Practice Essentials
Osteoporosis is common and the risk of fracture is high among patients who have undergone solid organ transplantation. [1] The pretransplant bone evaluation should include a careful history, with particular attention to risk factors for osteoporosis. Any personal history of fracture is particularly relevant (see the image below). Prophylaxis against bone loss should be given to all transplant recipients, regardless of baseline skeletal reserve.
Signs and symptoms
The following findings should be noted during the physical examination:
-
Loss of height or kyphosis, which could suggest occult compression fracture
-
Blue sclerae, which suggest underlying osteogenesis imperfecta
-
Bone tenderness or pain, which could suggest occult fracture, avascular necrosis, or osteomalacia
-
Incapacitating pain or tenderness in the lower extremities or bones of the feet following organ transplantation, which could suggest the calcineurin-inhibitor–induced pain syndrome
Assessment
Laboratory studies
The following laboratory studies are indicated in the pretransplant evaluation of all patients awaiting solid organs [2, 3] :
-
Serum calcium level
-
Phosphorus level
-
Bicarbonate level
-
Alkaline phosphatase level
-
Blood urea nitrogen (BUN)/Creatinine level
-
Intact parathyroid hormone (PTH)
-
Thyroid function tests
-
Testosterone (males only)
-
Estradiol and follicle-stimulating hormone (FSH) levels (in females with amenorrhea or irregular cycles)
-
Magnesium level
-
24-Hour urine calcium level
Imaging studies
Bone mineral density measurements by dual-energy X-ray absorptiometry (DEXA) of axial and appendicular bone should be performed on all patients prior to transplantation. Trabecular bone scores (TBS) would further help estimate bone integrity if they are available. Because the prevalence of occult, asymptomatic fractures (in particular, spinal compression fractures) is considerable, complete spine radiographs may also be obtained.
Management
Administer vitamin D at 800-1000 IU a day to all patients. Advise patients to maintain an adequate total elemental calcium intake (eg, 1000-1500 mg) including food and supplements.
Any patient who meets World Health Organization (WHO) criteria for osteoporosis should be considered for pharmacologic intervention.
Patients should avoid cigarette smoking and heavy alcohol consumption. Adequate nutrition is essential for optimal bone health in transplant recipients. Regular weight-bearing and muscle-strengthening exercises are recommended to improve bone strength and reduce the risk of fracture.
Fall risk should be assessed and any modifiable risk factors should be addressed routinely.
The Risk of Fracture and Skeletal Phenotype After Solid Organ Transplantation
The risk of fracture in patients with organ transplants is very high: almost 5 times and 20 times higher in male and female kidney transplant recipients compared with age- and sex-matched control groups. The risk is particularly high in perimenopausal women. [4, 5] Fracture seemed to occur frequently at an appendicular bone in kidney transplant recipients; yet the hip fracture risk, which is strongly associated with high mortality and poor prognosis, was concerningly high in those patients (30% higher than in patients on dialysis). [6, 7, 8]
The risk of fracture has been reported as particularly high during the early phase after the surgery. For example, the incidence of hip fracture was as high as 5 cases per 1000 person-year in the first year. [8] Fortunately, after this acute phase, the risk somewhat stabilizes based on data from multiple cohorts with various organ transplantation. [9, 8]
Skeletal phenotyping, such as measuring bone mineral density (BMD) and histomorphometry analysis with bone biopsy, has provided an explanation, in part, of the elevated fracture risk. The BMD in the lumbar spine (LS) decreased by 7% after 6 months and 9% after 12 months. [10] The acute bone loss was estimated at 1.6% per month in the first 5 months, more in trabecular bone than in cortical bone. [11, 12]
Histomorphometry findings were consistent with low bone turnover disease characterized by the decreased bone formation and resorption: increased mineralization lag time, increased osteoblasts apoptosis, decreased osteoblast surface, and resorptive surface. [13] In some patients with secondary hyperparathyroidism due to end-stage renal disease, findings of marrow fibrosis and woven bone disappeared as PTH levels normalized after transplantation. [10]
Consistent with the stabilized risk of fracture after an acute phase, the initial BMD loss seemed to slow down and even reverse almost to the pre-transplant levels in some patients. [4] The histomorphometry also showed improved bone formation rate and mineralization with time over 10 years. [14]
The pattern of acute bone loss followed by stabilization was observed in patients with various types of organ transplantation. In the first year following surgery, bone loss in the LS and femur neck (FN) was noted at about 2.5% and 6%, 3.5% and 9%, and 7% and 12% in liver, heart, and lung recipients, respectively. [15, 16, 17] Again, this initial acute bone loss stabilized and recovered in some patients over the following 4 years. [16, 17]
However, it should be noted that the risk of osteoporosis and long-term risk of fracture are still elevated in those patients despite some recovery. Data suggest that half of the renal transplant patients remained osteopenic or osteoporotic, [18] and the risk of osteoporosis almost doubled in liver transplant recipients. [19] More importantly, the long-term risk of fracture based on a 15-year observational study was ~60%, which was almost 3 times higher than the expected risk. [20]
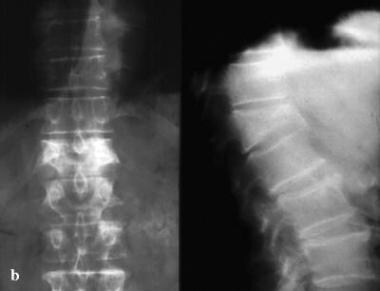
 Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Traditionally recognized risk factors for osteoporosis include white race, low body weight, estrogen or androgen deficiency, calcium and/or vitamin D deficiency, and thyroid hormone excess. Other risk factors, particularly immunosuppressant use, exist.
Even before transplantation, bone homeostasis may be adversely influenced by the underlying end-organ disease. Moreover, patients are often exposed to medications such as steroids, heparin, or loop diuretics, which promote negative calcium balance and bone loss. After transplantation, high-dose steroid and immunosuppressant use cause further bone loss and increase fracture risk.
