and possible benefits of surgery preoperatively.
Deep infiltrating endometriosis may be treated with surgical resection.
Diagnosis and treatment of vaginal infections
Asymptomatic women should not undergo screening for bacterial vaginosis with vaginal smears, nor should patients undergo acute chlamydia infection screening if asymptomatic. However, screening for chronic chlamydia infection may be performed with serology.
Clindamycin or metronidazole is suggested as treatment for bacterial vaginosis.
Infection prophylaxis is unwarranted in asymptomatic women and in the absence of pathogen confirmation.
Diagnosis and treatment of endocrine factors
The basic hormonal diagnostic workup in women with infertility consists of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, testosterone, dehydroepiandrosterone (DHEAS), sex hormone–binding globulin (SHBG), free androgen index, estradiol, and anti-müllerian hormone (AMH) on days 3-7 of the menstrual cycle (or when no follicle has a diameter >10 mm). Vaginal ultrasonography and thyroid evaluation are performed along with the basic diagnostic workup. Any additional testing is based on specific findings.
Progesterone levels may be assessed at approximately 7 days following presumed ovulation to determine ovulatory cycle.
Primary or secondary amenorrhea
A pregnancy test is the first step in evaluating for amenorrhea. After a basic diagnostic endocrine workup is performed, additional examinations are based on symptoms.
Women with a high BMI (>30 kg/m2) should be advised to lose weight.
Hyperprolactinemia
Confirmed hyperprolactinemia in women should be treated with dopamine agonists.
Polycystic ovary syndrome or hyperandrogenemia
If polycystic ovary syndrome (PCOS) is suspected, diagnostic criteria for PCOS must be evaluated clinically. Rotterdam criteria include abnormal periods with oligoovulation or anovulation, laboratory-confirmed or clinical hyperandrogenemia, and characteristic PCO sonomorphology findings.
Drug therapy to induce ovulation should be monitored with ultrasonography, especially in women with PCOS, to reduce the likelihood of multifollicular growth, multiple pregnancy, and overstimulation.
In women with PCOS and oligo-ovulation or anovulation, clomiphene stimulation or letrozole stimulation (off-label) is first-line therapy to induce ovulation.
Adrenogenital syndrome and congenital adrenal hyperplasia
If androgenital syndrome (AGS) is suspected, molecular-genetic testing must be performed. Partners with confirmed AGS must be provided with genetic counselling.
Glucocorticoid treatment should be administered to women with classic AGS. An endocrinologist must be consulted for treatment and monitoring.
Anti-müllerian hormone, age, and oocyte quality
Although the AMH level may be used to estimate ovarian activity and responsiveness to hormone stimulation treatment, it is not used for fertility evaluation.
Anovulatory cycle and luteal phase insufficiency
In women with a regular and unremarkable menstrual cycle duration, endometrial biopsy to evaluate the luteal phase is unwarranted.
Cyclical progestogens should not be given to women with spontaneous menstrual cycles.
Diabetes mellitus
Before conception, hemoglobin A1c (HbA1c) testing must be performed in women with diabetes. A planned pregnancy is appropriate only when blood sugar levels are within the reference range or near the reference range.
Thyroid dysfunction
All women who want children should undergo thyroid-stimulating hormone (TSH) testing. A TSH value exceeding 2.5 mU/L should prompt testing of anti-thyroid antibodies.
At least 100-150 µg iodine supplementation/day should be given to women before conception.
L-thyroxine should be used in women with a TSH level of 2.5 mU/L or more to decrease the TSH level to less than 2.5 mU/L.
Definitive thyroid treatment must be completed in women with hyperthyroidism before ART is initiated and prior to conception.
Treatment of immunological factors
Women with antiphospholipid syndrome or systemic lupus erythematosus (SLE) must undergo treatment by an interdisciplinary team prior to conception. Antibody status, disease activity, comorbidities, and an updated treatment approach are components of management.
Rheumatoid arthritis, chronic inflammatory bowel disease (IBD), multiple sclerosis (MS), and other autoimmune or immune disorders must be closely managed by an interdisciplinary team, with treatment initiated before conception.
