Overview
Aneuploid conceptions constitute the majority of implantation and pregnancy failures in women of advanced maternal age. One of the best ways to address the age-associated decline in fertility is through preimplantation genetic testing. Preimplantation genetic testing (PGT) is a technique used to identify chromosomal genetic abnormalities in embryos created through in vitro fertilization (IVF) before pregnancy. Preimplantation genetic testing is an umbrella term that refers to the assessment of embryos prior to implantation or pregnancy.
Currently, three different types of PGT exist: PGTa, which refers to techniques where embryos are screened for aneuploidy; PGTm, which refers specifically to when one or both genetic parents has a known genetic abnormality and testing is performed on an embryo to determine if it also carries a genetic abnormality; and PGTsr, which involves specifically screening for a structural rearrangment of chromosomes such as balanced translocations.
Because only unaffected embryos are transferred to the uterus for implantation, PGT is the only method available for screening embryos before pregnancy and provides an alternative to current postconception diagnostic procedures (ie, amniocentesis or chorionic villus sampling), which are frequently followed by the difficult decision of determining the pregnancy's disposition. PGT is presently the only option available for avoiding a high risk of having a child affected with a genetic disease prior to implantation. It is an attractive means of preventing heritable genetic disease, thereby eliminating the dilemma of pregnancy termination following unfavorable prenatal diagnosis.
PGTa allows for better embryo selection, which improves implantation rates with single embryo transfer and reduces miscarriage rates. Pregnancy complications such as multiple gestation, preterm or low birth weight infants can be reduced with single embryo transfers as only one embryo is transferred at a time.
Advancements in embryo culture, blastocyst biopsy techniques, 24-chromosome aneuploidy screening platforms, and improved genomic coverage of new sequencing platforms, such as next-generation sequencing, have made PGT safe and accessible for all patients who undergo in vitro fertilization. The modern approach to IVF involves blastocyst culture and biopsy followed by PGT and a single embryo transfer.
History
Edwards and Gardner successfully performed the first known embryo biopsy on rabbit embryos in 1968. In humans, PGT was developed in the United Kingdom in the mid 1980s as an alternative to current prenatal diagnoses. [1] Initially, PGT revolved around determination of gender as an indirect means of avoiding an X-linked disorder. In 1989 in London, Handyside and colleagues reported the first unaffected child born following PGT performed for an X-linked disorder.
The use of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening or PGS, has increased in recent years, now encompassing an estimated 40% of in vitro fertilization (IVF) cycles in the United States. [2] This technique has evolved throughout the years and is now largely performed by biopsy of the blastocyst trophectoderm cells with analysis using techniques such as next-generation sequencing (NGS) and comparative genomic hybridization (CGH) to test for aneuploidy. PGTm is currently available for most known genetic mutations. [3]
Indications and Conditions
Indications for preimplantation genetic testing
Preimplantation genetic testing (PGT) is recommended when couples risk transmitting a known genetic abnormality to their children. Only healthy and normal embryos are transferred into the mother's uterus, thus diminishing invasive prenatal diagnoses, late pregnancy termination, or the birth of a child with a serious genetic disease.
Primary candidates for PGT
These include the following:
-
Carriers of autosomal recessive diseases (for carriers of autosomal recessive diseases, the risk an embryo may be affected is 25%)
-
Carriers of autosomal dominant diseases (for carriers of autosomal dominant disease, the risk an embryo may be affected is 50%)
-
Couples with a family history of X-linked disorders (couples with a family history of X-linked disease have a 25% risk of having an affected embryo [half of the male embryos])
-
Couples with chromosomal translocation, which can cause implantation failure, recurrent pregnancy loss, or mental or physical problems in offspring [4]
Types of PGT
There are three different types of tests performed on embryos during IVF: preimplantation genetic testing for monogenic/single-gene diseases (PGT-M), preimplantation genetic testing for structural chromosome rearrangements (PGT-SR), and preimplantation genetic testing for aneuploidy (PGT-A). PGT should be offered for 3 major groups of disease: (1) sex-linked disorders, (2) single gene defects, and (3) chromosomal disorders.
PGT-A
Most early pregnancy losses can be attributed to aneuploidy. Because only chromosomally normal embryos are transferred into the uterus, the risk of first and second-trimester loss is markedly reduced. The value of PGT-A as a universal screening test has yet to be determined. PGT-A likely will be a part of multidimensional embryo selection in the future, but currently, there is insufficient evidence to recommend PGT-A for all patients experiencing infertility. [5]
Primary candidates for PGT-A can include the following:
-
Women of advanced maternal age
-
Couples with a history of recurrent pregnancy loss
-
Couples with repeated IVF failure
-
Male partner with severe male factor infertility
These patient populations are at risk of failure with IVF because of a high proportion of aneuploid embryos. PGT-A is believed to decrease this risk by selecting chromosomally normal embryos with a higher chance of implantation.