Long-term survival following organ transplantation has improved considerably. Because patients often wait 2 or more years before transplantation, this represents an opportunity to prevent further bone loss and to help restore what may already have been lost. The clinical focus should be to both optimize bone mass before transplantation and to prevent bone loss in the postoperative period. [21]
For other discussions on osteoporosis, see the topics Osteoporosis and Pediatric Osteoporosis, as well as Bone Markers in Osteoporosis and Nonoperative Treatment of Osteoporotic Compression Fractures.
Pathophysiology of Osteoporosis in Transplantation
Preexisting bone disease
The pre-transplant skeletal condition is a major contributing factor. The pre-transplant BMD, which reflects an underlying skeletal reserve, has shown a strong correlation with the risk of fracture in post-transplant patients. [22, 16, 18]
End-stage renal disease
Nearly all cross-sectional studies of renal transplant recipients demonstrate that BMD is below normal levels. [23] A fracture prevalence of 5-11% has previously been reported in cross-sectional studies, which is similar to or greater than rates observed in women with postmenopausal osteoporosis. [22, 6] Hyperparathyroidism preceding renal transplantation accelerates trabecular bone loss at the spine in the early transplant period. [24]
Most patients with end-stage renal disease (ESRD) are hypogonadal and have some degree of renal osteodystrophy. (For a comprehensive discussion of renal osteodystrophy, which is beyond the scope of this chapter, please see Goodman et al. [25] ) Most patients with ESRD have been exposed to drugs that negatively affect bone metabolism. In one large series of 250 such patients, risk factors for low bone mass included secondary amenorrhea, prior failed renal transplantation, chronic metabolic acidosis, and chronic heparin and aluminum exposure. Having had a prior failed renal transplant is probably associated with increased immunosuppressant exposure to prevent rejection and to hyperphosphatemia associated with osteomalacia and osteoporosis. [26]
In recent years, the continuous use of aluminum-containing phosphate binders has largely been abandoned; therefore, aluminum toxicity is not presently a significant problem in transplant-related bone disease. [27, 28] However, adynamic bone disease has become increasingly prevalent in the chronic kidney disease population, evident in 27% of transiliac bone biopsy specimens [29] and up to 50% of renal transplant patients prior to bisphosphonate treatment for osteoporosis. [30]
After successful renal transplantation, secondary hyperparathyroidism usually resolves gradually, with normalized vitamin D metabolism and creatinine clearance (CrCl). PTH levels frequently normalize or improve by 1-month post-transplant because of immediate improvement in phosphate retention. Cortical bone density improves secondarily, with significantly better Z-scores at the distal radius occurring by 6 months after renal transplantation. However, in approximately a third of cases, persistent hyperparathyroidism and hypercalcemia are noted. [31] In cases of refractory hyperparathyroidism in which surgery is indicated, parathyroidectomy has been associated with a marked improvement in BMD.
Overall, the transplant-related bone disease in kidney recipients does not appear to be as severe as in other solid organ transplant recipients, with the possible exception of kidney recipients with type 1 diabetes. [32] This is perhaps because kidney transplant recipients are younger on average than other organ recipients at the time of transplantation, and they may have had better recognition and management of pretransplant bone disease. Kidney transplant recipients may receive lower doses of immunosuppression overall. Rejection may also be more easily detected in renal recipients and, therefore, treated earlier than in other solid organ transplant recipients, resulting in lower total doses of immunosuppression.
End-stage lung disease
Osteoporosis is very common among patients awaiting lung transplantation. Shane et al studied 70 patients awaiting transplants for end-stage lung disease and found osteoporosis in 30% at the LS and in 49% at the FN. Osteopenia (low bone mass) was noted at those sites in 35% and 31% of patients, respectively. [33] In other words, only a minority of patients awaiting transplant had normal bone density. Ferrari et al also prospectively evaluated changes in bone mass in 21 consecutive lung transplant candidates and found similar findings of the high prevalence of osteoporosis. [34] It seems to become worse after transplantations, as nearly three-quarters (73%) of patients were at or below the fracture threshold after the surgery. [35] The prevalence rate of documented osteoporotic fractures was found to be 29% in patients with emphysema and 25% in patients with cystic fibrosis.
Cystic fibrosis, a common indication for transplantation, is itself associated with low bone mass and fragility fractures because of (1) delayed puberty and hypogonadism and (2) chronic malnutrition with pancreatic insufficiency causing calcium and vitamin D malabsorption. Despite the common practice of giving supplemental oral vitamin D in patients with cystic fibrosis, the usual daily doses of 400-800 IU of vitamin D are often insufficient due to malabsorption. Shane et al noted vitamin D deficiency in 36% of patients with cystic fibrosis awaiting transplantation; 40% of patients who received supplements were still deficient likely due to malabsorption. [33, 36]
Patients with end-stage lung disease are frequently exposed to systemic and inhaled steroids for managing flare-ups. The dose-dependent negative effect of steroids is well known, and it is associated with lower bone density and higher risk of vertebral and non-vertebral fracture. [37, 38] Of note, only a very few patients receiving long-term glucocorticoid therapy received an effective antiresorptive agent for osteoporosis prevention. [33, 5]
End-stage heart failure
As with patients awaiting lung transplantation, only a minority of patients awaiting cardiac transplantation have normal bone density. Only 50% and 47% had normal BMD at the LS spine and total hip, respectively; and the severity of heart failure is correlated with lower BMD. For example, patients with New York Heart Association (NYHA) classes III-IV had much lower BMD compared with those with NYHA I-II. [39]
The reasons for this are likely multifactorial. Older age, vitamin D deficiency, and coexisting chronic kidney disease all contribute to the increased risk of osteoporosis. Patients with end-stage congestive heart failure are uniformly exposed to potent loop diuretics that promote negative calcium balance, and they often have coexisting renal disease and hepatic congestion from their low-flow state. Vitamin D deficiency is common, [40] and secondary hyperthyroidism was noted to occur independently of renal function in those patients; patients with elevated PTH levels tended to show higher bone loss. [39, 41]