Vaccination status
The patient’s vaccination status should be evaluated. Rubella and varicella zoster immunity status must be confirmed and vaccination recommended, if necessary. Tetanus, diphtheria, and pertussis vaccinations should be given to women of childbearing age.
-
Infertility. Cervical os changes during the menstrual cycle. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Clear preovulatory cervical mucus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Fern pattern of preovulatory mucus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hysterosalpingogram image demonstrating normal findings with bilateral spillage. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Antero-retroflexion uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Anteroflexion or retroflexion of the uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bilateral cornual obstruction. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Salpingitis isthmica nodosa. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hydrosalpinx. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Tubal obstruction post–bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Tubal obstruction: Pomeroy bilateral tubal ligation. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Intravasation of the contrast medium due to myoma. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Extravasation or lymphatic penetration of the contrast medium. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Irregular cavity due to intramural fibroids intruding into the cavity. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Submucous fibroid. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Intrauterine synechiae. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Synechiae. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Endometrial polyp. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Placental polyp. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. T-shaped uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Fundal and right fibroid tubal obstruction. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Unicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Didelphys uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Double vagina. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Irregular endometrial cavity after myomectomy. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Sonogram: Sagittal view of the uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Sonogram: Sagittal view of the uterus. Three-laminar endometrial pattern. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Sonogram: Sagittal view of normal uterine cavity. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Sonogram: Transverse view of the uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Intramural fibroid. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Submucous myoma. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Endometrial polyp. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Ovarian cyst. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Dermoid cyst. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Endometrioma. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Corpus luteum. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Preovulatory follicle. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Multiple follicles during ovulation induction with human menopause gonadotropin. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Polycystic ovary. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Preovulatory follicle in polycystic ovary during clomiphene citrate ovulation induction. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Uterine septum. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Uterus didelphys. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Sonohysterogram. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hysteroscopy - Uterine synechiae. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hysteroscopy - Uterine synechiae. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hysteroscopy - Endometrial polyp. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hysteroscopy - Submucous fibroid. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Uterine septum. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Arcuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Basal body temperature chart. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Normal uterus, ovaries, and fallopian tubes. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus and ectopic pregnancy. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Uterine fibroids. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Hydrosalpinx. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Peritubal and ovarian adhesions. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Frozen pelvis. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Frozen pelvis. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Pelvic endometriosis. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Pelvic endometriosis. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Endometrioma. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Endometrioma. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Tubal ligation. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Uterus - Tubal ligation. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Bicornuate uterus - Ectopic pregnancy. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Ectopic pregnancy. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Ectopic pregnancy. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Paratubal cyst. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Septate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Septate uterus. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Septate uterus and pedunculated fibroid. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Polycystic ovaries. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Polycystic ovary. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Metroplasty - Strassman technique. Image courtesy of Clifford R. Wheeless, Jr, MD.
-
Infertility. Metroplasty - Jones technique. Image courtesy of Clifford R. Wheeless, Jr, MD.
-
Infertility. Diagram of oocyte aspiration by laparoscopy.
-
Infertility. Diagram of oocyte aspiration by transvaginal ultrasonography.
-
Infertility. Metaphase II preovulatory oocyte. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Atretic. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Metaphase I oocytes. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Fractured zona. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Fractured zona. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Two-pronuclei stage embryo - Eighteen hours postinsemination. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Polyspermia or second polar body. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Preembryo classification - Symmetry (8-cell embryo with equal-sized blastomeres). Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Fragments. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Blastomeres/embryo. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Clear blastomeres in a cleaving embryo. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Embryo (8-cell stage). Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Embryo (10- to 12-cell stage). Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Blastocysts. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Catheters for embryo transfer. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Preembryos being ejected. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Intracytoplasmic sperm injection loading tail first. Image courtesy of Jairo E. Garcia, MD.
-
Infertility. Intracytoplasmic sperm injection. Image courtesy of Jairo E. Garcia, MD.