Advanced maternal age
The risk of aneuploidy in children increases as women age. The chromosomes in the egg are less likely to divide properly, leading to an extra or missing chromosome in the embryo (see Table 1). The rate of aneuploidy in embryos is greater than 20% in mothers aged 35-39 years and is nearly 40% in mothers aged 40 years or older. The rate of aneuploidy in children is 0.6-1.4% in mothers aged 35-39 years and is 1.6-10% in mothers older than 40 years. The difference in percentages between affected embryos and live births is because an embryo with aneuploidy is less likely to be carried to term and will most likely be miscarried, some even before pregnancy is suspected or confirmed. Therefore, using PGD to determine the chromosomal constitution of embryos increases the chance of a healthy pregnancy and reduces the number of pregnancy losses and affected offspring. [6]
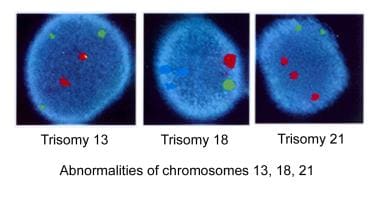
One of the most frequent aneuploidies, trisomy (ie, 3 identical chromosomes present in the embryo), is trisomy of chromosome 21, which leads to Down syndrome. This particular abnormality also frequently leads to spontaneous miscarriage, the precise frequency of which is difficult to determine. Thus, the only reliable information is on the frequency of babies born with Down syndrome. An informative article in the Journal of the American Medical Association [7] includes information on estimating the incidence of trisomy 21/Down syndrome in fetuses at 16 weeks of pregnancy (also see Table 2).
Table 1. Chromosomal Abnormalities (Open Table in a new window)
Age, y |
Embryos (Normal), % |
Embryos (Aneuploidy), % |
Other Abnormality, % |
25-35 |
61 |
8 |
31 |
36-37 |
60 |
10 |
30 |
38-39 |
47 |
18 |
35 |
40-41 |
43 |
26 |
31 |
42-44 |
39 |
30 |
31 |
Table 2. Frequency of Down Syndrome Per Maternal Age (Open Table in a new window)
Age, y |
Frequency of Fetuses With Down Syndrome to Normal Fetuses at 16 Weeks of Pregnancy |
Frequency of Live Births of Babies With Down Syndrome to Normal Births |
15-19 |
. . . |
1/1250 |
20-24 |
. . . |
1/1400 |
25-29 |
. . . |
1/1100 |
30-31 |
. . . |
1/900 |
32 |
. . . |
1/750 |
33 |
1/420 |
1/625 |
34 |
1/325 |
1/500 |
35 |
1/250 |
1/350 |
36 |
1/200 |
1/275 |
37 |
1/150 |
1/225 |
38 |
1/120 |
1/175 |
39 |
1/100 |
1/140 |
40 |
1/75 |
1/100 |
41 |
1/60 |
1/85 |
42 |
1/45 |
1/65 |
42 |
1/35 |
1/50 |
44 |
1/30 |
1/40 |
45 and older |
1/20 |
1/25 |
Early studies of PGT-A in patients with advanced maternal age seemed promising, with a decreased rate of miscarriages and a higher proportion of live births in the PGT-A group compared with the control group. Although data indicate an increased incidence of aneuploidy in older patients, several randomized controlled trials showed that routine PGT did not increase pregnancy rates after IVF in patients with advanced maternal age. [8, 9] Although PGT-A does not appear to statistically improve the chance of pregnancy in women with advanced maternal age, one study detected a trend toward decreasing the risk of miscarriage, thereby increasing the chance of live birth. [10]
Recurrent pregnancy loss
Recurrent pregnancy loss (RPL) is usually defined as 2 or more consecutive pregnancy losses before 20 weeks of gestation. The cause is frequently unknown but may be secondary to fetal anomalies or uterine abnormalities. Chromosomal abnormalities are noted in 50-80% of abortuses, [11] and couples with RPL have a higher percentage of aneuploid embryos than patients without RPL. [12] However, using PGT-A does not improve the pregnancy rate in this group of patients but increases the likelihood of delivery at term. [13] PGT-A also benefits the subgroup of patients who have proven abnormal concepts by cytogenetic analysis. [13]
Recurrent IVF failure
Recurrent IVF failure (RIF) is usually defined as 3 or more failed IVF attempts involving high-quality embryos. Evidence suggests that this patient population has more chromosomally abnormal embryos. [14] However, no study has shown an improvement in pregnancy rate with PGT in patients who have a history of RIF. Although most IVF failures can be accounted for by embryonic aneuploidy, various immunological and uterine factors likely contribute to implantation failure.
Male factor infertility
Gonadal failure in men has been linked to the generation of embryos with an increased incidence of inherited and de novo chromosomal abnormalities. Normal fertile men have approximately 3-8% of sperm that are chromosomally abnormal. This risk increases significantly in men with severe infertility (ie, low sperm count, poor morphology, and poor motility) to approximately 27-74% abnormal spermatozoa. [15] With the introduction of intracytoplasmic sperm injection (ICSI) in assisted reproductive techniques, clinicians have given men with poor sperm quality the opportunity to overcome natural selection and successfully produce a zygote.