Lastly, upregulated renin-angiotensin-aldosterone (RAA) axis in heart failure can increase RANKL expression and stimulate osteoclasts differentiation. [42, 43]
End-stage liver disease
In a large series of 243 consecutive patients undergoing evaluation for liver transplantation, only 15% had normal bone density. [44] Moreover, vertebral fractures were present in 35% of patients prior to transplantation in this same population. [45]
End-stage liver disease (ESLD) itself is associated with osteoporosis. Vitamin D deficiency is extremely common among patients with cirrhosis who are awaiting transplants. Cirrhosis is associated with significantly depressed levels of 25-hydroxyvitamin D-3, 1,25-dihydroxyvitamin D, osteocalcin, and PTH. [46] Cirrhosis is also associated with low osteocalcin levels and with histomorphometric evidence of decreased bone formation. In chronic liver disease, low vitamin D levels predict bone loss. [47] In one study of 27 patients awaiting orthotopic liver transplantation, 74% had subnormal 25-hydroxyvitamin D levels at baseline. Vitamin D levels were inversely associated with more advanced Child-Pugh classification, and more advanced Child-Pugh class was associated with increased bone loss at the LS spine. [48, 49]
Chronic obstructive liver disease appears to cause more bone loss than hepatitis. In one cross-sectional study, BMD Z-scores were more than twice as depressed in cholestatic patients than in patients with viral liver disease, although the viral liver disease is itself associated with significant osteopenia. [50] Also, bone loss was predicted by the degree of hyperbilirubinemia in a Swedish study. [48]
The cholestasis may interfere with the enterohepatic circulation of vitamin D metabolites. In addition, osteoblast function in patients with primary biliary cirrhosis (PBC) was shown to be depressed. Although the specific pathophysiological mechanisms in PBC have not been established. histomorphometry findings reveal suppressed bone formation and mineralization. [51, 52] It might have something to do with collagen synthesis and vitamin D action, as polymorphism in the gene encoding collagen type I alpha1 (COLA1) and vitamin D receptor may predict lower BMD in patients with PBD. [53, 54, 55]
In viral hepatitis, serum immunoglobulin F-1 levels were lower than in control subjects, and its levels differed significantly between cirrhotic patients with and without osteoporosis. This finding might suggest low immunoglobulin F-1 levels may play a role in osteopenia. [50, 56]
Other risk factors such as alcohol use and malnutrition, which result in vitamin D and magnesium deficiency, also play a significant role. [57, 58] Alcohol itself may directly suppress bone formation, as evidenced by a direct correlation between bone GLA protein levels and days of abstinence from alcohol. [59] Magnesium deficiency can cause hypocalcemia by inhibiting PTH secretion and PTH resistance. [60]
Hypogonadism is a known risk factor for osteopenia and occurs frequently in persons with alcoholism and ESLD. Both low testosterone and high sex hormone-binding globulin levels correlate with worsening Child-Pugh classification.
Diabetes-related bone disease
Diabetes mellitus is one of the most common causes of ESRD and is commonly seen in patients with other end-organ diseases. The patients with diabetes, in general, have a high risk of fracture: almost 7-fold in type 1 diabetes and 70% in type 2 diabetes. [61] Moreover, a history of diabetes before transplantation is an independent risk factor for the risk of fracture, [62] and almost 40% of the patients with diabetes had fractures compared with 11% in the non-diabetes group after transplantation. [32]
Most patients with type 1 diabetes mellitus have not yet achieved peak bone mass before the onset of diabetes, and long-standing insulin deficiency may compromise bone mass. Multiple studies have documented that patients with type 1 diabetes have reduced BMD at all sites measured, with a high prevalence of osteoporosis. Munoz-Torres et al examined a cohort of 94 consecutive Spanish patients (aged 20-56 y) with type 1 diabetes of 1-35 years' duration presenting to a diabetes clinic for therapy and observed reduced BMD at all sites; 19.1% of patients had osteoporosis. [63]
Poor glycemic control, the presence of diabetic complications, and smoking are also associated with a lower BMD in multiple studies. Patients with diabetic retinopathy, for example, were much more likely to exhibit osteopenia or osteoporosis. [64] Campos Pastor et al observed 62 patients with type 1 diabetes and found that after 7 years of intensive insulin therapy, BMD stabilized at all sites. A significant fall in tartrate-resistant alkaline phosphatase levels (4.3 vs 2.7 IU/L) and a significant rise in intact PTH levels (28 vs 40 ng/L) were also observed. [64]
Hyperglycemia seems to have a direct negative effect on bone. [65] The unregulated glycation process disrupts collagen crosslinking, [66] and increases inflammatory mediators, [67] which impair bone quality. In a murine model, aldose-reductase inhibitors prevented bone loss in diabetic mice. [68] Also, data suggest the low bone turnover in diabetes might be due to increased sclerostin and suppressed Wnt signaling pathway. [69]
Simultaneous pancreas-kidney transplantation (SPKT) successfully restores euglycemia and insulin independence while reversing uremia in patients with ESRD due to diabetic nephropathy. Whereas diabetic patients constitute approximately 20% of those receiving renal transplants, virtually all patients receiving both a pancreatic and renal transplant have type 1 diabetes mellitus. About 29% and 54% of patients who are awaiting SPKT had osteoporosis and osteopenia at the FN, and 75% among 20 patients already sustained a fracture while awaiting transplantation. [40]
A longitudinal follow-up study found that patients still had an elevated risk of fracture and osteoporosis after successful SPKT. Over 12 months of follow-up, 23% and 55% of patients had low bone mass at the LS and FN in the osteoporotic range, respectively. The fracture occurred in 45% of SPKT patients. Secondary hyperparathyroidism was noted in 55% of patients. As with other transplantation patients, BMD of SPKT recipients stabilized after the first 6 months posttransplant. [70] Notably, a study suggested that vitamin D supplements might have a protective skeletal effect after SPKT: alfacalcidol (0.25 mcg/d) improved BMD at the LS slightly in the first 6 months. [70]
Posttransplant immunosuppression
Routinely administered posttransplant immunosuppressants play a central role in the pathogenesis of bone loss and fracture. Regimens typically include glucocorticoids (at high dose initially), cyclosporin A (CsA), tacrolimus FK506, azathioprine, or mycophenolate mofetil. Because they are always administered simultaneously, sorting out the independent effects of immunosuppressants from those of glucocorticoids is difficult, if not impossible. Immunosuppressant doses are typically higher in liver and heart transplantations than in renal transplantation, contributing to the more advanced osteopenia seen in those groups.