Various genetic defects are associated with male factor infertility. This includes aneuploidy, most commonly Klinefelter syndrome, Robertsonian translocations, Y chromosome microdeletions, androgen receptor mutations, and other autosomal gene mutations (eg, cystic fibrosis transmembrane conductance regulator gene and sex hormone-binding globulin gene mutations). [16] Therefore, a high risk of transmission of genetic mutations to the patient's offspring is associated with IVF involving ICSI.
The use of PGT-A does not seem to be an independent factor associated with live birth per transfer in couples with severe male factor infertility (SMFI). [17]
Family balancing
Because PGT-A can determine the sex of the embryo, many couples request preimplantation genetic testing for family balancing or sex selection, which can be motivated by cultural, social, ethnic, psychological, and other reasons.
The use of PGT-A for sex selection and family balancing is unrelated to disease, is controversial, and has elicited moral outrage about not implanting normal embryos when they are found to be of the undesired sex. Frequent objections include the danger of sex discrimination, the perpetuation of oppression against females, the ethics of expanding control over nonessential characteristics (those not required for life) of offspring, and the relative importance of sex selection when weighed against medical and financial burdens to parents. Personal, religious, ethical, and moral norms vary among different populations, and proper respect must be given to these views when discussing the performance of PGT for sex selection. Much discussion is still necessary to achieve a reasonable consensus and acceptance of PGT for sex selection.
Human leukocyte antigen (HLA) matching
Among the new indications of PGT is preimplantation HLA matching. This technique can be applied to exclude the presence of a genetic disorder and provide a potential donor for stem cell or bone marrow transplantation to an affected child with recessive diseases, including thalassemias or acquired malignancies such as leukemia. This has been previously used to avoid the birth of a child with Fanconi anemia, an autosomal recessive disorder, whose HLA-matched cord blood stem cells were successfully transplanted to cure the affected sibling. [18]
PGT-M
Preimplantation genetic testing for monogenic/single-gene diseases is used to identify single-gene defects such as cystic fibrosis, neurofibromatosis type 1, Tay-Sachs disease, sickle cell anemia, and Huntington's disease. In such diseases, the abnormality is detectable with molecular techniques using polymerase chain reaction (PCR) amplification of the DNA from a single embryonic cell.
Although progress has been made, some single gene defects, such as cystic fibrosis, have multiple known mutations. In cystic fibrosis, there is a constantly changing number of known mutations, and third parties variably test different combinations of the known mutations. Because most of these rare mutations are not routinely tested, a parent without any clinical manifestations of cystic fibrosis could still be a carrier. This allows a parent carrying a rare mutation gene to be tested as negative but still have the ability to pass on the mutant cystic fibrosis gene.
PGT-M can also identify genetic mutations like BRCA-1, which does not cause a specific disease but increases the risk of a set of diseases.
PGT-M additionally detects sex-linked genetic disorders. X-linked diseases are passed to the child through a mother who is a carrier. They are passed by an abnormal X chromosome and manifest in sons, who do not inherit the normal X chromosome from the father. Because the X chromosome is transmitted to offspring/embryos through the mother, affected fathers have sons who are not affected. Still, their daughters have a 50% risk of being carriers if the mother is healthy. Sex-linked recessive disorders include hemophilia, fragile X syndrome, most neuromuscular dystrophies (currently, >900 neuromuscular dystrophies are known), and hundreds of other diseases. Sex-linked dominant disorders include Rett syndrome, incontinentia pigmenti, pseudohypoparathyroidism, and vitamin D–resistant rickets.
PGT-M for adult-onset conditions is ethically justified when the condition is serious, and no safe, effective interventions are available. Evidence shows that PGT-M is a low-risk procedure, but there are many scientific, psychological, and social issues involved in the use of PGT-M. [19] Patients considering PGT-M should be sure to meet with an experienced genetic counselor.
PGT-SR
Preimplantation genetic testing for structural chromosome rearrangements is used to identify potential chromosomal disorders. Chromosomal disorders involve various chromosomal rearrangements, including translocations, inversions, and deletions, and can be detected using next-generation sequencing (NGS). NGS platforms sequence small fragments of DNA in parallel, mapping the individual reads to a human reference genome. NGS can be used to sequence the entire genome or be limited to specific areas of interest. [20]
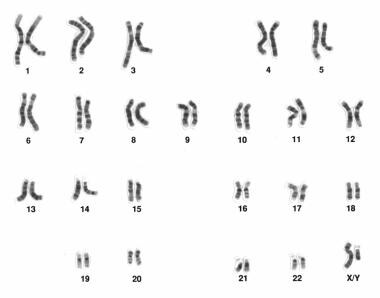 Human male karyotype. Courtesy of Wikimedia Commons [https://commons.wikimedia.org/wiki/File:Human_male_karyotpe_high_resolution.jpg, provided by the National Human Genome Research Institute, National Institutes of Health (NIH)].