Glucocorticoids
Although a detailed discussion of glucocorticoid-induced bone loss is beyond the scope of this article, glucocorticoids are known to induce osteoporosis. An increased risk of vertebral fracture has been associated with an oral dose of prednisolone of as low as 2.5 mg/d, which is approximately equipotent to prednisone at 2.5 mg/d. Glucocorticoid-induced bone loss is acute, and dose- and duration-dependent. Patients lost bone rapidly within 20 weeks, mainly in trabecular bone (8.2%) rather than cortical bone (2.1%). [71] The cumulative dose of 10 mg/kg per month (20 mg a day for a 60-kg adult) increased the risk of fracture by almost 10%. [72] Of note, glucocorticoids are commonly prescribed in high doses, up to 120 mg of prednisone or its equivalent daily during periods of acute rejection and immediately after transplantation. Fortunately, the risk reversed after the discontinuation, which stresses the importance of using the smallest effective dose for the shortest period. [73]
Glucocorticoids promote bone loss through a variety of simultaneously operating mechanisms, as follows:
-
Reduce GI calcium absorption
-
Increase urinary calcium excretion
-
Induce secondary hyperparathyroidism
-
Decrease production of skeletal growth factors
-
Decrease the responsiveness of luteinizing hormone (LH) to gonadotropin-releasing hormone, thereby decreasing gonadal hormone production; may also directly decrease gonadal hormone production
-
Suppress corticotropin, thereby suppressing the adrenal production of androstenedione, a substrate for both testosterone and estrone production
-
Decrease the osteoblast-mediated bone formation
-
Increase bone resorption
Glucocorticoids result in a disproportionate loss of cancellous or trabecular bone, possibly because the trabecular bone has an inherently greater rate of turnover than cortical bone. Glucocorticoid-induced osteoporosis is characterized by severely decreased bone turnover, particularly bone formation. Glucocorticoid-induced osteoporosis is characterized by severely decreased bone turnover, particularly bone formation. The differentiation of osteoblasts and osteoclasts was suppressed by almost 90% and 70% after prednisone use (2 mg/kg/d), respectively, based on histomorphometry study. Cell cycle arrest by upregulated cyclin-dependent kinase inhibitor 1B1N (p27kip1) after dexamethasone was reported. [74]
With the advent of the cyclosporines in the early 1980s, graft survival markedly improved owing to decreased organ rejection. The introduction of cyclosporines allowed steroid doses to be substantially reduced. At the time, the hope was that the harmful effects of immunosuppression on the skeleton would be ameliorated. Unfortunately, this was not the case. The lowest effective dose of glucocorticoids is recommended to minimize loss of bone mass and risk of osteonecrosis (a common complication in the first 2 years following surgery).
Calcineurin inhibitors (cyclosporin A and tacrolimus [FK506])
Like glucocorticoids, CsA causes severe and rapid bone loss in human and animal studies. Unlike glucocorticoids, CsA results in accelerated bone turnover with increased resorption. [75, 76, 77]
The bone histomorphology resembles that of the oophorectomized female rat. Antiresorptive agents, such as estrogen, alendronate, and calcitonin, can largely prevent this bone loss. Alendronate specifically prevents CsA-induced osteopenia in rats, maintaining trabecular bone volume at the tibia.
The skeletal effect of cyclosporine might be both direct and indirect. The null skeletal effect of the drug in athymic mice suggested the effect might be immune-mediated. [78] Some investigators have speculated that this effect of cyclosporine may be mediated through testosterone because cyclosporine suppresses the hypothalamic-pituitary-gonadal axis and lowers serum testosterone levels in rats and in human transplant patients. Some evidence suggests that cyclosporine may have a direct testicular effect. Examination of rat testes after CsA exposure has revealed decreased LH-receptor numbers and dramatically decreased serum and intratesticular testosterone. Altered testicular cytochrome P-450 activity is reported due to suppressed heme formation and the steroidogenic activities that rely on it, such as 17-hydroxylase and side-chain cleavage enzymes
However, the target of the drug- calcineurin Aα was found to express in both osteoblasts and osteoclasts. [79] Following studies using genetic-modification upregulation and downregulation of calcineurin Aα and pharmacologic inhibition using the drug showed that it causes increased osteoclastic bone resorption and decreased osteoblastic bone formation, resulting in significant bone loss. [80, 81]
Tacrolimus is a fungal macrolide that is less nephrotoxic and more potently immunosuppressive than CsA. In rats, this has been reported to cause high-turnover bone loss of even greater magnitude than that caused by CsA. [82, 83] Because tacrolimus is a more potent immunosuppressant, steroid doses may be reduced further with tacrolimus than with CsA.
A study in liver transplant recipients by Smallwood et al showed a nonsignificant tendency toward fewer patients with low bone density in the group receiving tacrolimus (n=112) compared with those receiving cyclosporine (n=25). Among the patients weaned from prednisone, the patients treated with tacrolimus were less likely to have low BMD (36.2% vs 68.8%). [84] Overall, rates of bone loss have been similar in heart, liver, and kidney transplant recipients receiving either tacrolimus or CsA.