Human male karyotype. Courtesy of Wikimedia Commons [https://commons.wikimedia.org/wiki/File:Human_male_karyotpe_high_resolution.jpg, provided by the National Human Genome Research Institute, National Institutes of Health (NIH)].
Some parents may have never achieved a viable pregnancy without using PGT-SR because previous conceptions resulted in chromosomally unbalanced embryos and were spontaneously miscarried. Prospective parents are routinely karyotyped—once a translocation, inversion, or deletion is found, it can take time for specific probes to determine if the embryos possess the same structural chromosome rearrangements.
Process
Approach considerations
While all patients should be offered preimplantation genetic testing for aneuploidy (PGT-A), there are certain groups which have been found to benefit from PGT-A. One group is women of advanced maternal age (AMA), greater than 35 years old. In this age group, PGT-A testing improved the live birth rate and decreased the miscarriage rate from 39% to 2.7%. This also demonstrated a shorter time to pregnancy. [21] .
The increasing availability of PGT-A also allows for utilization of elective single embryo transfer (eSET). For those patients who underwent eSET/PGT-A, there was a significantly increased live birth rate per embryo transfer cycle. However, less than half of PGT-A cycles had a euploid embryo to transfer. For those patients that undergo PGT-A, there is a natural subset of embryos that will not be eligible for transfer, as any patient is likely to have at least some percentage of aneuploid embryos.
Before requesting preimplantation genetic testing for monogeneic abnormalities (PGT-M), candidates should consult a geneticist or a genetic counselor to evaluate the risk of transferring their genetic abnormality to their offspring. Tests should be performed to confirm the diagnosis of the affected parent, to pinpoint the genetic change leading to the condition in question, and to ensure that the currently available technology can identify that genetic change in a polar body, cleavage state, or blastocyst embryo biopsy.
In order to have embryos to biopsy for PGT, patients must undergo in vitro fertilization (IVF). After fertilization of the egg with sperm, embryos are allowed to develop into day 5 blastomeres. On day 5 after egg retrieval, between three and five trophectoderm cells are removed from the developing embryo for genetic evaluation of the embryo. In most instances, the embryos are frozen after the biopsy is performed and transferred at a later date, given the time frame of completing PGT. Genetic evaluation is performed using PCR, FISH, comparative genomic hybridization (CGH), or most commonly, next generation sequencing (NGS). Nonaffected and/or euploid embryos are then transferred into the uterus for subsequent implantation/pregnancy.
In vitro fertilization
The IVF procedure consists of ovarian stimulation, egg retrieval, egg fertilization, embryo development, and embryo transfer (see the image below).
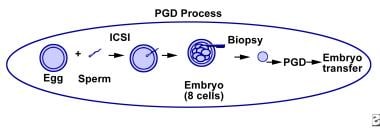 General process of steps required for preimplantation genetic diagnosis and preimplantation genetic screening.
General process of steps required for preimplantation genetic diagnosis and preimplantation genetic screening.
The steps can be summarized as follows:
Ovarian stimulation is needed in order to produce multiple eggs. During the 8- to 14-day hormonal stimulation period, frequent ultrasonographic examinations and laboratory tests are performed to monitor the development and maturation of follicles (egg-containing ovarian cysts).
The oocyte retrieval procedure is typically performed under anesthesia. Under sonographic guidance and a transvaginal approach, follicles are punctured and their follicular fluid aspirated. Oocytes are then identified in the embryology laboratory by embryologists. The procedure usually lasts less than 15 minutes.
The eggs are then cultured for a few hours after their retrieval to allow for final maturation to occur. If desired, though now rarely performed, a polar body can then be removed for PGT. For the PGT procedure at a later stage of embryonic development, intracytoplasmic sperm injection (ICSI), where a single sperm is injected into a single egg, is preferred. In this manner, ICSI prevents the chance of polyspermy and the accidental acquisition of “extra” chromosomal material from the sperm, which can then impact the results of the PGT (ie, give false positive results).
Sperm for purposes of egg fertilization are typically obtained from the male partner by masturbation on the day of egg retrieval.
The morning after ICSI, the eggs are examined for signs of fertilization, which is determined by the presence of 2 pronuclei, representing the male and female contribution to the embryo.
Embryos continue to divide into multicellular entities. Five days after egg retrieval, when the embryo is normally at the blastocyst stage, the embryos can be prepared for a trophectoderm biopsy.
Biopsy techniques
Most clinics perform a trophectoderm biopsy. However, one of the 3 techniques of polar body biopsy, cleavage-stage embryo biopsy, or blastocyst biopsy can be used for PGT.
Polar body biopsy
Polar body biopsy works only for female chromosomal disorders. The mature metaphase II egg extrudes a single polar body. This polar body can be removed and tested, providing information on only the chromosomal content of the egg. Importantly, this does not provide any information regarding the chromosomal constitution of the subsequent embryo.
Because only information about the mother can be obtained by analyzing polar bodies, chromosomal abnormalities occurring after fertilization (when the sperm meets the egg) are not detected.
This technique is infrequently used given the limitations listed above.
Cleavage-stage embryo biopsy
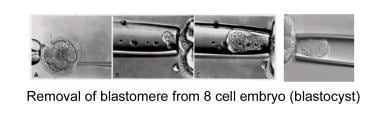
Another approach for PGT is to biopsy a single blastomere from day 3 embryos; this allows extraction of a single blastomere from a developing embryo. The removal of the blastomere is a technically challenging procedure. The embryologist's goal, accomplished using a special microscope and micromanipulators, is to remove an intact cell with minimal trauma to the remaining embryo (see the image below).
Before extracting the single cell from a 6-10 cell embryo, the embryo is incubated in calcium- and magnesium-free medium for approximately 20 minutes in order to reduce blastomere-to-blastomere adherence.
The embryo is then anchored on one side with a holding pipette; simultaneously, a small opening within the zona pellucida is made in order to readily access the blastomeres. This opening procedure is called assisted hatching. Assisted hatching can be performed with either a dilute acidic Tyrode solution, with a laser, or with a sharp curette. After the small opening is made, a pipette is placed through the opening and focused on the blastomere of choice, containing a visible nucleus. The blastomere is subsequently gently aspirated into the pipette and expelled into the surrounding medium.
The embryo, now containing one less blastomere, is returned to the incubator into the appropriate culture medium. The blastomere is then processed as described below.
A limitation of the cleavage stage biopsy is that the acquired blastomere may not be completely representative of the entire embryo in that embryos can be mosaic (ie, the embryos may be composed of more than one population of cells).
Blastocyst biopsy
This technique has become the most popular for PGT. Blastocyst formation begins on day 5 post-egg retrieval and is defined by the presence of an inner cell mass and the outer cell mass or trophectoderm. At this stage of development, the embryo is formed of more than 100 cells. A hole is breached in the zona pellucida in a similar manner as described for a cleavage-stage embryo biopsy, and cells are removed from the trophectoderm using a fine biopsy pipette. The inner cell mass is left undisturbed. Genetic analysis is performed via FISH, PCR analysis, CGH, or NGS as described below.
A limitation of this procedure is the potential acquisition of cells from the trophectoderm that are not representative of the developing embryo (inner cell mass) due to mosaicism (having multiple different types of cell lines). In addition, genetic/aneuploidy testing is completed approximately 24-48 hours of the embryo biopsy; due to the limited viability of embryos in the laboratory (≤6 d after egg retrieval), biopsied blastocysts must be frozen.
Methods of assessing chromosomal constitution
Polymerase chain reaction
PCR is used for the diagnosis of single gene defects, including dominant and recessive disorders. PCR, sometimes called DNA amplification, is a technique in which a particular DNA sequence is copied many times in order to facilitate its analysis. PCR rapidly multiplies a single DNA molecule into billions of molecules.
The DNA is immersed in a solution containing the DNA polymerase enzyme, unattached nucleotide bases, and primers. The solution is heated to break the bonds between the strands of the DNA. When the solution cools, the primers bind to the separated strands, and the DNA polymerase quickly builds new strands by joining the free nucleotide bases to the primers. By repeating this process, a strand that was formed with one primer binds to the other primer, resulting in a new strand that is specific solely to the desired segment. Further repetitions of the process can produce billions of copies of a small piece of DNA in several hours.
PCR is a relatively fast and convenient way to test DNA. The method has been used in a variety of preimplantation genetic testing protocols. However, it requires sufficient amounts of a pure, high-quality sample of DNA, which is sometimes difficult to obtain from a single cell such as a polar body or blastomere. In addition, laboratory contamination and allele dropout are possible complications.
Only one cell should be amplified; however, if another cell or piece of DNA enters the tube, it is also amplified. ICSI must be used to minimize this problem and to ensure that no excess sperm are present (paternal contamination) and that all the cumulus cells have been removed (maternal contamination).
The laboratory environment must be strictly controlled to avoid the introduction of contaminants to the tested material. The laboratory technicians must be trained extremely well to avoid all types of outside interferences.
Errors in PCR can result in misdiagnoses leading to an affected embryo being transferred or the discarding of a normal embryo. One error is caused by a phenomenon known as allele dropout. This refers to the preferential amplification of one allele over another during the PCR process and is mainly a problem for PGD of dominant disorders or when 2 different mutations are carried for a recessive disorder and only one mutation is being analyzed. In autosomal dominant diseases, the risk of transferring an affected embryo is 11% and 2% for recessive disorders.