Other immunosuppressants
Rapamycin inhibits downstream signaling from the mammalian target of rapamycin (mTOR) proteins, a signaling pathway promoting tumor growth. Rapamycin binds to the FK506 binding protein, and this complex then binds to mTOR and prevents the interaction of mTOR with target proteins in the signaling pathway. Short-term administration of rapamycin causes no trabecular bone loss and potentially has bone-sparing effects. [85]
Studies have shown that inhibiting mTOR complex 1 signaling decreased osteoclast differentiation by upregulating OPG. [86, 87, 88]
Two open-label, randomized, phase 2 studies comparing sirolimus versus cyclosporine examined bone metabolism with markers of bone turnover, osteocalcin, and urinary N-telopeptides measured over a 1-year period in 115 patients receiving CsA or sirolimus with azathioprine and glucocorticoids or mycophenolate with glucocorticoids. In the patients treated with CsA, serum osteocalcin and urine N-telopeptides were consistently higher compared with those receiving sirolimus. [89]
In a noncontrolled report, the incorporation of mycophenolate mofetil in the immunosuppressant regimen has successfully reduced steroid requirements in a subset of patients with symptomatic osteoporosis after cardiac transplantation. However, in the small number (12) of patients studied, this did not result in an improvement of BMD after 1 year. [90]
Clinical Presentation
Patient history
The pretransplant bone evaluation should include a careful history with particular attention to risk factors for osteoporosis. Any personal history of fracture is particularly relevant because prior fracture predicts future fracture. [82] Any family history of osteoporosis or fragility fractures is also relevant. A history of loss of height suggests established osteoporosis and occult thoracic compression fracture.
The review of systems should include a review of gonadal function because a history of amenorrhea, decreased libido, or erectile or orgasmic dysfunction could suggest underlying hypogonadism, predisposing to osteopenia.
The review of systems should specifically inquire about bone pain, myalgias, or myopathic symptoms, which could suggest occult vitamin D deficiency or osteomalacia.
Significant constitutional symptoms or symptomatic anemia could suggest occult multiple myeloma as a cause of osteopenia, in the appropriate clinical context.
Because immobility is known to promote negative bone balance, any history of periods of prolonged bed rest is also relevant.
A careful medication history should be taken for past anticonvulsant use because these medications can derange vitamin D metabolism. Heparin, loop diuretics, and steroids (both oral and inhaled) are associated with negative calcium balance. Any history of tobacco or alcohol abuse is relevant because the use of these substances is an established risk factor for osteoporosis.
A dietary history should be obtained. This should include an estimate of daily calcium intake. An overview of lifelong dairy intake or avoidance, lactose intolerance, malabsorption, or celiac disease, [83] which predispose the patient to vitamin D deficiency, is also relevant. A history of sun avoidance is similarly important. Overall nutritional status, including any prior periods of malnutrition or energy (caloric) restriction (eg, anorexia nervosa), should be assessed.
A history of dietary supplement intake is also relevant. Observational data suggest that excess vitamin A intake (eg, as retinol) is associated with an increased hip fracture risk. [91]
Physical examination
Record height for any loss or kyphosis, which could suggest occult compression fracture.
The examiner should be alert for the presence of blue sclerae, which suggest underlying osteogenesis imperfecta.
Any scoliosis or other regions of bony deformity including osteoarthritic changes, and previous vertebroplasty should be noted. These deformities have the potential to render bone density determinations inaccurate in these regions, and the physician or technician performing bone densitometry should be made specifically aware of them.
Any areas of bone tenderness or pain, which could suggest occult fracture, avascular necrosis, or osteomalacia, should prompt further diagnostic evaluation.
Incapacitating pain or tenderness in the lower extremities or feet following organ transplantation could suggest the calcineurin-inhibitor–induced pain syndrome (CIPS), an unusual side effect of cyclosporine or tacrolimus.
CIPS may be accurately diagnosed by its typical presentation, negative inflammatory markers (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), and characteristic magnetic resonance imaging findings with altered bone marrow signal. The reduction of cyclosporine or tacrolimus trough levels and the administration of calcium channel blockers can relieve pain. In some cases, bone marrow edema and pain resolve spontaneously in approximately 3 months. [92, 93]
Assessment of Risk Factors and the Risk of Fracture
Laboratory workup
Because even patients with entirely normal pretransplant BMD experience fractures following solid organ transplantation, it is not possible to reliably predict who will sustain a fracture. Thus, it has been recommended that all transplant candidates undergo an evaluation of bone health. [94] The following laboratory studies are indicated in the pretransplant evaluation of all patients awaiting solid organs [2, 3] :
-
Serum calcium level
-
Phosphorus level
-
Bicarbonate level
-
Alkaline phosphatase level
-
BUN/creatinine levels
-
Intact PTH assay, to assess for hyperparathyroidism (primary or secondary)
-
25-Hydroxyvitamin D value to assess total body vitamin D stores
-
Thyroid function tests (eg, thyroid-stimulating hormone, free levothyroxine)
-
Testosterone level (males only), to ensure eugonadism
-
Estradiol and FSH levels (in females with amenorrhea or irregular cycles)
-
Magnesium level
-
24-Hour urine calcium level
-
Bone turnover markers
-
Serum aluminum
-
Protein electrophoresis
Dual-energy X-ray absorptiometry (DEXA)
Bone densitometry measurements by dual-energy X-ray absorptiometry (DEXA) scanning of the LS spine and hips should be performed on all patients prior to transplantation.
DEXA scanning should be performed not only to screen for preexisting bone loss in this medically ill population predisposed to osteoporosis but also to establish a baseline in patients who will require long-term administration of glucocorticoids as part of an immunosuppressive posttransplantation regimen. [95]
Guidelines established by the American College of Rheumatology and the UK Consensus Group recommend that patients receiving daily glucocorticoid at doses of 7.5 mg or more of prednisolone for 6 months or longer should have a BMD measurement. [96]
Bone density of the distal radius should also be measured in patients with renal osteodystrophy and any evidence of hyperparathyroidism.
The Kidney Disease Outcomes Quality Initiative has recommended a DEXA scan at the time of renal transplantation to assess for the presence of development of osteoporosis. [97] DEXA scans are recommended at the time of transplantation and 1 and 2 years following transplantation. Parenteral bisphosphonate should be considered when the BMD T-score is less than or equal to –2 SD.