Fluorescence in situ hybridization (FISH)
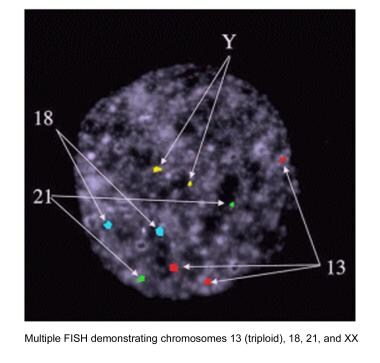
FISH is used for the determination of sex for X-linked diseases, chromosomal abnormalities, and aneuploidy screening. FISH is used more commonly in PGS secondary due to its utility as an aneuploidy screen. Probes (ie, small pieces of DNA that are a match for the chromosomes being analyzed) bind to a particular chromosome. Each probe is labeled with a different fluorescent dye. These fluorescent probes are applied to the cell biopsy sample and are expected to attach to the specific chromosomes. They can be visualized under a fluorescent microscope. The number of chromosomes of each type (color) present in that cell is counted. The geneticist can thus distinguish normal cells from abnormal cells, such as those with aneuploidy (see the images below).
Chromosomes that can be analyzed with FISH probes include X, Y, 1, 13, 16, 18, and 21.
While both PCR and FISH were highly utilized in the early stages of PGT, they have become less commonly performed, as newer techniques are more accurate and precise. These new technologies are comparative genomic hybridization (CGH) and next generation sequencing (NGS).
Comparative genomic hybridization (cGH)
A human cell contains 23 pairs of chromosomes; however, FISH analysis allows accurate assessment of only 7-9 chromosomes in each biopsied cell. Consequently, many abnormal embryos, incapable of forming a successful pregnancy, remain undetected and may be transferred.
Using CGH, the embryo nucleus is labeled with a fluorescent dye and a control cell is labeled using another color (ie, red or green). The two cells are then cohybridized onto a control metaphase spread, and the ratio between the 2 colors is compared. If the chromosomal analysis shows an excess of red, the embryo nucleus contains an extra chromosome. If an excess of green is apparent, then the embryo nucleus is missing one of these chromosomes. CGH enables not only enumeration of all 23 chromosomes but provides a more detailed picture of the entire length of the chromosome, which may detect imbalance of chromosomal segments.
Currently, this technique takes 72 hours, and, given the limited duration of embryo viability in culture, embryo cryopreservation is necessary to provide the time necessary to obtain a diagnosis. Even with a high cell survival rate, cryopreservation can lead to a 30% loss of viable embryos. [22]
Studies have shown CGH protocols that avoid cryopreservation and are compatible with embryo transfer on day 4-5 after fertilization. This is achieved by either using array CGH, an accelerated CGH protocol providing results in 24 hours for all chromosomes, or by using polar body biopsy. Few laboratories currently offer this technology.
Next generation sequencing (NGS)
The newest technology available for performing PGT is known as next generation sequencing. This technique uses capillary electrophoresis; fragmented DNA strands are identified using emitted signals and ligated against a template strand. Amplification methods such as PCR are used to amplify the DNA library. Sequencing is then performed using several different techniques, including genome, exome, and targeted gene panel sequencing. Of these, genome sequencing is the most expensive; however, it also has the lowest accuracy. Using targeted gene panel sequencing, the accuracy is much higher and the process is much quicker and more cost-effective.
NGS allows for comparatively rapid sequencing of entire genomes, as well as extremely detailed sequencing of targeted regions. This allows it to be useful in PGT-A, PGT-M, and PGT-SR.
Noninvasive PGT (niPGT)
Given that embryo biopsy has inherent risks, new technology has targeted noninvasive techniques of performing PGT. One technique involves using a ICSI pipette to pierce into the embryo opposite of the inner cell mass, to aspirate the fluid inside the blastocyst cavity. This fluid contains DNA that can be amplified to perform PGT. This process should not be harmful to the embryo; in fact, removal of the fluid may lead to improved embryo freezing, given that the fluid filled cavity is at increased risk of cellular damage from ice crystal formation when vitrified. However, it takes a large amount of skill to perform these techniques, but spending effort to learn these techniques may be advantageous in the long term. [23]
One of the issues that has arisen wtih niPGT is that when retrieving DNA using these techniques, it is often found to be of low quality and quantity, which can cause issues with DNA amplification. It is thought that the DNA inside the blastocyst may be degraded and hence why there are issues with using the DNA for PGT. There has also been found to be a discordance between the traditional invasive PGT-A result and the result obtained from the niPGT. In one study, 70% of embryos tested had a euploid biopsy; however, their respective niPGT results demonstrated aneuploidy in 86%. Another study demonstrated at 40% concordance between traditionally biopsied PGT-A specimens and their paired niPGT results. [23]
Another technique to perform niPGT is using spent culture medium. This is a completely noninvasive technique that avoids any manipulation of the embryo. DNA has been found in spent media even at day 2-3 of culture. [23] However as with blastocyst fluid niPGT, studies have not shown consistent concordance between spent culture media and traditional biopsy specimens. This may be due in part to embryo self-correction, a phenomenon in which embryos expel abnormal blastomeres to correct for some level of cellular aneuploidy. [24]
While these noninvasive techniques are novel and have advanced in the last few years, there is still more development and research that needs to be done in order to ensure there is accurate and concordant results with traditional biopsy methods. These techniques are attractive to those undergoing PGT as it avoids a large amount of the manipulation that must be done with traditional PGT methods.