Newer imaging tool
Physicians should be aware that the BMD measurement and currently available biomarkers do not provide a whole picture of bone integrity or risk of fracture in those patients. Trabecular bone score (TBS), which indirectly analyzes trabecular bone microarchitecture, may add further insight in fracture assessment. [98, 99] It is shown that low TBS scores were also significantly associated with the risk of fracture in kidney transplant recipients, independently of the FRAX score. [100] Other newer imaging modalities such as high resolution peripheral quantitative computed tomography (HR-qCT), micro-magnetic resonance imaging (MRI), finite element modeling (FEM), and microindentation provide a better understanding of the mechanical properties. Micro-MRI and FEM measurements in kidney transplant recipients showed that stiffness and failure strength was significantly lower in the early phase in those patients. [101]
Vertebral X-ray
Because the prevalence of occult, asymptomatic fractures (in particular, spinal compression fractures) is considerable, complete spine radiographs may be obtained (see images below). [2] Further, radiographs of any painful bone sites should be obtained to help exclude the presence of Looser zones or milkman fractures, which are pathognomonic for osteomalacia.
 Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Overview of Management
Because chronic pain and immobilization from fractures can significantly diminish the quality of life, it has been recommended that patients with extremely low bone mass or osteoporotic fractures documented prior to transplantation be counseled about the increased fracture risk that follows transplantation. [95] Since even an entirely normal bone density pretransplantation is not protective against posttransplantation fracture, prophylaxis against bone loss should be given in all transplant recipients, without regard to baseline bone density. [94, 102, 103, 104]
Any patient who meets World Health Organization (WHO) criteria for low bone mass (osteoporosis) should receive pharmacologic treatment. There are no specific therapies for posttransplantation osteoporosis approved by the US Food and Drug Administration. Therapeutic strategies are extrapolated from nontransplant situations and based on relatively small numbers of patients in clinical trials. Vitamin D and calcium alone are clearly insufficient to prevent transplant-related bone loss.
The aim of medical therapy should be to prevent bone loss and (if possible) restore bone loss before transplantation. Guidelines established by the American College of Rheumatology and the UK Consensus Group [96, 105] recommend that patients who receive daily glucocorticoid at doses of 7.5 mg of prednisolone or more for 6 months or longer should begin preventive therapy. Transplant recipients clearly meet this criterion.
Postrenal transplant bone disease reflects the complexity of preexisting renal osteodystrophy, although many aspects of renal osteodystrophy improve with transplantation. Hyperparathyroidism may persist in a subset of patients. [106] Patients should be advised to take an adequate total elemental calcium intake (ie, 1000-1500 mg), but the dose of supplemental calcium should be individualized on the basis of dietary calcium intake, menopausal status, and underlying medical issues. For example, a pharmacologic dose of calcium administered to a renal transplant recipient with persistent secondary hyperparathyroidism could worsen hypercalciuria because of excess PTH action and could be contraindicated.
Vitamin D Therapy
A study examined vitamin D metabolites in a cohort of 61 renal transplant recipients and found that nearly half of the patients demonstrated abnormal 1,25-D levels for at least 6 months after transplant, the period associated with the steepest decline in BMD. [107] 1,25-Dihydroxy vitamin D levels were low at transplantation in all patients and remained subnormal in 64% of patients at 3 months and 47% of patients at 6 months after transplant. After the transplantation, PTH levels responded quickly after surgery from 520.6 (197-770) to 169.2 (71.8-265.4) during the first month, and further down to 57.4 at 12-month follow-up. [108] Vitamin D supplementation at 800-1000 IU a day should be encouraged for all patients. However, patients with malabsorption, cystic fibrosis, or PBC have higher vitamin D requirements, [36, 109] and 25-hydroxyvitamin D levels should be monitored to assess the adequacy of replacement.
Calcidiol (25-hydroxyvitamin D)
In an uncontrolled 12-month study in 77 renal transplant recipients, Talalaj et al found that BMD remained stable at the LS spine (–0.2%) or modestly increased at the femur neck (1.3%) in subjects who received calcidiol 40 mcg with calcium 3 g daily, whereas the control group lost BMD by 7.1% at the LS and 5.5% at FN. A significant decrease in vertebral deformities was noted in the calcidiol-treated group. [110] In post-cardiac transplant patients, calcidiol 32,000 IU per week for 18 months increased BMD at the LS compared with calcitonin- or etidronate-treated groups. [111]
Alfacalcidol (1-α hydroxyl Vitamin D)
Alfacalcidol increases trabecular BMD and prevents vertebral fracture in early postmenopausal osteoporosis and glucocorticoid-induced osteoporosis. [112, 113] In cardiac transplant recipients, spine and femoral bone loss were decreased with alfacalcidol plus calcium. Moreover, fewer vertebral fractures were seen in alfacalcidol-treated patients, compared with a control group receiving etidronate and calcium. [114]
Renal transplant recipients receiving alfacalcidol (0.25 mcg/d) with calcium over 6 months had diminished bone loss at the LS and trochanter and almost complete prevention of bone loss at the femoral neck. [107] In addition, alfacalcidol may prevent fractures due to falls by improving muscle power. [115]
Calcitriol (1,25-dihydroxyvitamin D)
Kidney transplant and SPKT patients may continue to require posttransplant calcitriol as 1-α hydroxylase activity is slowly recovered approximately a year after transplantation. [116] However, studies showed mixed results. A study showed negative findings where a low dose of calcitriol (0.25 - 0.5 mcg/48hrs) did not prevent bone loss in heart and kidney recipients. [94] However, another study suggested that starting calcitriol 0.5 mcg immediately after heart or lung transplantation for the first 6 months can preserve bone mass compared with the non-intervention group. [117] This benefit did not persist beyond 12-24 months. In a randomized double-blind 2-year study using higher doses of calcitriol at 0.5-0.75 mcg daily, beginning by 4 weeks posttransplantation, less bone loss was noted at the FN but both groups similarly lost BMD at the LS over a year.
As might be anticipated with this activated form of vitamin D, hypercalcemia and hypercalciuria can occur, seen in 18% and 59% of patients treated with calcitriol. Routine monitoring of urine and serum calcium is indicated if calcitriol is prescribed. Therefore, therapy must be individualized because a significant proportion of patients have persistent hyperparathyroidism.
Calcitriol may have significant nonosteogenic benefits, which include recognized immunomodulatory and steroid-sparing actions. In a Turkish study of renal transplant recipients, patients who received calcitriol had lower PTH levels in the third year posttransplantation, as well as decreased requirements for pulse steroid doses. The increase in creatinine levels was also less in the calcitriol group. The authors concluded that calcitriol may reduce the rate of loss of renal function after renal transplant and protect renal allograft function. [118]
Anti-Osteoporosis Drugs
Bisphosphonates
The evidence of bisphosphonate use in preventing or treating transplant-associated bone loss is limited by small numbers of patients and differing immunosuppressant regimens and underlying conditions. The timing of the study is particularly important because it is not appropriate to compare interventions in the early posttransplant period (within 6 mo of transplant) when bone loss is greatest, with later interventions. [94]
A small study in heart transplant recipients demonstrated a benefit of pamidronate. The treatment group of 18 patients received intravenous pamidronate 60 mg within 2 weeks of transplant with calcitriol at 0.25 mcg/d. This was followed by etidronate 400 mg/d for 14 days every 3 months. [119] A second group only received calcium and vitamin D. After 12 months, spinal BMD was preserved in the pamidronate-treated group whereas the control group lost 6-7%. FN BMD fell 2.7% in the antiresorptive group, whereas the comparator group lost 10.6%. [120]
In a prospective, randomized, controlled study, 26 male renal transplant patients received either placebo or intravenous pamidronate at 0.5 mg/kg at the time of transplant and 1 month later. At 12 months posttransplant, spine and femur-neck BMD were preserved in the pamidronate group, whereas in the control group, spine and femur-neck BMD fell 6.4% and 9%, respectively. In this small study, transient hypocalcemia was the only noted adverse effect, seen in 2 patients. [121] Another study from the University of North Carolina also showed pamidronate improved BMD (8.8% BMD at LS and 8.2% at FN) in patients with cystic fibrosis after lung transplantation. [122]
Zoledronic acid was also used in patients with renal transplantation and showed efficacy in preventing bone loss in the first 6 months in a small (n=20) randomized, placebo-controlled study. BMD at the femoral neck showed no change in the zoledronic acid group but fell in the placebo group. BMD at the lumbar spine increased in the zoledronic acid group and was unchanged in the placebo group. In addition, the zoledronate-treated group showed Improved trabecular mineralization and architecture. [123]
A meta-analysis of 9 studies including 625 subjects summarized that bisphosphonate use after various organ transplantations (4 liver, 3 kidney, and 2 heart) was associated with a lower risk of fractures (OR 0.53, CI 0.30-0.91) and increased BMD in LS (3.34%, 1.10-5.58) and FN (3.04%, 1.42-5.65). [26] Two additional meta-analyses, which specifically examined the efficacy in post-kidney transplant patients, also found increased BMD in LS (7.4%) and FN (6.0%); however, the risk of fracture was not reduced. [124] There was no significant change in terms of kidney function or calcium levels with bisphosphonate use. [124, 125]
Lastly, a randomized controlled trial demonstrated that monthly nesidronate use in patients with various organ transplants increased BMD in LS after 12 months. [119]
Overall, bisphosphonates seem efficacious and safe, and the National Kidney Foundation (K/DOQI) recommends that if a patient has a BMD T-score of –2 or lower at the time of transplantation or at subsequent evaluations, therapy with a parenteral amino-bisphosphonate should be considered. [97] However, the risk of rare side effects such as atypical femur fracture or jaw osteonecrosis in post-transplant patients with decreased bone turnover is not known. There remain significant concerns for the use of bisphosphonates in renal patients with preexisting low bone turnover disease, wherein bisphosphonates could further slow bone turnover and potentially increase fracture rate. [107] The optimal duration and dose of bisphosphonate still need to be further studied.
Denosumab
Denosumab also increased BMD in patients with organ transplantation. A randomized controlled trial showed that denosumab increased BMD by 4.6% in LS and 5.1% in the total hip after 12 months. Another study including kidney, kidney/pancreas, and liver transplant recipients also showed an increased BMD in LS by 11.5% and FN by 10.4%. [126]
A meta-analysis of 5 studies that included 162 kidney transplant recipients showed a significant increase in BMD in LS and FN without any changes in PTH or Ca levels. [127] Interestingly, denosumab improved cortical microarchitecture based on HR-qCT. [128]
However, clinicians should be cautioned about the risk of increased infection and reported cases of severe hepatoxicity with denosumab, given the immunocompromised state and underlying conditions of patients. [129, 130] Also, the rapid decline in BMD after denosumab withdrawal was also observed in this group of patients, which emphasizes the importance of compliance, coordination, and alternative treatment upon discontinuation. [131]
Anabolic agents
Teriparatide showed promising efficacy in glucocorticoid-induced bone loss, [132] which is a major contributor in acute severe bone loss in post-transplant patients. Unfortunately, a small trial of teriparatide in transplant recipients did not increase BMD or lower the risk of fracture. [133]
Romosozumab is a new agent exerting dual effects—anabolic and antiresorptive—by blocking sclerostin. Considering the elevated sclerostin in patients with transplantation, which is associated with mortality, romosozumab should be examined as a potential therapeutic candidate. [134, 135, 136]
Estrogen replacement
Transdermal estrogen therapy provides protection against osteoporosis in postmenopausal women with liver transplants, as it does in healthy postmenopausal women. [137] Estrogen is also known to improve BMD in women receiving glucocorticoids and to prevent CsA-mediated bone loss in animals. If estrogen is prescribed together with progesterone, fixed daily doses are preferred over cyclic regimens because estrogen can enhance the hepatic metabolism of cyclosporine, resulting in erratic blood levels. Estrogen alone is probably insufficient to prevent transplant-induced bone loss, particularly in the first year following transplantation. [138]
Diet, Lifestyle, and Activity
Diet and lifestyle
Smoking and alcohol cessation should be counseled. Adequate nutrition is essential for optimal bone health and essential for overall well-being in transplant recipients. A significant number of patients have compromised nutritional status after successful organ transplantation. Malnutrition has been associated with increased morbidity and higher rates of hospitalization.