Clinical Considerations
Improving IVF success
Even with a successful IVF-PGT procedure, pregnancy is not guaranteed after transfer, and a term or near-term delivery is also not guaranteed.
For an unselected population: The use of PGT-a for the improvement of live birth rates is controversial, and a randomized controlled trial found no difference in the ongoing pregnancy per embryo transfer after randomization to PGT-a or assessing developmental patterns/morphology alone. [25] A Cochrane review found, in an unselected population of patients, insufficient good-quality evidence that live birth rates after the first embryo transfer, cumulative live birth rates, and miscarriage rates are significantly different between IVF with and without PGT-a; the study concluded that there is currently insufficient evidence to support the use of PGT-a in routine clinical practice. [26] Despite this, nearly 40% of all IVF cycles in the United States have PGT-a performed. [2]
Furthermore, a randomized controlled trial of 1212 patients showed similar live births occurred in 468 women (77.2%) in the PGT-a group and in 496 (81.8%) in the non-PGT-a-IVF group. [27] Specifically, among women with three or more good-quality blastocysts, non-PGTa-IVF resulted in a cumulative live-birth rate that was noninferior to the rate with PGT-a. In addition, a study by Mejia et al showed that the cumulative live birth rate is no different in women 35–37 years of age who transfer PGT-a tested vs PGT-a untested embryos (66.6% vs 62.5%; aOR: 0.92, 95% CI: 0.83–1.01). Among patients aged < 35 years, PGT-A was associated with reduced clinical live birth rates (70.6% vs 71.1%; aOR, 0.82; 95% CI [confidence interval], 0.72–0.93). Overall, there was no significant difference in the miscarriage rate (aOR, 0.97; 95% CI, 0.82–1.14). The average time to pregnancy resulting in a live birth was 2.37 months (SD, 3.20) for untested transfers vs 4.58 months (SD, 3.53) for PGT-a transfers. [28]
For patients of advanced maternal age: In a randomized controlled trial that focused on women of advanced maternal age (38-41 years old), the live birth rate was significantly higher in the PGT-a group when analyzed per transfer (52.9% vs 24.2%, P=.0002) and per cycle (36% vs 21.9%, P=.031) compared to the non-PGT-a group. [29] Because embryos were being tested, only 68% of the PGT-A patients had a transfer vs 95% in the control group (P=.001). The miscarriage rate was significantly lower in the PGT-A group (2.7% vs 39%, P=.0007). Time to pregnancy resulting in live birth was estimated at 7.7 weeks for PGT-A group vs 14.9 weeks for controls.
Reducing miscarriage
Interestingly, an important finding from the Mejia study is the reduction in miscarriage rate with PGT-a in women 35–37 years of age (aOR: 0.77, 95% CI: 0.61–0.98). [28] The decision to use PGT-a for the sole purpose of reducing, not preventing, miscarriage in this age group appears to be a reasonable option. That being said, even though embryo aneuploidy accounts for a large portion of reproductive loss, it is not the only cause. Factors that need to be considered include the added financial cost and increased time to pregnancy.
Current recommendations from the Society for Assisted Reproductive Technology (SART) and American Society for Reproductive Medicine (ASRM) state that available evidence does not support the use of PGS to improve live-birth rates for advanced maternal age, recurrent pregnancy loss, or implantation failure and recommends that patients be counseled about the limitations of the technique and should not make future treatment decisions based solely on PGS results. [30]
Reducing risk of multiple pregnancy
A study compared IVF success before and after a change in clinic protocol designed to decrease the number of embryos transferred in patients older than 35 years, with the hopes of reducing a multiple pregnancy. [31] eSET was offered to patients with fewer than two implantation failures and who undertook PGTa. The study noted a significant increase in live birth rates per embryo transfer cycle for the eSET/PGT-A recipients compared to those without eSET of PGTa embryos.
Technical considerations
Removal of cells is technically difficult and requires skill and experience. Damage to the embryo (projected to be 0.1%) may accidentally occur during removal of cells.
In the past, FISH offered evaluation of less than half of the 23 chromosomes; usually 9-11 were analyzed. Studies using comparative genetic hybridization (CGH) and FISH demonstrate that as many as 25% of aneuploid embryos are characterized as normal because the abnormal chromosomes were not analyzed. NGS allows for evaluation of all 23 pairs of chromosomes which significantly improves both sensitivity and specificity of the test as it relates to the chromosomal composition of the cells being biopsied. However, embryo mosaicism, which refers to two or more cell populations with different chromosomal complements being present within the same embryo, can result in false-positive or false-negative results of an embryo's chromosomal constitution.
Mosaicism
Self-correction refers to evidence that mosaic embryos are able to halt the proliferation of abnormal cells and that many embryos identified as aneuploid will survive and be reidentified as normal. [32] Mosaic embryos could implant and generate euploid offspring; however, these embryos tend to implant at a lower success rate.
A statement by the Practice Committee and Genetic Counseling Professional Group of the American Society for Reproductive Medicine (ASRM) emphasizes the necessity for appropriate patient education and counseling before undertaking any form of PGT. [33] It is important that providers review the expected frequency of mosaic results at the laboratory used as well as the challenges associated with interpreting these results to ensure that patients are prepared for results that may not be straightforward.
Viotti et al reported on outcomes associated with 1000 mosaic embryos transferred, and found that mosaic results only affected implantation or early pregnancy viability, with no reports of fetal mosaicism in this large cohort. [34] Thus far, there has been one report of fetal mosaicism resulting from an embryo identified as mosaic for the same chromosome. [35] These publications suggest that the vast majority of mosiac embryos that result in viable pregnancies are actually chromosomally normal.
Additional insight into the self-correction of aneuploid and mosaic embryos, either by post-zygotic chromosome loss, chromosome gain, mitotic nondisjunction, or trisomic rescue, requires further investigation.
Neonatal outcomes
Thousands of infants have been born following PGT worldwide. To date, there are no reports of increased fetal malformation rates or other identifiable problems in babies born from IVF with PGT. However, the presentation of other abnormalities later in life as a consequence of the PGT remains theoretically possible.
Obstetric, neonatal, and early childhood outcome data seem reassuring thus far; however, much has focused on PGT-m (single gene) rather than PGT-a (aneuploidy). As previously noted, PGT-m vs PGT-a parental groups are often inherently different in that most patients undergoing PGT-m do not have concomitant infertility. Nonetheless, kindergarten-aged PGT-Mm offspring perform as well as their IVF/ICSI and naturally conceived peers on measures of cognition (Wechsler Preschool and Primary Scale of IntelligenceTM), motor skills (Movement ABC), and psychosocial development (Child Behavior Checklist [CBCL]. [36]
Due to a reduction in the number of chromosomally normal embryos available for embryo transfer, patients should be counseled that IVF with PGT may result in fewer opportunities at pregnancy compared to non-PGT cycles. Specific patient cohorts, such as those of advanced maternal age, specifically >37 years old, appear to benefit from PGT-a.
Single gene disorders
Currently available technology can help eliminate some genetic diseases in the future (eg, Tay-Sachs disease, cystic fibrosis, Huntington disease, X-linked dystrophies). Complete cures for many genetic diseases are not likely to be found soon; therefore, preventing the disease is often preferred by patients.
Prenatal testing for genetic diseases is currently performed through noninvasive prenatal testing (NIPT) or more invasive chorionic villus testing (CVS) at 10 weeks estimated gestational age (EGA) or with an invasive amniocentesis at 16 weeks EGA. If the examination findings reveal a genetically affected fetus, the options available to parents are to have a child with a genetic disease or to undergo a pregnancy termination. This is a difficult and often traumatic decision, especially in advanced pregnancy. However, PGT-m is performed before pregnancy begins, thus allowing patients to become pregnant with an unaffected embryo, thus eliminating this difficult decision. In this manner, PGT-m allows these couples the opportunity to have a child free of their particular disease.
Concluding Thoughts
The first successful cases of preimplantation genetic diagnosis (PGT) in humans were performed in 1988. However, the development and acceptance of PGT since then has been slow, mainly due to the time necessary to develop and learn single-cell diagnostic techniques and to the costs involved. Given the technical considerations associated with PGT, these procedures should be limited to centers experienced with micromanipulation.
Although PGT has been incorporated into the care of patients undergoing IVF treatment, its indications, utility, benefit, and outcomes remain an active area of research in reproductive medicine. As preimplantation screening for medical disorders at the embryonic level optimizes, its place in medicine and society will continue to grow, thus generating controversy and ethical debate.
-
General process of steps required for preimplantation genetic diagnosis and preimplantation genetic screening.
-
Aspiration, fertilization, and transfer.
-
Removal of blastomere from an 8-cell embryo (cleavage-stage embryo).
-
Abnormalities of chromosomes 13, 18, and 21.
-
Multiple fluorescence in situ hybridization demonstrating chromosomes 13 (triploid), 18, 21, and Y.
-
Instruments used for preimplantation genetic diagnosis compared with a human hair (upper part of picture). To the lower-left side is the holding pipette and to the right side is the glass needle used for aspiration of the blastomere.
-
Intracytoplasmic sperm injection. Puncture of the oocyte.
-
Intracytoplasmic sperm injection. Injection of sperm.
-
Human male karyotype. Courtesy of Wikimedia Commons [https://commons.wikimedia.org/wiki/File:Human_male_karyotpe_high_resolution.jpg, provided by the National Human Genome Research Institute, National Institutes of Health (NIH)].
-
Day 5 blastocyst biopsy.