Low pretransplant body weight that remains low posttransplant negatively affects bone density. Renal transplant recipients with osteoporosis have lower posttransplant cholesterol and high-density lipoprotein (HDL) levels (likely attributable to nutritional deficiencies). [118] Global assessment of nutritional status by a certified dietician to detect malnutrition followed by appropriate nutritional interventions may be necessary for the transplant recipient. [139]
Exercise
Exercise that provides a mechanical load to bone represents an osteogenic stimulus. A 6-month, randomized, controlled clinical trial in heart transplant recipients showed that resistance exercise training in addition to alendronate can prevent bone loss. In this study, 8 different resistance training such as lumbar extension exercises added the benefit of BMD of the femur neck and lumbar spine to 2.1% and 3.4% of pretransplantation levels, respectively. [140] Also, a structured resistance exercise program demonstrated skeletal benefit in lung transplant patients. [141]
Similar exercise-associated preservation of bone mass was demonstrated in the same population in a prospective study of nasal calcitonin with and without resistance exercise in 18 heart transplant recipients. Lumbar BMD declined to 16.9% below pretransplant levels in the calcitonin-only group, whereas the calcitonin-plus-exercise group achieved BMD results to within 5% of their pretransplant levels by 8 months after transplant. [142]
It has not been studied in post-transplant patients, but high-intensity resistance and impact training improved bone mass at the lumbar spine and femoral neck in postmenopausal women. [143] Overall, regular weight-bearing and muscle-strengthening exercises are recommended to reduce the risk of fracture when medically possible. Improving overall fitness is recommended to minimize the risk of falling. Therefore, following transplantation, weight-bearing exercise should be resumed as soon as possible, and a prescribed rehabilitation program encouraged. [94]
Long-Term Monitoring
In the transplant recipient, annual assessments of clinical risk factors for osteoporosis and fracture including DEXA are important. Clinicians should pay extra attention to fall risk. Polypharmacy particularly CNS depressants or pain killers, postural hypotension from autonomic neuropathy and vascular insufficiency, hypoglycemia in diabetic patients, and certain drugs with negative skeletal effects such as thiazolidinedione and SGLT-2 inhibitors (canagliflozin) should be addressed. Any clinical suggestion of fracture should prompt bone radiographs. If an antiresorptive agent such as a bisphosphonate is used, significant increases in spine BMD may be observed within 1 year. An increase in femoral neck BMD may not be seen until after an average of 4-5 years and probably longer with weaker antiresorptive agents.
Consultations
Given the medical complexity of the typical patient awaiting solid organ transplantation, a referral to an endocrinologist or bone metabolism expert should be considered.
Patient education
For patient education information, see the Osteoporosis and Bone Health Center and Procedures Center, as well as Understanding Osteoporosis Medications, Heart and Lung Transplant, Kidney Transplant, and Liver Transplant. For further information, see Mayo Clinic - Kidney Transplant Information.
Questions & Answers
Overview
Which transplant recipients should receive prophylaxis against osteoporosis?
What are the signs and symptoms of organ transplantation-related osteoporosis?
What is the role of lab testing in the pretransplantation evaluation for solid organs?
How is organ transplantation-related osteoporosis treated?
What is the pathophysiology of lung transplantation-related osteoporosis?
What is the pathophysiology of heart transplantation-related osteoporosis?
What is the pathophysiology of liver transplantation-related osteoporosis?
What is the pathophysiology of kidney transplantation-related osteoporosis?
What is the role of cyclosporin A in the pathogenesis of organ transplantation-related osteoporosis?
What is the role of tacrolimus in the pathogenesis of organ transplantation-related osteoporosis?
What is the role of rapamycin in the pathogenesis of organ transplantation-related osteoporosis?
What is the focus of the clinical history for organ transplantation-related osteoporosis?
What is the focus of the physical exam for organ transplantation-related osteoporosis?
What is the role of exercise in the treatment of organ transplantation-related osteoporosis?
What is included in the long-term monitoring of organ transplantation-related osteoporosis?
What is included in patient education about organ transplantation-related osteoporosis?
What is the prevalence of organ transplantation-related osteoporosis?
What are the risk factors for organ transplantation-related osteoporosis?
Which patients are at highest risk for organ transplantation-related osteoporosis?
Which patient groups have the highest prevalence of organ transplantation-related osteoporosis?
Which blood tests are performed in the workup of organ transplantation-related osteoporosis?
What is the role of bone densitometry in the workup of organ transplantation-related osteoporosis?
What is the role of plain radiography in the workup of organ transplantation-related osteoporosis?
How is organ transplantation-related osteoporosis treated?
-
Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
-
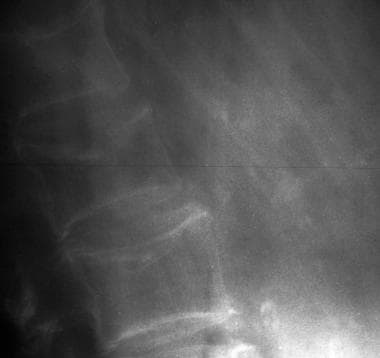
Anteroposterior and lateral radiographs of an L1 osteoporotic wedge compression fracture.
Tables
What would you like to print?
- Practice Essentials
- The Risk of Fracture and Skeletal Phenotype After Solid Organ Transplantation
- Pathophysiology of Osteoporosis in Transplantation
- Presentation
- Assessment of Risk Factors and the Risk of Fracture
- Overview of Management
- Vitamin D Therapy
- Anti-Osteoporosis Drugs
- Diet, Lifestyle, and Activity
- Long-Term Monitoring
- Consultations
- Questions & Answers
- Show All
- Media Gallery
- References